Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raghad Alghazali | -- | 3928 | 2024-02-05 10:43:52 | | | |
| 2 | Fanny Huang | Meta information modification | 3928 | 2024-02-07 06:35:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alghazali, R.; Nugud, A.; El-Serafi, A. Glycan Modifications as Regulators of Stem Cell Fate. Encyclopedia. Available online: https://encyclopedia.pub/entry/54753 (accessed on 07 February 2026).
Alghazali R, Nugud A, El-Serafi A. Glycan Modifications as Regulators of Stem Cell Fate. Encyclopedia. Available at: https://encyclopedia.pub/entry/54753. Accessed February 07, 2026.
Alghazali, Raghad, Ahmed Nugud, Ahmed El-Serafi. "Glycan Modifications as Regulators of Stem Cell Fate" Encyclopedia, https://encyclopedia.pub/entry/54753 (accessed February 07, 2026).
Alghazali, R., Nugud, A., & El-Serafi, A. (2024, February 05). Glycan Modifications as Regulators of Stem Cell Fate. In Encyclopedia. https://encyclopedia.pub/entry/54753
Alghazali, Raghad, et al. "Glycan Modifications as Regulators of Stem Cell Fate." Encyclopedia. Web. 05 February, 2024.
Copy Citation
Glycosylation is a process where proteins or lipids are modified with glycans. The presence of glycans determines the structure, stability, and localization of glycoproteins, thereby impacting various biological processes, including embryogenesis, intercellular communication, and disease progression. Glycans can influence stem cell behavior by modulating signaling molecules that govern the critical aspects of self-renewal and differentiation. Furthermore, being located at the cell surface, glycans are utilized as markers for stem cell pluripotency and differentiation state determination.
glycosylation
synthetic glycans
stem cell differentiation
1. Introduction
Glycans are ubiquitous sugar molecules on the outer surface of all cells in nature and serve as essential markers for the identification and isolation of distinct cell types [1]. Beyond their role as markers, the complexity of glycans is multifaceted, as their structures are not only unique at every level of biological organization—from species level down to individual molecules—but they also exhibit dynamic changes throughout development and disease [2][3]. The complex biosynthesis of glycans further contributes to their enigmatic nature, as they are not directly encoded within the genome. Instead, these compounds are synthesized in correspondence to the activity of glycosidases and glycosyltransferases on the cytosolic and luminal faces of the Endoplasmic Reticulum (ER) and within the Golgi apparatus (GA) [4][5]. There are over 300 identified human glycosyltransferases and glycosidases, and their expression and activity are influenced by internal and external factors [6][7]. Nearly all cell surface proteins undergo glycosylation, with approximately 50% of glycosylated proteins being secreted [4]. These protein-bound glycans decisively govern the structure, stability, and localization of glycoproteins, thereby holding paramount importance in biological processes such as protein folding and quality control. The presence or absence of glycans exerts significant influence over an array of biological processes, encompassing development, tumorigenesis, and inflammation [8]. In many cases, specific functions of glycans remain elusive, and the same glycan may serve different functions based on the type of aglycone (protein or lipid) to which it attaches [9]. In multicellular organisms, glycan components of matrix molecules, including proteoglycans, are pivotal for maintaining tissue structure, porosity, and integrity [9]. Thick layers of glycans serve as a crucial physical protective barrier. For instance, the dense layer of mucins coating many epithelial surfaces, present in the inner linings of airways and intestines, provides protection against pathogen invasion [10][11]. Certain glycans can also act as a storage depot for biologically important molecules. Hydrophilic glycans on cell surfaces and extracellular matrices can serve as a depot for water molecules [12], while extracellular matrix glycosaminoglycans and polysialic acid can locally store growth factors and other bioactive molecules and release them as needed, particularly during processes like injury and wound healing [13][14][15]. Furthermore, glycans play an essential role as mediators of cell–cell interactions, cell–extracellular matrix interactions, and, most notably, interactions between ligands and receptors. Examples include Wnt receptor, fibroblast growth factor (FGF) receptor, Hedgehog (Hh) receptor, and bone morphogenetic protein (BMP) receptor interactions [1].
Notably, genetic mutations linked to glycosylation processes have been pinpointed in several inherited disorders, collectively referred to as congenital disorders of glycosylation [16]. Furthermore, cell surface glycans regulate immune responses, inflammatory reactions, and host–pathogen recognition, as pathogens often exploit specific sialic acid linkages to facilitate their entry into host cells [17][18][19]. Remarkably, dysregulated glycosylation machinery is associated with tumor development and progression, where the aberrant glycome of tumors is thought to explain the heterogeneity seen in numerous cancers [20][21]. The implications of glycosylation in cancer and cancer stem cells have been comprehensively reviewed in the other literature [21][22]. Given the multitude of roles that glycans play in maintaining distinct biological functions, it is unsurprising that glycans are regarded as universal in their nature as other major macromolecular building blocks (nucleic acids, proteins, and lipids), and as indispensable for the existence of all known living organisms [13][23][24].
