| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hyeonseok Yoon | -- | 8821 | 2024-02-05 11:01:02 | | | |
| 2 | Mona Zou | Meta information modification | 8821 | 2024-02-07 08:20:46 | | |
Video Upload Options
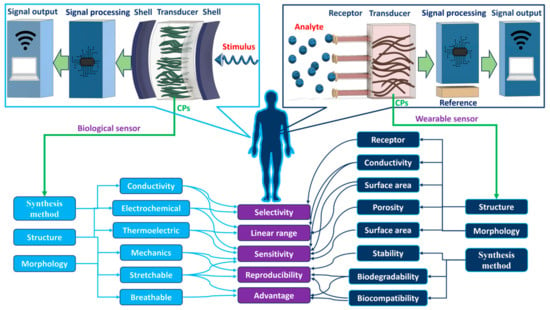
Conducting polymers (CPs) are an innovative class of materials recognized for their high flexibility and biocompatibility, making them an ideal choice for health monitoring applications that require flexibility. They are active in their design. Advances in fabrication technology allow the incorporation of CPs at various levels, by combining diverse CPs monomers with metal particles, 2D materials, carbon nanomaterials, and copolymers through the process of polymerization and mixing. This method produces materials with unique physicochemical properties and is highly customizable. In particular, the development of CPs with expanded surface area and high conductivity has significantly improved the performance of the sensors, providing high sensitivity and flexibility and expanding the range of available options. However, due to the morphological diversity of new materials and thus the variety of characteristics that can be synthesized by combining CPs and other types of functionalities, choosing the right combination for a sensor application is difficult but becomes important.
1. Overview of the Use of CP in Sensor Design
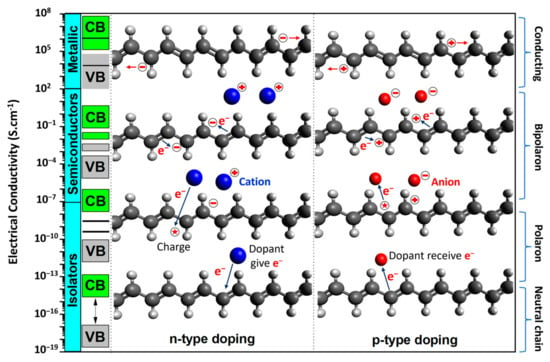
1.1. CP Characterization
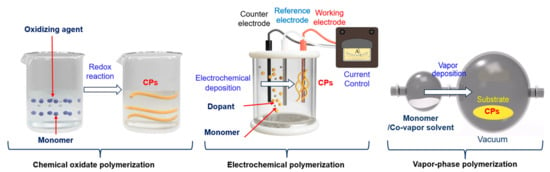
1.2. CP Processing
1.2.1. Chemical Oxidate Polymerization
1.2.2. Electrochemical Polymerization
1.2.3. Vapor-Phase Polymerization
| Method | Advantages | Disadvantages |
|---|---|---|
| Chemical | Scalable production, easy to functionalize, involves chemical doping, straightforward and efficient route, yields a composite material, easy to control the morphology |
Time-consuming, only thick films and powders can be synthesized, CPs may impurify, heterogeneity optimization, indirect approach |
| Electrochemical | No oxidizing agent is required, requires less time, produced CPs have high electrical conductivity, thin films can be produced with dimensional control. Eco-friendly, solventless, efficient, requires a small amount of chemical |
Large-scale production is not possible, it is difficult to remove the grown film from the electrode surface, larger reactors are required |
| Vapor-phase polymerization | Covers many surfaces, monomer easily combines with volatile substances, no solvent required, few impurities | Long duration, low yield, hard to control synthesis, CPs heterogeneity |
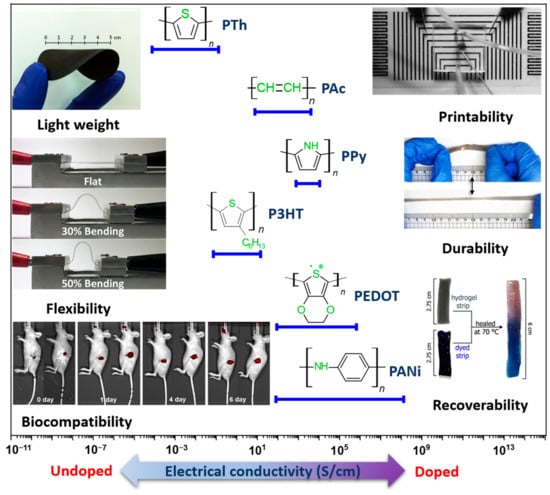
2. Role and Advantages of CPs in Enhancing Sensor Performance
| Role of CP/Analyte Detection | Polymerization Method | Conductivity | Young’s Modulus | CP Advantages | CP Disadvantages |
|---|---|---|---|---|---|
| Transducer PPA/interleukin 6 sensing [47] |
Vapor-phased deposition | 10 to 104 S/cm | 1.2 to 2.0 GPa | Easy synthesis, controllable, high electrical conductivity | Poor stability, non-thermoplastic |
| Transducer PPy/dopamine detection [48] |
Chemical oxidative | 40 to 200 S/cm [49] | 430 to 800 MPa [49] | Biocompatibility, promotes cell proliferation, high conductivity, environmental friendliness, low cost, compatibility | Rigid, brittle, non-thermoplastic, nondegradable, insoluble in acetone, methanol, and ethanol solvents |
| Transducer PANi/pH detection [50] |
Chemical oxidative | 5 S/cm [51] | 2.0 to 4.0 GPa [51] | Reversible doping, properties controllable by pH, wide range of conductivity, controllable conductivity, colored and transparent, various synthesis ways, stability with environment |
Low processing capacity, inflexibility, lack of biodegradability, poor solubility, complex process |
| Transducer PTh/parathyroid hormone detection [52] | Vapor-phased deposition | 10 to 100 S/cm [53] | 3.0 GPa [53] | Low cost, high electrical, mechanical, and optical properties, high thermal and environmental constancy, smaller bandgap energy (2.0 eV) compared with PANi and PPy | Poor solubility with ordinary solvents, difficult to synthesize, poor chemical stability and processibility, poor flexibility |
| Transducer P3HT/immunoglobulin G detection [54] |
Chemical, 3D printing | 10−2, 10−5 to 10 S/cm [55] |
28 MPa [56] |
Low cost, high electrical, mechanical, and optical properties, high thermal and environmental constancy | Difficult process, poor solubility with common solvents, difficult to synthesize |