Stem cells are attracting considerable attention due to their ability to differentiate and regenerate lost or damaged tissues. Despite decades of research, harnessing the differentiation of stem cells is still a target to be achieved, while the role of glycans in this process is often underestimated. Interestingly, glycans are pivotal for modulating signaling molecules that govern self-renewal and differentiation [25]. Glycans have proven particularly valuable as markers for discerning the pluripotent status of mouse embryonic stem cells and human induced pluripotent stem cells due to their presence on the cell surface. Additionally, there is growing evidence suggesting that glycans play a role in maintaining stem cell pluripotency [1][26]. Furthermore, glycans offer a distinctive opportunity for steering or manipulating stem cell differentiation. Innovative strategies in cell surface engineering have emerged, providing opportunities to control stem cell differentiation. These strategies encompass chemoenzymatic methods for editing existing cell surface glycan structures [27], as well as metabolic approaches to introduce non-natural monosaccharide modifications across the glycome [28].
2. Glycosylation
Glycosylation is an ubiquitous and indispensable co- and/or post-translational modification required for the normal biological functioning of cells. Glycosylation occurring midway through folding, significantly contributes to the accurate three-dimensional structure of the protein [29]. To date, several types of protein glycosylation have been identified, each characterized by unique protein–sugar linkages [30].
3. Glycosylation Effect on Stem Cells and Their Differentiation
The link between cell surface glycans and the status of stem cells, whether in their naïve status or throughout the differentiation process, has been extensively explored (Table 1). The change of glycome signature was studied in accordance with the lineage of interests. While comprehensive global characterization is still warranted, examples highlighting alterations in glycosylation patterns of different proteins and their impact on stem cells and their differentiation are summarized in Table 2. Furthermore, the role of glycans in epigenetics is well established. The addition of O-GlcNAc residues to histone proteins is a key component of the histone code that regulates gene expression. O-GlcNAcylation targets key transcriptional and epigenetic regulators including RNA polymerase II, histones, histone deacetylase complexes, and members of the Polycomb and Trithorax groups. As O-GlcNAc cycling relies on cytosolic UDP–N-acetyl–glucosamine (UDP–GlcNAc) levels, it is considered a homeostatic mechanism linking nutrient availability to higher-order chromatin organization [31][32][33]. Evidence also suggests that this glycosylation mechanism can also influence X chromosome inactivation and genetic imprinting, given that the O-GlcNAc transferase is encoded on the X chromosome [13]. Researchers have shown the effect of histone modification on the differentiation of stem cells into the chondrogenic and adipogenic lineages as well as to insulin-secreting cells [34][35][36]. Unfortunately, the glycosylation effect has not been investigated in those studies.
Table 1. Examples of glycosylation use/potential use as markers in relation to stem cells and their differentiation.
| Glycosylation Marker | Use/Potential Use |
|---|---|
| α–1–2–fucosylation | Adipogenic differentiation of ESC |
| α–1–6–fucosylation | Adipogenic differentiation of ESC |
| α–1–6–mannosylation | Adipogenic differentiation of ESC |
| α2–6 Sia | Chondrocyte differentiation of hMSC |
| GlcNAc | Adipogenic differentiation of ESC |
| H3N4F1 | Adipogenic differentiation |
| H5N4F3 | Adipogenic differentiation |
| H6N5F1 | Undifferentiated MSCs |
| H7N6F1 | Undifferentiated MSCs |
| rBC2LCN | Marker of stemness |
| SSEA-5 | Marker of stemness |
H: Hex, N: HexNAc, F: Fuc.
Table 2. Glycosylation and its effect on stem cells and their differentiation.
| Tissue | Glycosylation Effect |
|---|---|
| Adipose tissue |
|
| Cardiac tissue |
|
| Central nervous system |
|
| Epidermis |
|
| Hematopoiesis |
|
| Naïve stem cells |
|
| Osteogenic tissue |
|
CNS: Central Nervous System, CS: Chondroitin Sulfate, C1GALT1: Core–1 Synthase Gene, CM–hPSC: Cardiomyocytes Derived Human Pluripotent Stem Cells, DS: Dermatan Sulfate, ESC: Embryonic Stem Cells, mESC: Murine Embryonic Stem Cells, iMSC: Immortalized Mesenchymal Stem Cell, iPSC: Induced Pluripotent Stem Cells, iPS–MBMC: iPS generated from Menstrual Blood-derived Mesenchymal Cells, PKC: Protein Kinase C, PI3K/Akt: Phosphoinositide–3–Kinase/Akt Pathway.
4. Harnessing Synthetic Glycans to Control Stem Cell Differentiation
As researchers' understanding of the factors influencing stem cell behavior deepens, greater capabilities can be gained to manipulate their abilities and maximize their therapeutic benefits. Among these factors, cell surface glycans are increasingly identified as co-regulators or stabilizers of growth factor signaling essential for stem cell fate decision [1][37]. Synthetic glycans emerge as a versatile toolset for studying and harnessing the complex mechanisms that govern stem cell fate determination, opening novel avenues within regenerative medicine and tissue engineering. Biologically functionalized, engineered materials have the capacity to influence stem cell behavior through a synergistic blend of biological, mechanical, and topographical cues.
While genetic approaches to manipulate the expression of glycosyltransferase genes are available, their utility in glycan engineering has limitations due to the combinatorial nature of glycan biosynthesis and the functional redundancy of glycosyltransferase genes [38]. Additionally, genetic transfection using viral vectors may cause unpredictable risks, and irreversible gene modifications may raise safety concerns for clinical applications. Moreover, not all cell types can adapt to genetic alteration without side effects, particularly in stem cells [39][40]. Therefore, biochemical and chemical strategies offer valuable complements to these genetic approaches, notably by enabling the introduction of unnatural functionalities, such as fluorophores, into cell surface glycans.
4.1. Biochemical and Chemical Strategies in Glycan Engineering
Numerous studies have explored the application of synthetic glycans to direct stem cell differentiation. One such example is the use of engineered glycans to drive the differentiation of mESCs towards the mesodermal lineage [41]. The transition of mESCs from their pluripotent state to mesodermal cell lineage is orchestrated by the growth factors FGF2 and BMP4, respectively [42]. BMP4, via the Smad protein signaling pathway, downregulates FGF and Wnt signaling, thereby suppressing neuroectoderm formation and promoting mesoderm formation [42][43]. Remarkably, both the extracellular matrix (ECM) and cellular glycans play significant co-regulatory roles in this process. HS has been identified as a class of glycans involved in spatially patterning growth factors and facilitating signal transduction at the cell surface [44][45]. Consequently, the precise chemical manipulation of HS activity within the cellular glycocalyx of stem cells presents a promising effective control of cellular differentiation.
Naticchia et al. (2018) reported a novel method to enhance differentiation, utilizing lipid-functionalized synthetic HS-mimetic glycopolymers. These synthetic glycans exhibited a dual affinity for both FGF2 and BMP4, facilitating the mesodermal differentiation of mESCs in embryoid body culture. These glycans were introduced into the plasma membrane of mutant mESCs deficient in exostosin 1 and 2 (Ext1/2) glycotransferases, which are responsible for HS biosynthesis by adding alternating N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA) residues to the growing polysaccharide chain [41]. Remodeling the glycocalyx of these mutant Ext1−/− mESCs showcased an increased association of BMP4 at the cell surface, leading to enhanced mesodermal differentiation through the associated MAPK and Smad signaling pathways [41]. This study demonstrated the feasibility of using synthetic glycans to engineer the glycocalyx of Ext1−/− mESCs within three-dimensional embryoid body structures, providing valuable insights into the complex mechanisms governing stem cell differentiation and fostering potential therapeutic advancements.
On the other hand, collagen–GAG scaffolds have emerged as promising tools for bone tissue engineering. Synthetic GAGs possess a few advantages over natural counterparts, offering structural homogeneity, purity, and controlled sulfation to circumvent limitations [46][47]. Farrell et al. (2006) exemplified this potential by utilizing a collagen–glycosaminoglycan scaffold to promote the differentiation of adult rat mesenchymal stem cells towards the osteogenic and chondrogenic lineages [48]. Cultivating these cells on the collagen–GAG scaffold combined with the addition of osteogenic factors (dexamethasone, ascorbic acid, and beta-glycerophosphate) induced osteogenesis, as evident by the temporal induction of the bone-specific proteins, collagen I and osteocalcin, as well as subsequent matrix mineralization and the activation of the extracellular-regulated protein kinase (ERK), which is involved in the osteogenic process. Conversely, exposing the scaffold-seeded cells to chondrogenic factors, dexamethasone and transforming growth factor–1 beta, enhanced collagen II immunoreactivity, suggesting that the scaffold can be used to generate a suitable three-dimensional environment that supports chondrogenesis [48].
Sulfated glycosaminoglycans play pivotal roles in regulating stem cell lineage commitment and differentiation within the bone marrow stem cell niche and mature bone tissue. An interesting study by Hempel et al. (2014) provided valuable insights into the utilization of artificial extracellular matrices (aECMs) as influential factors in shaping the differentiation of osteoblast precursor cells and early osteoblasts. This investigation’s premise involved the preparation of aECMs through the gradual sulfation of chondroitin sulfate and hyaluronan derivatives [49]. Human bone marrow stromal cells were used to identify the most potent aECM formulation that drives pro-osteogenic effects, as evaluated by the influence of sulfate groups, as well as the type of disaccharide integrated into aECM. The results of the study revealed that over-sulfated GAG derivatives, characterized by a sulfate group positioned at the C–6 site of N-acetylglycosamine, exhibited the most pronounced and effective pro-osteogenic impact, as evaluated by tissue nonspecific alkaline phosphatase activity and calcium deposition. Subsequent analysis encompassing a subset of aECMs in association with primary osteoblasts and cell lines representing diverse maturation stages reaffirmed the notable pro-osteogenic influence specifically on early osteoblasts [49]. Through a comprehensive approach that encompasses molecular positioning, structure, and biological response, this study highlights the significance of over-sulfated GAG derivatives as influential players in steering early osteoblast differentiation. The findings underscore the potential of tailored aECMs in modulating stem cell behavior within their niche, thereby advancing researchers' understanding of osteogenesis.