| Transducer PEDOT/temperature sensing [57] | Chemical oxidative | 1200 S/cm [58] | 1.2 to 4.0 GPa [58] | Transparent conductor, low redox potential, moderate bandgap, environmentally and electrochemically stable | Limited solubility, acidic nature, anisotropic charge injection, hygroscopicity |
2.1. The Use of PAc in the Fabrication of the Transducer
2.1.1. Effect of PAc Polymerization on Transducer Fabrication
2.1.2. Effect of PAc Structure and Morphology on Transducer Fabrication
2.2. The Use of PPy in the Fabrication of the Transducer
2.2.1. Effect of PPy Polymerization on Transducer Fabrication
2.2.2. Effect of PPy Structure and Morphology on Transducer Fabrication
2.3. The Use of PANi in the Fabrication of the Transducer
2.3.1. Effect of PANi Polymerization on Transducer Fabrication
2.3.2. Effect of PANi Structure and Morphology on Transducer Fabrication
2.4. The Use of PTh and P3HT Derivatives in the Fabrication of the Transducer
2.4.1. Effect of the Polymerization of PTh and P3HT Derivatives on Transducer Fabrication
2.4.2. Effect of the Morphology and Structure of the Polymerization of PTh and P3HT Derivatives on Transducer Fabrication
2.5. The Use of PEDOT in the Fabrication of the Transducer
2.5.1. Effect of PEDOT Polymerization on Transducer Fabrication
2.5.2. Effect of PEDOT Morphology and Structure on Transducer Fabrication
References
- Heeger, A. Nobel lecture: Semiconducting and metallic polymers: The fourth generation of polymeric materials. Rev. Mod. Phys. 2001, 73, 681–700.
- Jacobs, I.; Lin, Y.; Huang, Y.; Ren, X.; Simatos, D.; Chen, C.; Tjhe, D.; Statz, M.; Lai, L. High-Efficiency Ion-Exchange Doping of Conducting Polymers. Adv. Mater. 2022, 34, 2102988.
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350.
- Yoon, H.; Sungrok, K.; Jyongsik, J.Y. Field-effect-transistor sensor based on enzyme-functionalized polypyrrole nanotubes for glucose detection. J. Phys. Chem. B 2008, 112, 9992–9997.
- Jang, J.; Yoon, H. Multigram-scale fabrication of monodisperse conducting polymer and magnetic carbon nanoparticles. Small 2005, 1, 1195–1199.
- Yu, Y.; Peng, S.; Blanloeuil, P.; Wu, S.; Wang, C. Wearable temperature sensors with enhanced sensitivity by engineering microcrack morphology in PEDOT: PSS–PDMS sensors. ACS Appl. Mater. Interfaces 2020, 12, 36578–36588.
- Shaari, A.; Ramli, M.; Mohtar, M.; Rahman, N.; Ahmad, A. Synthesis and conductivity studies of poly (Methyl Methacrylate)(PMMA) by co-polymerization and blending with polyaniline (PANi). Polymers 2021, 13, 1939.
- Ditte, K.; Perez, J.; Chae, S.; Hambsch, M.; Al-Hussein, M.; Komber, H.; Formanek, P.; Mannsfeld, S.C.; Fery, A.; Kiriy, A.; et al. Ultrasoft and high-mobility block copolymers for skin-compatible electronics. Adv. Mater. 2021, 33, 2005416.
- Jang, J.; Nam, Y.; Yoon, H. Fabrication of Polypyrrole–Poly (N-vinylcarbazole) Core–Shell Nanoparticles with Excellent Electrical and Optical Properties. Adv. Mater. 2005, 17, 1382–1386.
- Reichstein, P.; Gödrich, S.; Papastavrou, G.; Thelakkat, M. Influence of composition of amphiphilic double-crystalline P3HT-b-PEG block copolymers on structure formation in aqueous solution. Macromolecules 2016, 49, 5484.
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-doped conducting polymers for electrochemical sensors and biosensor. J. Mater. Chem. B 2018, 6, 4173–4190.
- Jeong, J.; Jo, G.; Choi, S.; Kim, Y.; Yoon, H.; Ryu, S.; Jung, J.; Chang, M. Solvent additive-assisted anisotropic assembly and enhanced charge transport of π-conjugated polymer thin films. ACS Appl. Mater. Interfaces 2018, 10, 18131–18140.
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, P.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. Rsc Adv. 2015, 5, 37553–37567.
- Maziz, A.; Özgür, E.; Bergaud, C.; Uzun, L. Progress in conducting polymers for biointerfacing and biorecognition applications. Sens. Actuators Rep. 2021, 3, 100035.
- Pooja, A.; Prasher, P.; Mudila, H. Factors affecting the electrical conductivity of conducting polymers. Carbon Lett. 2023, 33, 307–324.
- Abu-Thabit, N. Chemical oxidative polymerization of polyaniline: A practical approach for preparation of smart conductive textiles. J. Chem. Educ. 2016, 93, 1606–1611.
- Thanasamy, D.; Jesuraj, D.; Kannan, S.; Avadhanam, V. A novel route to synthesis polythiophene with great yield and high electrical conductivity without post doping process. Polymers 2019, 175, 32–40.
- Kim, K.; Kim, H.; Zhang, H.; Park, W.; Meyer, D.; Kim, M.K.; Kim, B.; Park, H.; Xu, B.; Kollbaum, P.; et al. All-printed stretchable corneal sensor on soft contact lenses for noninvasive and painless ocular electrodiagnosis. Nat. Commun. 2021, 12, 1544.