Apart from osteogenic differentiation, synthetic GAGs exhibit a remarkable potential to induce neural differentiation. Wang et al. (2015) introduced an innovative strategy to prepare GAGs analogs by splitting and recombining sulfated saccharide units found in natural GAGs. They employed monomers (SS and MAG) containing essential GAG structural units as building blocks to synthesize polymers with well-defined chemical structures and adjustable ratios of functional units through living radical polymerization [46]. The synthetic polymers exhibited robust bioactivity, promoting both cell proliferation and neural differentiation of ESCs. The results of the study further revealed distinct roles played by unit S and unit G in influencing GAG bioactivities. Significantly, these synthetic polymers demonstrated superior bioactivity compared to heparin, highlighting their potential to enhance researchers' comprehension of biomacromolecule structure–function relationships and create alternatives to complex natural macromolecules [46].
Furthermore, novel strategies in cell surface engineering have harnessed the function of HS, which mediates interactions between growth factors and their receptors, to promote the differentiation of ESCs. In an interesting example, Huang et al. (2014) generated a synthetic neoproteoglycan with an affinity for FGF2 and integrated it into the plasma membrane of HS-deficient ESCs to induce neuronal differentiation. The study revealed that neoproteoglycan retained the function of native HS, effectively rescuing FGF2 activity and promoting neural specification, which demonstrates the versatility of glycocalyx remodeling for potential application in diverse differentiation processes [50]. Another innovative approach involved the functionalization of electrospun scaffolds with GAGs through ionic immobilization onto fiber surfaces [51]. This binding strategy preserved GAGs’ interaction capability with binding molecules and showcased essential GAG sulfation motifs pivotal for orchestrating stem cell behavior. These GAGs successfully rescued the neural differentiation capacity of HS-deficient mESCs and, in synergy with FGF4, facilitated extensive neural process formation across the scaffold surface [51]. The combination of GAGs with electrospun scaffolds establishes a potent biomaterial platform for stem cell propagation and differentiation, holding great promise for tissue engineering and regenerative medicine applications.
Collectively, these studies underscore the potential of synthetic glycans in driving stem cell differentiation and suggest a foundation for tailored stem cell differentiation strategies with promising therapeutic applications. Continued investigations and refinements in the design and application of synthetic glycans will undoubtedly lead to even greater advancements in the field of stem cell-based therapies, potentially revolutionizing the treatment of various medical conditions.
4.2. Metabolic Glycoengineering
Metabolic glycoengineering (MGE) is another approach that is being exploited to control stem cell activities. Although MGE technique was introduced over three decades ago [52], it is currently finding renewed interest in modeling stem cell niches and controlling their fate [38]. The primary goal of MGE is to augment the expression of natural glycans and incorporate non-natural monosaccharides into cell surface glycoconjugates, such as ketone-, azide-, thiol-, or alkyne-modified glycans [53][54].
Since MGE exploits the innate metabolic pathway of cells, the modification process minimally disrupts other cellular functions [55]. Additionally, the MGE strategy possesses several distinctive advantages, being an easy, yet highly efficient process, and achieved through straightforward coculturing of cells with metabolic precursors. Remarkably, MGE exhibits no cytotoxicity even under high treatment concentration and its applicability extends to nearly all cell types, rendering it a versatile tool in the field. Furthermore, the modifications introduced by MGE are nonpermanent, enabling controlled reversibility. Bioorthogonal click chemistry and the wide array of sugar analogs available further contribute to the versatility of MGE by offering diverse options for membrane modification [38].
The sialic acid pathway was the first glycosylation pathway harnessed in MGE [56], and it remains the most frequently utilized pathway to date. The suitability of the sialic acid pathway for MGE lies in the notable substrate versatility of sialyltransferases [57], which enables the modified analogs to effectively intercept glycosylation pathways, resulting in chemically altered sialic [38]. Among human cells, N-Acetylneuraminic acid (Neu5Ac) is the most common form of sialic acid, while N-acetyl–D–mannosamine (ManNAc) serves as the physiological precursor of all sialic acids. Once internalized as a precursor within a cell, ManNAc undergoes conversion to Neu5Ac with the help of specific sialyltransferases, ultimately becoming anchored to the residues of cell surface sialic acid (Figure 1) [58].
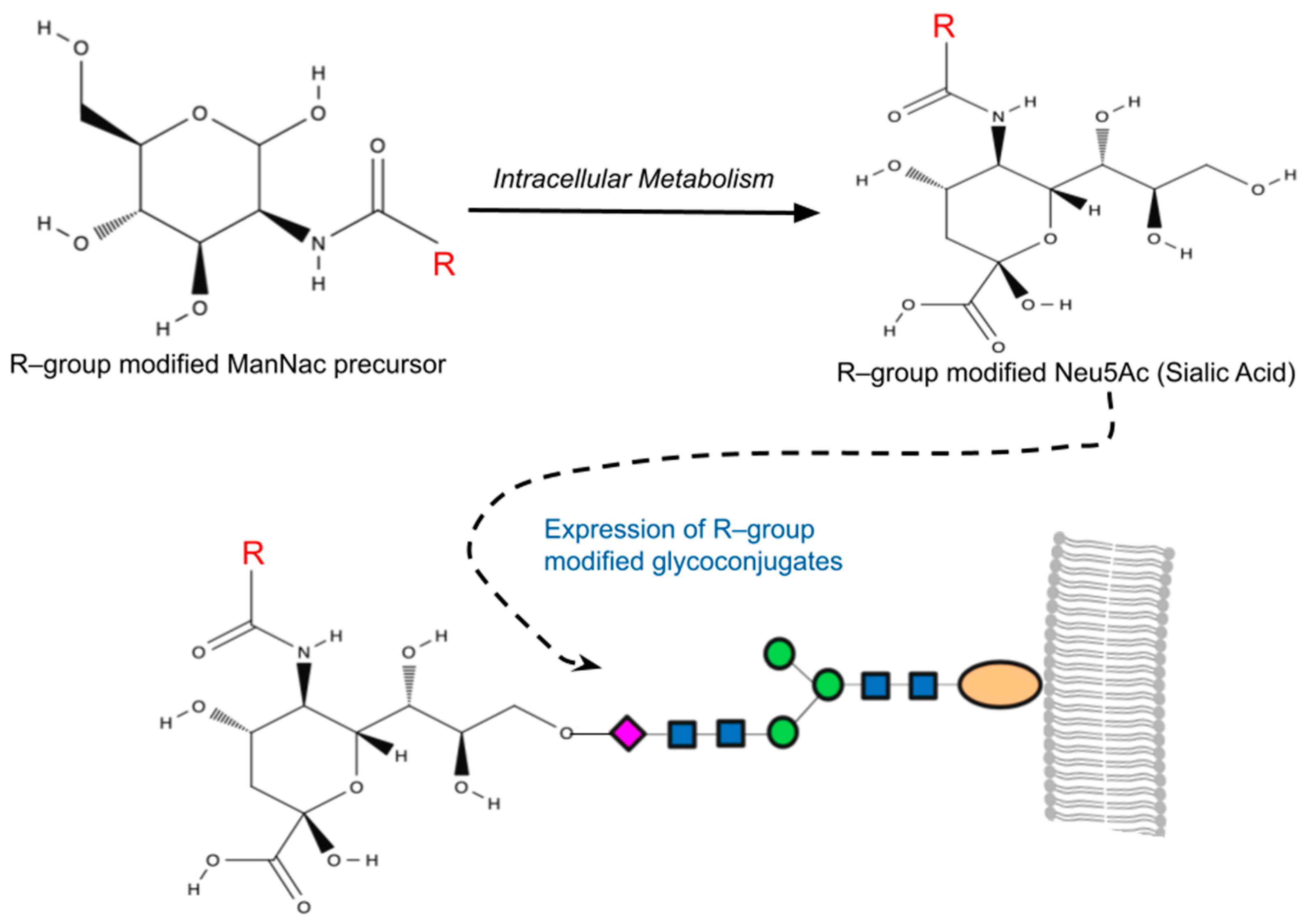
Figure 1. A simplified overview of metabolic glycoengineering (MGE). MGE involves the introduction of diverse chemical groups into cellular glycans through artificially modified monosaccharides containing unnatural functionalities (R-groups). Mammalian cells incubated with the R-group-modified N-Acetylmannosamines (ManNAc) metabolize these precursors intracellularly, resulting in the production of non-natural sialic acids (Neu5Ac). This process leads to the presentation of R-group-modified glycans on cell surfaces or their secretion as glycoconjugates.
Numerous studies have highlighted the applicability of MGE analogs in modulating stem cell behavior. For example, pretreatment of peracetylated N-thiolglycolyl–d–mannosamine (Ac5ManNTGc), a hyperacetylated ManNAc analog with a thiol group on its N-acyl side chain, significantly enhanced the adhesion capabilities of Jurkat cells—a property previously absent in this T–lymphoma-derived cell line [59]. This pretreatment also induced their expression of ECM components and upregulated the expression of β1–integrin, MMP–9, and CD44 [60]. Beyond adhesion, MGE extends its influence on cellular differentiation. Notably, the application of Ac5ManNTGc was shown to promote neural lineage differentiation in human embryoid body-derived (hEBD) stem cells, even in the absence of Wnt signaling proteins that are essential for neural differentiation [59][61]. Noteworthily, Wnt pathway upregulation and the response of Jurkat cells to Ac5ManNTGc treatment were scaffold dependent, occurring only when the cells were cultured on gold- or maleimide-covered surfaces where the thiol-modified cell surface sialic acids could form high-affinity bonds with the substrate. While scientifically intriguing, this approach faced limitations for translational research due to the challenges associated with developing in vivo applications dependent on a gold-plated surface or other high-affinity scaffolds. To address this issue, Du et al. (2021) developed two novel thiolated analogs, namely Ac5ManNTProp and Ac5ManNTBut, which install thiol on an elongated N-acyl side chain, effectively substituting natural cell surface sialic acids with their thiolated counterparts [62]. Treatment of human neural stem cells (hNSCs) with these thiolated analogs enhanced the ability of glycans to interact with naturally occurring endogenous thiols present in the cellular nano and microenvironment. This, in turn, enhanced the differentiation of hNSCs as well as their adhesion to extracellular matrix components in the absence of a complementary high-affinity scaffold [62]. Thereby, advancing the in vivo applications and potentially paving the way for clinical translation of these MGE analogs. Building on the previous studies, the group further expanded the applications of thiol-modified MGE analogs by demonstrating the ability of Ac5ManNTProp (tProp) to facilitate Schwann cells (SCs) differentiation from Adipose-derived stem cells (ASCs) [63]. SCs are myelinating cells essential for peripheral nerve regeneration [64]. SCs are often depleted when nerve lesions occur, hindering the repair process [65]. Addressing the limited and slow expansion capacity of SCs, ASCs have emerged as a promising therapeutic avenue for peripheral nerve injuries [66]. While ASCs possess SC differentiation potential, their natural transdifferentiation period exceeds two weeks [67]. To overcome this limitation, Du et al. (2023) harnessed MGE technology to expedite ASC differentiation into SCs. Specifically, the sugar analog tProp significantly enhanced ASC differentiation, leading to elevated expression of SC proteins S100β and p75NGFR, along with heightened levels of neurotrophic factors such as nerve growth factor beta (NGFβ) and glial cell-line-derived neurotrophic factor (GDNF). Remarkably, tProp treatment reduced the SC transdifferentiation period from approximately two weeks to just two days in vitro [63]. This breakthrough holds the potential to significantly improve neuronal regeneration.