- Bamgbopa, M.; Edberg, J.; Engquist, I.; Berggren, M.; Tybrandt, K. Understanding the characteristics of conducting polymer-redox biopolymer supercapacitors. J. Mater. Chem. A 2019, 41, 23973–23980.
- Veeramuthu, L.; Venkatesan, M.; Benas, J.S.; Cho, C.J.; Lee, C.; Lieu, F.K.; Lin, J.H.; Lee, R.H.; Kuo, C.C. Recent progress in conducting polymer composite/nanofiber-based strain and pressure sensors. Polymers 2021, 13, 4281.
- Shen, H.; Liu, H.; Wang, X. Surface construction of catalase-immobilized Au/PEDOT nanocomposite on phase-change microcapsules for enhancing electrochemical biosensing detection of hydrogen peroxide. Appl. Surf. Sci. 2023, 612, 155816.
- Heinze, J.; Frontana-Uribe, B.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771.
- Dong, H.; Li, C.; Chen, W.; Zhou, Q.; Zeng, Z.; Luong, J. Sensitive amperometric immunosensing using polypyrrolepropylic acid films for biomolecule immobilization. Anal. Chem. 2006, 78, 7424–7431.
- Coelho, M.; Giarola, J.; Silva, A.; Tarley, C.; Borges, K.; Pereira, A. Development and application of electrochemical sensor based on molecularly imprinted polymer and carbon nanotubes for the determination of carvedilol. Chemosensors 2016, 4, 22.
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and electrochemical synthesis of polypyrrole using carrageenan as a dopant: Polypyrrole/multi-walled carbon nanotube nanocomposites. Polymers 2018, 10, 632.
- Husain, A. Electrical conductivity based ammonia, methanol and acetone vapour sensing studies on newly synthesized polythiophene/molybdenum oxide nanocomposite. J. Sci. Adv. Mater. Devices 2021, 6, 528–537.
- Lawal, A.; Wallace, G. Vapor phase polymerization of conducting and non-conducting polymers: A review. Talanta 2014, 119, 133–143.
- Majumdar, S.; Sarmah, K.; Mahanta, D. A simple route to prepare polypyrrole-coated filter papers via vapor phase polymerization and their gas sensing application. ACS Appl. Polym. Mater. 2020, 2, 1933–1942.
- Kwon, G.; Kim, S.; Kim, D.; Lee, K.; Jeon, Y.; Park, C.; You, J. Vapor phase polymerization for electronically conductive nanopaper based on bacterial cellulose/poly (3,4-ethylenedioxythiophene). Carbohydr. Polym. 2021, 257, 117658.
- Jun, T.S.; Kim, C.K.; Kim, Y.S. Vapor phase polymerization of polyaniline nanotubes using Mn3O4 nanofibers as an oxidant. Mater. Lett. 2014, 133, 17–19.
- Yang, Y.; Zhang, L.; Li, S.; Wang, Z.; Xu, J.; Yang, W.; Jiang, Y. Vapor phase polymerization deposition conducting polymer nanocomposites on porous dielectric surface as high performance electrode materials. Nano-Micro Lett. 2013, 5, 40–46.
- So, J.; Mayevsky, D.; Winther-Jensen, O.; Winther-Jensen, B. A novel route for polymerisation of thiophene based conducting polymers using trace-free oxidants. Polym. Chem. 2014, 5, 361–364.
- Yoon, H.; Choi, M.; Lee, K.; Jang, J. Versatile strategies for fabricating polymer nanomaterials with controlled size and morphology. Macromol. Res. 2008, 16, 85–102.
- Lee, J.; Lee, Y.; Ahn, K.; Huh, J.; Shim, H.; Sampath, G.; Im, W.; Huh, Y.; Yoon, H. Role of co-vapors in vapor deposition polymerization. Sci. Rep. 2015, 5, 8420.
- Kwon, O.; Park, S.; Park, H.; Kim, T.; Kang, M.; Jang, J.; Yoon, H. Kinetically controlled formation of multidimensional poly (3,4-ethylenedioxythiophene) nanostructures in vapor-deposition polymerization. Chem. Mater. 2012, 24, 4088–4092.
- Luong, J.; Narayan, T.; Solanki, S.; Malhotra, B. Recent advances of conducting polymers and their composites for electrochemical biosensing applications. J. Funct. Biomater. 2020, 11, 71.
- Park, C.; Ha, T.; Kim, M.; Raja, N.; Yun, N.; Sung, M.; Kwon, O.; Yoon, H.; Lee, C. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158.
- Ahn, K.; Lee, Y.; Choi, H.; Kim, M.; Im, K.; Noh, S.; Yoon, H. Surfactant-templated synthesis of polypyrrole nanocages as redox mediators for efficient energy storage. Sci. Rep. 2015, 5, 14097.
- Lee, K.; Oh, Y.; Yoon, H.; Chang, M.; Kim, H. Multifunctional role of MoS2 in preparation of composite hydrogels: Radical initiation and cross-linking. ACS Appl. Mater. Interfaces 2020, 12, 8642–8649.
- Onggar, T.; Kruppke, I. Techniques and processes for the realization of electrically conducting textile materials from intrinsically conducting polymers and their application potential. Polymers 2020, 12, 2867.
- Ding, L.; Yu, Z.; Wang, X.; Yao, Z.; Lu, Y.; Yang, C.; Wang, J.; Pei, J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123, 7421–7497.
- Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical design of functional polymer structures for biosensors: From nanoscale to macroscale. Polymers 2018, 10, 551.
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697.
- Nguyen, D.; Yoon, H. Recent Advances in Nanostructured Conducting Polymers: From Synthesis to Practical Applications. Polymers 2016, 8, 118.
- Yoon, H.; Jang, J. Conducting-polymer nanomaterials for high-performance sensor applications: Issues and challenges. Adv. Funct. Mater. 2009, 19, 1567–1576.
- Lakard, B. Electrochemical biosensors based on conducting polymers: A review. Appl. Sci. 2020, 10, 6614.
- Aydın, E.; Aydın, M.; Sezgintürk, M. A novel electrochemical immunosensor based on acetylene black/epoxy-substituted-polypyrrole polymer composite for the highly sensitive and selective detection of interleukin 6. Talanta 2021, 222, 121596.
- Vellaichamy, B.; Periakaruppan, P.; Paulmony, T. Evaluation of a new biosensor based on in situ synthesized PPy-Ag-PVP nanohybrid for selective detection of dopamine. J. Phys. Chem. B 2017, 121, 1118–1127.
- Sevil, B.; Zuhal, K. Synthesis and characterization of polypyrrole nanoparticles and their nanocomposites with poly(propylene). Macromol. Symp. 2010, 295, 59–64.
- Li, Y.; Mao, Y.; Xiao, C.; Li, X. Flexible pH sensor based on a conductive PANI membrane for pH monitoring. RSC Adv. 2010, 10, 21–28.
- Beygisangchin, M.; Rashid, S.; Shafie, S.; Sadrolhosseini, A.; Lim, H. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003.
- Li, S.; Liu, Y.; Ma, Q. A novel polydopamine electrochemiluminescence organic nanoparticle-based biosensor for parathyroid hormone detection. Talanta 2019, 202, 540–545.
- Wang, X.; Feng, X. Effects of thickness on mechanical properties of conducting polythiophene films. J. Mater. Sci. Lett. 2002, 21, 715–717.
- Runfang, H.; Yangfan, Y.; Leilei, L.; Jianlong, J.; Qiang, Z.; Lifeng, D.; Shengbo, S.; Qiang, L. P3HT-based organic field effect transistor for low-cost, label-free detection of immunoglobulin G. J. Biotechnol. 2022, 359, 75–81.
- Hynynen, J.; Kiefer, D.; Yu, L.; Kroon, R.; Munir, R.; Amassian, A.; Kemerink, M.; Müller, C. Enhanced electrical conductivity of molecularly p-doped poly (3-hexylthiophene) through understanding the correlation with solid-state order. Macromolecules 2017, 50, 8140–8148.
- Müller, C.; Goffri, S.; Breiby, D.; Andreasen, J.; Chanzy, H.D.; Janssen, R.A.; Nielsen, M.M.; Radano, C.P.; Sirringhaus, H.; Smith, P.; et al. Tough, semiconducting polyethylene-poly (3-hexylthiophene) diblock copolymers. Adv. Funct. Mater. 2017, 15, 17.
- Wang, Y.; Fei, Y.; Sekine, T.; Takeda, Y.; Yokosawa, K.; Matsui, H.; Kumaki, D.; Shiba, T.; Nishikawa, T.; Tokito, S. Fully printed PEDOT: PSS-based temperature sensor with high humidity stability for wireless healthcare monitoring. Sci. Rep. 2020, 10, 2467.
- Rahimzadeh, Z.; Naghib, S.; Zare, Y.; Rhee, K. An overview on the synthesis and recent applications of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) in industry and biomedicine. J. Mater. Sci. Lett. 2020, 55, 7575–7611.
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab A Chip 2018, 18, 217–248.
- Kim, J.; Campbell, A.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- Yoon, H.; Chang, M.; Jang, J. Sensing behaviors of polypyrrole nanotubes prepared in reverse microemulsions: Effects of transducer size and transduction mechanism. J. Phys. Chem. B 2006, 110, 14074–14077.
- Jiang, L.; Luo, J.; Dong, W.; Wang, C.; Jin, W.; Xia, Y.; Wang, H.; Ding, H.; Jiang, L.; He, H. Development and evaluation of a polydiacetylene based biosensor for the detection of H5 influenza virus. J. Virol. Methods 2015, 219, 38–45.
- You, J.; Heo, J.; Kim, J.; Park, T.; Kim, B.; Kim, H.; Choi, Y.; Kim, H.; Kim, E. Noninvasive photodetachment of stem cells on tunable conductive polymer nano thin films: Selective harvesting and preserved differentiation capacity. ACS Nano 2013, 7, 4119–4128.
- Gliga, E.; Iacob, B.; Cheșcheș, B.; Florea, A.; Tudoran, L.; Bodoki, E.; Oprean, R. Electrochemical platform for the detection of adenosine using a sandwich-structured molecularly imprinted polymer-based sensor. Electrochim. Acta 2020, 354, 136656.
- Tang, T.; Lu, S.; Ahumada, G.; Bielawski, C. Megadalton Macromolecules Made-to-Order in Minutes: A Highly Active Nanosphere Catalyst for Preparing High-Molecular Weight Polymers. Macromolecules 2022, 55, 9943–9950.
- Qi, M.; Wang, P.; Cong, R.; Yang, J. Simultaneous determination of roxithromycin and ambroxol hydrochloride in a new tablet formulation by liquid chromatography. J. Pharm. Biomed. Anal. 2004, 35, 1287–1291.
- Wang, X.; Gao, Q.; Schubert, D.W.; Liu, X. Review on Electrospun Conductive Polymer Composites Strain Sensors. Adv. Mater. Technol. 2023, 8, 2300293.
- Gui, M.; Jiang, J.; Wang, X.; Yan, Y.; Li, S.; Xiao, X.; Liu, T.; Liu, T.; Feng, Y. Copper ion-mediated glyphosate detection with N-heterocycle based polyacetylene as a sensing platform. Sens. Actuators B Chem. 2017, 243, 696–703.
- Gu, Y.; Liu, L.; Wang, Y.; Zhang, C.; Satoh, T. Chromaticity sensor for discriminatory identification of aliphatic and aromatic primary amines based on conformational changes of polyacetylene. Talanta 2024, 268, 25361.