Beyond sialic acid, additional glycosylation pathways have been harnessed in MGE. In a seminal study, Sackstein et al. (2008) demonstrated the profound impact of introducing fucose to cell-surface glycoprotein receptors in enhancing the trafficking of mesenchymal stem cells (MSCs) to bone. The group addressed a critical limitation in the clinical effectiveness of MSCs, which show promise in treating skeletal diseases but suffer from poor homing to bone [68]. The recruitment of cells to bone takes place within specialized marrow vessels expressing vascular E–selectin, a lectin that recognizes sialofucosylated determinants on its ligands [69][70]. Notably, it was observed that human MSCs lack E–selectin ligands but instead express a CD44 glycoform bearing alpha–2,3–sialyl modifications [71]. Through glycan engineering using an alpha–1,3–fucosyltransferase preparation, the research team successfully fucosylated the native CD44 glycoform on MSCs, transforming it into a hematopoietic cell E–selectin/L–selectin ligand (HCELL). This modification enhanced E–selectin binding without compromising cell viability or multipotency. Real-time intravital microscopy in immunocompromised mice revealed that intravenously infused HCELL(+) MSCs swiftly infiltrated marrow, leading to rare foci of endosteally localized cells and the generation of human osteoid [68]. This innovative approach not only provided a blueprint for programming cellular trafficking, but also underscored the broader potential of glycan engineering, particularly fucosylation, in directing the homing of various stem cell types to specific tissues. The study marks a significant leap forward in the field, offering a promising strategy for advancing stem cell-based therapies, not only for skeletal diseases but also for broader applications.
Overall, the aforementioned advances in MGE demonstrate its potential in providing fine-tuned control over stem cell fate as well as opening new avenues for the study of cellular niches and developmental pathways. Continued research and refinement in synthetic glycans and metabolic glycoengineering will undoubtedly lead to greater advancements in the field, ushering in a new era of tailored stem cell differentiation strategies with broad therapeutic applications.
References
- Nishihara, S. Glycans in stem cell regulation: From Drosophila tissue stem cells to mammalian pluripotent stem cells. FEBS Lett. 2018, 592, 3773–3790.
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867.
- Tateno, H.; Saito, S.; Hiemori, K.; Kiyoi, K.; Hasehira, K.; Toyoda, M.; Onuma, Y.; Ito, Y.; Akutsu, H.; Hirabayashi, J. α2-6 sialylation is a marker of the differentiation potential of human mesenchymal stem cells. Glycobiology 2016, 26, 1328–1337.
- Berger, R.P.; Dookwah, M.; Steet, R.; Dalton, S. Glycosylation and stem cells: Regulatory roles and application of iPSCs in the study of glycosylation-related disorders. Bioessays 2016, 38, 1255–1265.
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462.
- Kelly, M.I.; Albahrani, M.; Castro, C.; Poon, E.; Yan, B.; Littrell, J.; Waas, M.; Boheler, K.R.; Gundry, R.L. Importance of evaluating protein glycosylation in pluripotent stem cell-derived cardiomyocytes for research and clinical applications. Pflug. Arch. 2021, 473, 1041–1059.
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749.
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015.
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022.
- Bergstrom, K.S.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037.
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20.
- Espinosa-Marzal, R.M.; Fontani, G.; Reusch, F.B.; Roba, M.; Spencer, N.D.; Crockett, R. Sugars communicate through water: Oriented glycans induce water structuring. Biophys. J. 2013, 104, 2686–2694.
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49.
- Andersson-Sjöland, A.; Hallgren, O.; Rolandsson, S.; Weitoft, M.; Tykesson, E.; Larsson-Callerfelt, A.K.; Rydell-Törmänen, K.; Bjermer, L.; Malmström, A.; Karlsson, J.C.; et al. Versican in inflammation and tissue remodeling: The impact on lung disorders. Glycobiology 2015, 25, 243–251.