- Foulds, N.; Lowe, C.R. Enzyme entrapment in electrically conducting polymers. Immobilisation of glucose oxidase in polypyrrole and its application in amperometric glucose sensors. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1259–1264.
- Qi, G.; Huang, L.; Wang, H. Highly conductive free standing polypyrrole films prepared by freezing interfacial polymerization. Chem. Commun. 2012, 48, 8246–8248.
- Cetiner, S. Electrospun nanofibers of polypyrrole-poly (acrylonitrile-co-vinyl acetate). Text. Res. J. 2010, 80, 1784–1792.
- Jo, H.; Le, T.H.; Lee, H.; Lee, J.; Kim, M.; Lee, S.; Chang, M.; Yoon, H. Macrocyclic ligand-embedded graphene-in-polymer nanofiber membranes for lithium ion recovery. Chem. Eng. J. 2023, 6, 139274.
- Chronakis, S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymers 2006, 47, 159–1603.
- Kim, D.; Hendricks, J.; Sequera, C. Effect of immobilized nerve growth factor on conductive polymers: Electrical properties and cellular response. Adv. Funct. Mater. 2007, 17, 79–86.
- Liang, Y.; Goh, C. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119.
- Ramachandran, R.; Chen, T.; Chen, S.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439.
- Ferraz, N.; Strømme, M.; Fellström, B.; Pradhan, S.; Nyholm, L.; Mihranyan, A. In vitro and in vivo toxicity of rinsed and aged nanocellulose–polypyrrole composites. J. Biomed. Mater. Res. Part A 2012, 100, 2128–2138.
- Mobarakeh, G.; Prabhakaran, M.; Morshed, M.; Esfahani, M.; Baharvand, H.; Kiani, S.; Deyab, S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 17–35.
- Zheng, L.; Congdon, R.; Sadik, O.; Marques, C.; Davies, D.; Sammakia, B.; Lesperance, L.; Turner, J. Electrochemical measurements of biofilm development using polypyrrole enhanced flexible sensors. Sens. Actuators B Chem. 2013, 182, 725–732.
- Niţă, I.; Rabeah, K.; Tencaliec, A.; Cosnier, S.; Marks, R. Amperometric biosensor based on the electro-copolymerization of a conductive biotinylated-pyrrole and alginate-pyrrole. Synth. Met. 2009, 159, 1117–1122.
- An, Q.Q.; Feng, X.Z.; Zhan, T.; Cheng, Y.Y.; Han, G.C.; Chen, Z.; Kraatz, H.B. A simple synthesis of a core-shell structure PPy-Au nanocomposite for immunosensing of C-reactive protein. Talanta 2024, 267, 125158.
- Shamaeli, E.; Alizadeh, N. Nanostructured biocompatible thermal/electrical stimuli-responsive biopolymer-doped polypyrrole for controlled release of chlorpromazine: Kinetics studies. Int. J. Pharm. 2014, 472, 327–338.
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. Part A 2010, 93, 164–174.
- Tajik, S.; Beitollahi, H.; Nejad, F.; Shoaie, I.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856.
- Shi, W.; Han, G.; Chang, Y.; Song, H.; Hou, W.; Chen, Q. Using stretchable PPy@ PVA composites as a high-sensitivity strain sensor to monitor minute motion. ACS Appl. Mater. Interfaces 2020, 12, 45373–45382.
- Li, Y.; Neoh, G.; Cen, L.; Kang, E. Porous and electrically conductive polypyrrole–poly (vinyl alcohol) composite and its applications as a biomaterial. Langmuir 2005, 21, 10702–10709.
- Shi, G.; Zhang, Z.; Rouabhia, M. The regulation of cell functions electrically using biodegradable polypyrrole–polylactide conductors. Biomaterials 2008, 29, 3792–3798.
- Xiong, Z.; Yu, H.; Gong, X. Designing photothermal superhydrophobic PET fabrics via in situ polymerization and 1,4-conjugation addition reaction. Langmuir 2022, 38, 8708–8718.
- Wang, X.; Wan, A.; Jiang, G.; Raji, R.; Yu, D. Preparation of polypyrrole/silver conductive polyester fabric by UV exposure. Autex Res. J. 2021, 21, 231–237.
- Zhang, Z.; Roy, R.; Dugré, F.; Tessier, D.; Dao, L. In-vitro biocompatibility study of electrically conductive polypyrrole-coated polyester fabrics. J. Biomed. Mater. Res. 2001, 57, 63–71.
- Xu, H.; Holzwarth, J.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235.
- Shi, G.; Rouabhia, M.; Wang, Z.; Dao, L.; Zhang, Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004, 25, 2477–2488.
- Jang, J.; Oh, J. Novel crystalline supramolecular assemblies of amorphous polypyrrole nanoparticles through surfactant templating. Chem. Commun. 2002, 19, 2200–2201.
- Li, M.; Wu, J.; Su, H.; Tu, Y.; Shang, Y.; He, Y.; Liu, H. Ionic Liquid-polypyrrole-gold composites as enhanced enzyme immobilization platforms for hydrogen peroxide sensing. Sensors 2019, 19, 640.
- Jang, J.; Yoon, H. Facile fabrication of polypyrrole nanotubes using reverse microemulsion polymerization. Chem. Commun. 2003, 6, 720–721.
- Jang, J.; Yoon, H. Formation mechanism of conducting polypyrrole nanotubes in reverse micelle systems. Langmuir 2005, 21, 11484–11489.
- Zhou, D.X.; Cui, T.; Hines, A.; Greenberg, R. Implant Neural Prostheses 2; Techniques and Engineering Approaches; Springer Science + Business Media: Berlin, Germany, 2010; pp. 217–252.
- Salas, F.; Pardo, I.; Salavagione, H.; Aza, P.; Amougi, E.; Vind, J.; Martinez, A.T.; Camarero, S. Advanced synthesis of conductive polyaniline using laccase as biocatalyst. PLoS ONE 2016, 11, 0164958.
- Yoo, J.E.; Cross, J.L.; Bucholz, T.L.; Lee, K.S.; Espe, M.P.; Loo, Y.L. Improving the electrical conductivity of polymer acid-doped polyaniline by controlling the template molecular weight. J. Mater. Chem. 2007, 17, 1268–1275.
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153.
- Prabhakaran, M.; Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507.
- Borriello, A.; Durante, C.; Lusvardi, G.; Malavasi, G.; Menabue, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011, 22, 1053–1062.
- Zhang, Q.; Yan, Y.; Li, S.P.; Feng, T. Synthesis of a novel biodegradable and electroactive polyphosphazene for biomedical application. Biomed. Mater. 2009, 4, 035008.
- Oztekin, Y.; Ramanaviciene, A.; Yazicigil, Z.; Solak, A.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline-modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546.
- Abad, B.; Alda, I.; Díaz-Chao, P.; Kawakami, H.; Almarza, A.; Amantia, D.; Gutierrez, D.; Aubouy, L.; Martín-González, M. Improved power factor of polyaniline nanocomposites with exfoliated graphene nanoplatelets (GNPs). J. Mater. Chem. A 2013, 1, 10450–10457.
- Yan, H.; Sada, N.; Toshima, N. Thermal transporting properties of electrically conductive polyaniline films as organic thermoelectric materials. J. Therm. Anal. Calorim. 2002, 69, 881–887.
- Liu, C.; Hayashi, K.; Toko, K. Au nanoparticles decorated polyaniline nanofiber sensor for detecting volatile sulfur compounds in expired breath. Sens. Actuators B 2012, 161, 504–509.
- Lu, C.; Chen, X. Electrospun polyaniline nanofiber networks toward high-performance flexible supercapacitors. Adv. Mater. Technol. 2019, 4, 1900564.
- Park, S.; Park, S.; Yoon, H. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 2017, 9, 155.
- Dong, H.; Prasad, S.; Nyame, V.; Jones, W.E. Sub-micrometer conducting polyaniline tubes prepared from polymer fiber templates. Chem. Mater. 2004, 16, 371–373.
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319, 128293.
- Sajedi, S.A.; Bagheri-Mohagheghi, M.M.; Shirpay, A. PANI/SnO2 nanoparticle, FTO/PET and Al/PET as hybrid nanocomposite soft electrodes synthesized by sol–gel, spray pyrolysis and thermal vacuum evaporation methods. Bull. Mater. Sci. 2024, 47, 9.
- Yamamoto, T.; Sanechika, K.; Yamamoto, K. Preparation of thermostable and electric-conducting poly (2,5-thienylene). J. Polym. Sci. Polym. Lett. Ed. 1980, 18, 9–12.
- Li, K.; Gao, H.; Ren, X.; Gao, G. Regulatable thermochromic hydrogels via hydrogen bonds driven by potassium tartrate hemihydrat. ACS Sustain. Chem. Eng. 2019, 7, 15036–15043.
- Park, C.-S.; Kim, D.Y.; Jung, E.Y.; Jang, H.J.; Bae, G.T.; Kim, J.Y.; Shin, B.J.; Lee, H.-K.; Tae, H.-S. Ultrafast Room temperature synthesis of porous polythiophene via atmospheric pressure plasma polymerization technique and its application to NO2 gas sensors. Polymers 2021, 13, 1783.
- Scicchitano, P.; Iacoviello, M.; Passantino, A.; Gesualdo, M.; Trotta, F.; Basile, M.; De Palo, M.; Guida, P.; Paolillo, C.; Riccioni, G.; et al. Plasma levels of intact parathyroid hormone and congestion burden in heart failure: Clinical correlations and prognostic role. J. Cardiovasc. Dev. Dis. 2022, 9, 334.
- Kulkani, G.; Kandesar, P.; Velhal, N.; Phadtare, V.; Jatratkar, A.; Shinde, S.K.; Kim, D.Y.; Puri, V. Exceptional electromagnetic interference shielding and microwave absorption properties of room temperature synthesized polythiophene thin films with double negative characteristics (DNG) in the Ku-band region. Chem. Eng. J. 2019, 355, 196–207.
- Sarkar, S.; Kandesar, P.; Velhal, N.; Phadtare, V.; Jatratkar, A.; Shinde, S.; Kim, D.; Puri, V. Synthesis of graphene nanoplatelets/polythiophene as a high performance supercapacitor electrode material. New J. Chem. 2021, 45, 16187–16195.
- Zhang, Q.; Chang, M.; Fan, Z.; Deng, L.; Lu, Y. Direct (hetero) arylation polymerization, electrochemical and optical properties of regioregular 3-substituted polythiophenes with alkylsulphanyl and alkylsulfonyl groups. Eur. Polym. J. 2022, 166, 111032.
- Qu, K.; Kondengaden, S.M.; Li, J.; Wang, X.; Sevilla, M.D.; Li, L.; Zeng, X. Carbohydrate-functionalized polythiophene biointerface: Design, fabrication, characterization and application for protein analysis. Appl. Surf. Sci. 2019, 486, 561–570.
- Li, J.; Zhou, G.; Jin, X.; Hong, Y.; He, W.; Wang, S.; Chen, Y.; Yang, W.; Su, X. Direct activation of copper electroplating on conductive composite of polythiophene surface-coated with nickel nanoparticles. Compos. Part B Eng. 2018, 154, 257–262.
- Okada, N.; Sato, K.; Yokoo, M.; Kodama, E.; Kanehashi, S.; Shimomura, T. Thermoelectric properties of poly (3-hexylthiophene) nanofiber aerogels with a giant Seebeck coefficient. ACS Appl. Polym. Mater. 2020, 3, 456–463.
- Inagaki, S.; Higashihara, T. Synthesis of an ABC triblock copolymer by a bilateral Click reaction using α, ω-bifunctionalized poly (3-hexylthiophene) as an inner segment. Polym. Chem. 2022, 13, 3613–3618.