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471.
- Chang, I.J.; He, M.; Lam, C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018, 6, 477.
- DeLuca, G.M.; Donnell, M.E.; Carrigan, D.J.; Blackall, D.P. Plasmodium falciparum merozoite adhesion is mediated by sialic acid. Biochem. Biophys. Res. Commun. 1996, 225, 726–732.
- Dolan, S.A.; Proctor, J.L.; Alling, D.W.; Okubo, Y.; Wellems, T.E.; Miller, L.H. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 1994, 64, 55–63.
- Orlandi, P.A.; Klotz, F.W.; Haynes, J.D. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal- sequences of glycophorin A. J. Cell Biol. 1992, 116, 901–909.
- Chugh, S.; Gnanapragassam, V.S.; Jain, M.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim. Biophys. Acta 2015, 1856, 211–225.
- Barkeer, S.; Chugh, S.; Batra, S.K.; Ponnusamy, M.P. Glycosylation of Cancer Stem Cells: Function in Stemness, Tumorigenesis, and Metastasis. Neoplasia 2018, 20, 813–825.
- Khan, T.; Cabral, H. Abnormal Glycosylation of Cancer Stem Cells and Targeting Strategies. Front. Oncol. 2021, 11, 649338.
- Marth, J.D. A unified vision of the building blocks of life. Nat. Cell Biol. 2008, 10, 1015–1016.
- Varki, A. Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect. Biol. 2011, 3, a005462.
- Lanctot, P.M.; Gage, F.H.; Varki, A.P. The glycans of stem cells. Curr. Opin. Chem. Biol. 2007, 11, 373–380.
- Hasehira, K.; Tateno, H.; Onuma, Y.; Ito, Y.; Asashima, M.; Hirabayashi, J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell Proteom. 2012, 11, 1913–1923.
- Lopez Aguilar, A.; Briard, J.G.; Yang, L.; Ovryn, B.; Macauley, M.S.; Wu, P. Tools for Studying Glycans: Recent Advances in Chemoenzymatic Glycan Labeling. ACS Chem. Biol. 2017, 12, 611–621.
- Dube, D.H.; Bertozzi, C.R. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr. Opin. Chem. Biol. 2003, 7, 616–625.
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261.
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R.
- Lewis, B.A.; Hanover, J.A. O-GlcNAc and the epigenetic regulation of gene expression. J. Biol. Chem. 2014, 289, 34440–34448.
- Olivier-Van Stichelen, S.; Hanover, J.A. You are what you eat: O-linked N-acetylglucosamine in disease, development and epigenetics. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 339–345.
- Hanover, J.A.; Krause, M.W.; Love, D.C. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 2012, 13, 312–321.
- Elsharkawi, I.; Parambath, D.; Saber-Ayad, M.; Khan, A.A.; El-Serafi, A.T. Exploring the effect of epigenetic modifiers on developing insulin-secreting cells. Hum. Cell 2020, 33, 1–9.
- El-Serafi, A.T.; Sandeep, D.; Abdallah, S.; Lozansson, Y.; Hamad, M.; Khan, A.A. Paradoxical effects of the epigenetic modifiers 5-aza-deoxycytidine and suberoylanilide hydroxamic acid on adipogenesis. Differentiation 2019, 106, 1–8.
- El-Serafi, A.T.; Oreffo, R.O.; Roach, H.I. Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: Differential effects of 5-aza-deoxycytidine and trichostatin A. Differentiation 2011, 81, 35–41.
- Nakato, H.; Li, J.P. Functions of Heparan Sulfate Proteoglycans in Development: Insights From Drosophila Models. Int. Rev. Cell Mol. Biol. 2016, 325, 275–293.
- Li, Y.; Zhang, Y.; Tao, Y.; Huang, X.; Yu, C.; Xu, H.; Chen, J.; Xia, K.; Shi, K.; Wang, J.; et al. Metabolic Glycoengineering: A Promising Strategy to Remodel Microenvironments for Regenerative Therapy. Stem Cells Int. 2023, 2023, 1655750.
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016, 3, 16011.
- Csizmar, C.M.; Petersburg, J.R.; Wagner, C.R. Programming Cell-Cell Interactions through Non-genetic Membrane Engineering. Cell Chem. Biol. 2018, 25, 931–940.
- Naticchia, M.R.; Laubach, L.K.; Tota, E.M.; Lucas, T.M.; Huang, M.L.; Godula, K. Embryonic Stem Cell Engineering with a Glycomimetic FGF2/BMP4 Co-Receptor Drives Mesodermal Differentiation in a Three-Dimensional Culture. ACS Chem. Biol. 2018, 13, 2880–2887.
- Varga, A.C.; Wrana, J.L. The disparate role of BMP in stem cell biology. Oncogene 2005, 24, 5713–5721.
- Malaguti, M.; Nistor, P.A.; Blin, G.; Pegg, A.; Zhou, X.; Lowell, S. Bone morphogenic protein signalling suppresses differentiation of pluripotent cells by maintaining expression of E-Cadherin. eLife 2013, 2, e01197.