- Li, X.; Zhao, M.; Yu, X.; Chua, J.; Yang, Y.; Lim, K.; Zhai, W. Multifunctional and customizable lattice structures for simultaneous sound insulation and structural applications. Mater. Des. 2023, 234, 112354.
- Ghoorchian, A.; Madrakian, T.; Afkhami, A.; Bagheri, H. Spectroelectrochemical and electrochromic behavior of poly (methylene blue) and poly (thionine)-modified multi-walled carbon nanotubes. J. Solid State Electrochem. 2021, 25, 1217–1229.
- Hira, S.M.; Yusuf, M.; Annas, D.; Nagappan, S.; Song, S.; Park, S.; Park, K.H. Recent advances on conducting polymer-supported nanocomposites for nonenzymatic electrochemical sensing. Ind. Eng. Chem. Res. 2021, 60, 1325–13437.
- Abdelnasser, S.; Park, G.; Han, H.; Toth, R.; Yoon, H. Enhanced photocatalytic performance of poly (3,4-ethylenedioxythiophene)-coated TiO2 nanotube electrodes. Synth. Met. 2019, 251, 120–126.
- Nagamatsu, S.; Pandey, S. Ordered arrangement of F4TCNQ anions in three-dimensionally oriented P3HT thin film. Sci. Rep. 2020, 10, 20020.
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Thermoelectric properties of conducting polymers: The case of poly (3-hexylthiophene). Phys. Rev. B 2010, 82, 115454.
- Mori, H. Development of semiconducting polymers based on a novel heteropolycyclic aromatic framework. Polym. J. 2021, 53, 975–987.
- Xie, R.; Weisen, A.R.; Lee, Y.; Aplan, M.A.; Fenton, A.M.; Masucci, A.E.; Kempe, F.; Sommer, M. Glass transition temperature from the chemical structure of conjugated polymers. Nat. Commun. 2020, 11, 893.
- Irikura, K.K. Theoretical dissociation, ionization, and spin–orbit energetics of the diatomic platinum hydrides PtH, PtH+, and PtH−. J. Chem. Phys. 2022, 10, 157.
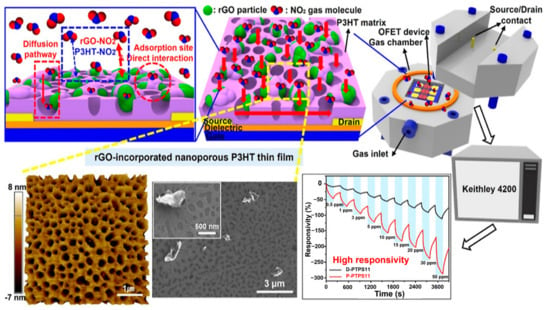
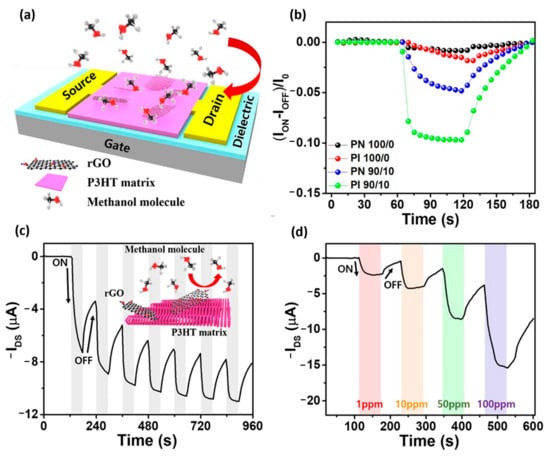
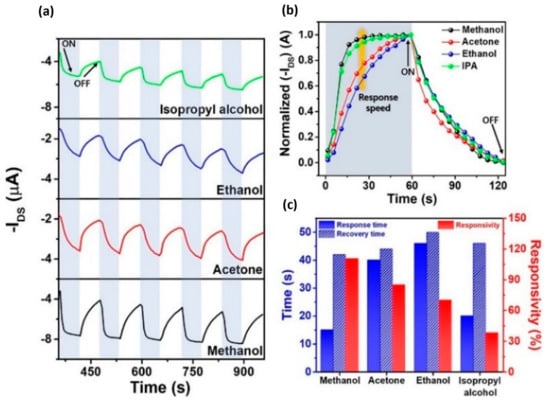
- Shin, S.; Jeong, G.; Phu, N.; Cheon, H.; Tran, V.; Yoon, H.; Chang, M. Improved NO2 Gas-Sensing Performance of an Organic Field-Effect Transistor Based on Reduced Graphene Oxide-Incorporated Nanoporous Conjugated Polymer Thin Films. Chem. Mater. 2023, 35, 7460–7474.
- Lin, Y.; Huang, W.; Li, J.; Yang, Y.; Chen, W.; Chueh, C. Improving mobility–stretchability properties of polythiophene derivatives through ester-substituted, biaxially extended conjugated side chains. ACS Appl. Polym. Mater. 2021, 3, 1628–1637.
- Shen, J.; Kashimoto, M.; Matsumoto, T.; Mori, A.; Nishino, T. Structural deformation of elastic polythiophene with disiloxane moieties under stretching. Polym. J. 2020, 52, 1273–1278.
- Coote, J.; Kim, J.; Lee, B.; Han, J.; Kim, J.; Stein, G. Crystallization modes of poly (3-dodecylthiophene)-based block copolymers depend on regioregularity and morphology. Macromolecules 2018, 51, 9276–9283.
- Shin, M.; Oh, J.; Byun, K.; Lee, Y.; Kim, B.; Baik, H.; Park, J.; Jeong, U. Polythiophene nanofibril bundles surface-embedded in elastomer: A route to a highly stretchable active channel layer. Adv. Mater. 2015, 27, 1255–1261.