- Kraushaar, D.C.; Dalton, S.; Wang, L. Heparan sulfate: A key regulator of embryonic stem cell fate. Biol. Chem. 2013, 394, 741–751.
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021.
- Wang, M.; Lyu, Z.; Chen, G.; Wang, H.; Yuan, Y.; Ding, K.; Yu, Q.; Yuan, L.; Chen, H. A new avenue to the synthesis of GAG-mimicking polymers highly promoting neural differentiation of embryonic stem cells. Chem. Commun. 2015, 51, 15434–15437.
- Wang, M.; Liu, X.; Lyu, Z.; Gu, H.; Li, D.; Chen, H. Glycosaminoglycans (GAGs) and GAG mimetics regulate the behavior of stem cell differentiation. Colloids Surf. B Biointerfaces 2017, 150, 175–182.
- Farrell, E.; O’Brien, F.J.; Doyle, P.; Fischer, J.; Yannas, I.; Harley, B.A.; O’Connell, B.; Prendergast, P.J.; Campbell, V.A. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006, 12, 459–468.
- Hempel, U.; Preissler, C.; Vogel, S.; Möller, S.; Hintze, V.; Becher, J.; Schnabelrauch, M.; Rauner, M.; Hofbauer, L.C.; Dieter, P. Artificial extracellular matrices with oversulfated glycosaminoglycan derivatives promote the differentiation of osteoblast-precursor cells and premature osteoblasts. Biomed. Res. Int. 2014, 2014, 938368.
- Huang, M.L.; Smith, R.A.; Trieger, G.W.; Godula, K. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J. Am. Chem. Soc. 2014, 136, 10565–10568.
- Meade, K.A.; White, K.J.; Pickford, C.E.; Holley, R.J.; Marson, A.; Tillotson, D.; van Kuppevelt, T.H.; Whittle, J.D.; Day, A.J.; Merry, C.L. Immobilization of heparan sulfate on electrospun meshes to support embryonic stem cell culture and differentiation. J. Biol. Chem. 2013, 288, 5530–5538.
- Kayser, H.; Zeitler, R.; Kannicht, C.; Grunow, D.; Nuck, R.; Reutter, W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J. Biol. Chem. 1992, 267, 16934–16938.
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401.
- Mahal, L.K.; Yarema, K.J.; Bertozzi, C.R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 1997, 276, 1125–1128.
- Wratil, P.R.; Horstkorte, R.; Reutter, W. Metabolic Glycoengineering with N-Acyl Side Chain Modified Mannosamines. Angew. Chem. Int. Ed. Engl. 2016, 55, 9482–9512.
- Gross, H.J.; Brossmer, R. Enzymatic introduction of a fluorescent sialic acid into oligosaccharide chains of glycoproteins. Eur. J. Biochem. 1988, 177, 583–589.
- Gross, H.J.; Rose, U.; Krause, J.M.; Paulson, J.C.; Schmid, K.; Feeney, R.E.; Brossmer, R. Transfer of synthetic sialic acid analogues to N- and O-linked glycoprotein glycans using four different mammalian sialyltransferases. Biochemistry 1989, 28, 7386–7392.
- Nischan, N.; Kohler, J.J. Advances in cell surface glycoengineering reveal biological function. Glycobiology 2016, 26, 789–796.
- Sampathkumar, S.G.; Li, A.V.; Jones, M.B.; Sun, Z.; Yarema, K.J. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2006, 2, 149–152.
- Du, J.; Che, P.L.; Wang, Z.Y.; Aich, U.; Yarema, K.J. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials 2011, 32, 5427–5437.
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999.
- Du, J.; Agatemor, C.; Saeui, C.T.; Bhattacharya, R.; Jia, X.; Yarema, K.J. Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs. Cells 2021, 10, 377.
- Du, J.; Wang, Z.; Liu, X.; Hu, C.; Yarema, K.J.; Jia, X. Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering. Cells 2023, 12, 1190.
- Balakrishnan, A.; Belfiore, L.; Chu, T.H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair from Studies of Development and Injury. Front. Mol. Neurosci. 2020, 13, 608442.
- Wang, Q.; Chen, F.Y.; Ling, Z.M.; Su, W.F.; Zhao, Y.Y.; Chen, G.; Wei, Z.Y. The Effect of Schwann Cells/Schwann Cell-Like Cells on Cell Therapy for Peripheral Neuropathy. Front. Cell Neurosci. 2022, 16, 836931.
- Xu, Y.; Liu, L.; Li, Y.; Zhou, C.; Xiong, F.; Liu, Z.; Gu, R.; Hou, X.; Zhang, C. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008, 1239, 49–55.
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274.
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187.
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Côté, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005, 435, 969–973.
- Schweitzer, K.M.; Dräger, A.M.; van der Valk, P.; Thijsen, S.F.; Zevenbergen, A.; Theijsmeijer, A.P.; van der Schoot, C.E.; Langenhuijsen, M.M. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am. J. Pathol. 1996, 148, 165–175.
- Dimitroff, C.J.; Lee, J.Y.; Rafii, S.; Fuhlbrigge, R.C.; Sackstein, R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J. Cell Biol. 2001, 153, 1277–1286.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
601
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