- Wang, J.T.; Satoh, K.; Chen, W.; Wu, H.C.; Sun, H.S.; Hung, C.C.; Chen, Y.; Isono, T.; Kakuchi, T.; Satoh, T.; et al. High-performance stretchable resistive memories using donor–acceptor block copolymers with fluorene rods and pendent isoindigo coils. NPG Asia Mater. 2016, 8, 298.
- Higashihara, T.; Fukuta, S.; Ochiai, Y.; Sekine, T.; Chino, K.; Koganezawa, T.; Osaka, I. Synthesis and deformable hierarchical nanostructure of intrinsically stretchable ABA triblock copolymer composed of poly (3-hexylthiophene) and polyisobutylene segments. ACS Appl. Polym. Mater. 2019, 1, 315–320.
- Wang, J.; Takshima, S.; Wu, H.; Shih, C.; Isono, T.; Kakuchi, T.; Satoh, T.; Chen, W.C. Stretchable conjugated rod–coil poly (3-hexylthiophene)-block-poly (butyl acrylate) thin films for field effect transistor applications. Macromolecules 2017, 50, 1442–1452.
- Watts, K.; Neelamraju, B.; Ratcliff, E.L.; Pemberton, J.E. Stability of charge transfer states in F4TCNQ-doped P3HT. Chem. Mater. 2019, 31, 6986–6994.
- Jacobs, I.E.; Aasen, E.W.; Oliveira, J.L.; Fonseca, T.N.; Roehling, J.D.; Li, J.; Zhang, G.; Augustine, M.P.; Mascal, M.; Moulé, A.J. Comparison of solution-mixed and sequentially processed P3HT: F4TCNQ films: Effect of doping-induced aggregation on film morphology. J. Mater. Chem. C 2016, 4, 3454–3466.
- Hynynen, J.; Kiefer, D.; Müller, C. Influence of crystallinity on the thermoelectric power factor of P3HT vapour-doped with F4TCNQ. RSC Adv. 2018, 8, 1593–1599.
- Prunet, G.; Pawula, F.; Fleury, G.; Cloutet, E.; Robinson, A.J.; Hadziioannou, G.; Pakdel, A. A review on conductive polymers and their hybrids for flexible and wearable thermoelectric applications. Mater. Today Phys. 2021, 18, 100402.
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150.
- Sun, J.; Yeh, M.L.; Jung, B.J.; Zhang, J.; Feser, J.; Majumdar, A.; Katz, H.E. Simultaneous increase in seebeck coefficient and conductivity in a doped poly (alkylthiophene) blend with defined density of states. Macromolecules 2010, 43, 2897–2903.
- Zuo, G.; Li, Z.; Andersson, O.; Abdalla, H.; Wang, E.; Kemerink, M. Molecular doping and trap filling in organic semiconductor host–guest systems. J. Phys. Chem. C 2017, 121, 7767–7775.
- Cheon, H.J.; Shin, S.Y.; Tran, V.V.; Park, B.; Yoon, H. Preparation of conjugated polymer/reduced graphene oxide nanocomposites for high-performance volatile organic compound sensors. Chem. Eng. J. 2021, 425, 131424.
- Oh, W.K.; Kim, S.; Yoon, H.; Jang, J. Shape-dependent cytotoxicity and proinflammatory response of poly (3,4-ethylenedioxythiophene) nanomaterials. Small 2010, 6, 872–879.
- Jang, J.; Chang, M.; Yoon, H. Chemical sensors based on highly conductive poly (3,4-ethylenedioxythiophene) nanorods. Adv. Mater. 2005, 17, 1616–1620.
- Zhang, Y.; Lee, G.; Li, S.; Hu, Z.; Zhao, K.; Rogers, J.A. Advances in Bioresorbable Materials and Electronics. Chem. Rev. 2023, 123, 11722–11773.
- Wang, X.; Zhang, X.; Sun, L.; Lee, D.; Lee, S.; Wang, M.; Zhao, J.; Shao-Horn, Y.; Dincă, M.; Palacios, T.; et al. High electrical conductivity and carrier mobility in oCVD PEDOT thin films by engineered crystallization and acid treatment. Sci. Adv. 2018, 9, 4.
- Li, H.; Luo, R.; Hu, J.; Yang, K.; Du, B.; Zhou, S.; Zhou, X. Self-assembled gel-assisted preparation of high-performance hydrophobic PDMS@ MWCNTs/PEDOT: PSS composite aerogels for wearable piezoresistive sensors. J. Mater. Sci. Technol. 2024, 182, 22–32.
- Gamboa, J.; Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741.
- Ferlauto, L.; D’Angelo, A.; Vagni, P.; Airaghi Leccardi, M.J.I.; Mor, F.M.; Cuttaz, E.A.; Heuschkel, M.O.; Stoppini, L.; Ghezzi, D. Development and characterization of PEDOT: PSS/alginate soft microelectrodes for application in neuroprosthetics. Front. Neurosci. 2018, 12, 648.
- Lu, M.L.; Zhang, X.Y.; Song, J.Q.; Peng, Q.Y.; Xiao, H.X.; Yang, Y.; Liang, W.B.; Sun, S.; Yuan, R.; Xiao, D.R. In-situ growth of hydrogen-bonded organic framework on conductive PEDOT: PSS polymer to realize conductivity-enhanced electrochemiluminescence for fabricating ultrasensitive biosensor. Sens. Actuators B Chem. 2024, 401, 134974.
- Kushwaha, C.; Singh, P.; Shukla, S.; Chehimi, M. Advances in conducting polymer nanocomposite based chemical sensors: An overview. Mater. Sci. Eng. B 2022, 284, 115856.
- Li, Z.; Mu, H.; Larsen, S.; Jensen, H.; Østergaard, J. An in vitro gel-based system for characterizing and predicting the long-term performance of PLGA in situ forming implants. Int. J. Pharm. 2021, 609, 121183.
- Leau, S.; Lete, C.; Lupu, S. Nanocomposite materials based on metal nanoparticles for the electrochemical sensing of neurotransmitters. Chemosensors 2023, 11, 179.