Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md. Abul Kalam Azad | -- | 3882 | 2024-02-03 08:24:36 | | | |
| 2 | Jason Zhu | Meta information modification | 3882 | 2024-02-04 02:38:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Azad, A.K.; Lai, J.; Sulaiman, W.M.A.W.; Almoustafa, H.; Alshehade, S.A.; Kumarasamy, V.; Subramaniyan, V. Approaches to Overcoming Existing Barriers of Curcumin. Encyclopedia. Available online: https://encyclopedia.pub/entry/54726 (accessed on 06 February 2026).
Azad AK, Lai J, Sulaiman WMAW, Almoustafa H, Alshehade SA, Kumarasamy V, et al. Approaches to Overcoming Existing Barriers of Curcumin. Encyclopedia. Available at: https://encyclopedia.pub/entry/54726. Accessed February 06, 2026.
Azad, Abul Kalam, Joanne Lai, Wan Mohd Azizi Wan Sulaiman, Hassan Almoustafa, Salah Abdalrazak Alshehade, Vinoth Kumarasamy, Vetriselvan Subramaniyan. "Approaches to Overcoming Existing Barriers of Curcumin" Encyclopedia, https://encyclopedia.pub/entry/54726 (accessed February 06, 2026).
Azad, A.K., Lai, J., Sulaiman, W.M.A.W., Almoustafa, H., Alshehade, S.A., Kumarasamy, V., & Subramaniyan, V. (2024, February 03). Approaches to Overcoming Existing Barriers of Curcumin. In Encyclopedia. https://encyclopedia.pub/entry/54726
Azad, Abul Kalam, et al. "Approaches to Overcoming Existing Barriers of Curcumin." Encyclopedia. Web. 03 February, 2024.
Copy Citation
Turmeric contains curcumin, a naturally occurring compound with noted anti-inflammatory and antioxidant properties that may help fight cancer. Curcumin is readily available, nontoxic, and inexpensive. At high doses, it has minimal side effects, suggesting it is safe for human use. However, curcumin has extremely poor bioavailability and biodistribution, which further hamper its clinical applications. It is commonly administered through oral and transdermal routes in different forms, where the particle size is one of the most common barriers that decreases its absorption through biological membranes on the targeted sites and limits its clinical effectiveness.
curcumin
encapsulation
targeted drug delivery
colon cancer
1. Introduction
Optimizing oral curcumin’s bioavailability and stability directly impacts its plasma concentration and therapeutic benefits. The overarching principle for overcoming issues like rapid metabolism is improving bioavailability. This can be achieved by formulating a pH-sensitive colon-targeted drug delivery system (CTDDS) to preserve and release an adequate amount of curcumin at the preferred colon target site [1]. CTDDS provides prolonged retention time, decreased upper stomach absorption, and reduced first-pass metabolism. Consequently, properties like bioavailability and stability are enhanced, while dosing frequency is reduced [2][3]. Due to the small size of CTDDS, curcumin particles readily diffuse in the colon without irritation. Simultaneously, keeping serum concentrations low reduces adverse reactions, further improving patient compliance [1]. Microencapsulation, producing 0.5–1 mm diameter microbeads, is an attractive CTDDS method [1][4][5]. It also improves oral curcumin’s sensory experience by eliminating the bitter taste without altering its nature [6]. Studies show that micronized curcumin has a nine-fold higher bioavailability than unformulated curcumin due to the increased surface-area-to-drug ratio with a smaller diameter, improving the dissolution rate and bioavailability. Notably, 0.5 g of micronized curcumin led to a significant plasma level in humans, at 0.6 μg/mL, a remarkable outcome for curcumin [7]. The hydrogen donor site, α,β-unsaturated β-diketone moiety, is believed to be the breakdown point during curcumin hydrolysis and degradation in water [8]. Binding curcumin’s diketo reaction site with proteins, lipids, polymers, or macromolecules improves its solubility in water by protecting the site from hydrolysis. Other approaches to improving chemical stability include the synthetic modifications of oxidation sites like phenolic OH and enolic OH, and encapsulation in lipids [8].
Using nanosized particles to deliver curcumin to tumor sites either alone [9] or more likely in conjunction with conventional chemotherapeutic agents is a common approach in cancer-targeting strategies, with investigators searching for synergistic combinations and a reduction in side effects or studying how to overcome drug resistance with different chemotherapeutics like platinum compounds [10] in nonsmall cell lung cancer (NSCLC) and colon cancer and paclitaxel [11] in breast cancer. Injectable nanoparticles delivering curcumin to tumors have to be coated with a hydrophilic coating, almost exclusively composed of polyethylene glycol (PEG), and they provide a wide array of options for positive targeting techniques [12].
2. Microencapsulation and Nanoencapsulation in Polymeric Materials
Any drug delivery system should aim to achieve and maintain the target therapeutic drug concentration at the site in the body. This can be accomplished using a multiparticulate dosage form like microbeads or nanoparticles, which are divided into smaller subunits that each have the desired properties [5]. Microencapsulation is an attractive, advanced process commonly used to produce controlled drug release systems [5][13]. It involves the permanent or temporary coating of an unstable active substance with a polymeric or nonpolymeric material. This forms small spheres with diameters ranging from one to several hundred micrometers, enabling targeted and controlled drug release under specific circumstances [6][13][14][15].
The encapsulation of curcumin in polymer-based particulates is not without limitations. The main ones include the lack of mature methods for industrial scale-up, shelf stability issues for many formulations, difficulties in coencapsulating more hydrophilic drugs [16], the lack of sufficient toxicology data for most polymers being investigated, high immunogenicity for older generations of particles and high costs [17].
According to several studies, microencapsulation protects sensitive core materials from degradation under harsh conditions like heat, oxygen, acids, or alkalis by incorporating them within a protective wall [6][13][15]. It enables controlled or sustained drug release at a particular location in the body over time and under desired environmental conditions. This preserves an adequate drug amount to have the intended effect at a specific site [5][14][15]. Subsequently, core substance stability is improved, along with enhanced efficiency in factors like dose-dependent antioxidant and anti-inflammatory effects. Bioavailability is also boosted, reducing dosing frequency and dose amount while maintaining uniform delivery [5][6][13][18]. The coating material type is crucial in this regard [15]. Additionally, microencapsulation enhances the sensory experience of medication by eliminating unpleasant tastes, aromas, or odors like bitterness without altering the drug’s nature [6] Azad et al., 2020 [14]. As noted by [6], microencapsulation also aids core substance solubility and improves permeability.
3. Microencapsulation and Nanoencapsulation Techniques
There are various microencapsulation techniques, classified into three main groups: physical, chemical, and physicochemical methods. Physical methods include spray drying, spray chilling, fluidized bed coating, centrifugal extrusion, solvent evaporation, and supercritical fluid precipitation [4][13]. Chemical methods involve in situ interfacial polymerization and molecular inclusion complexation. Physicochemical techniques include micro- and nanoparticle preparation techniques that depend on high sheer homogenization like emulsion solvent displacement technique, as well as low energy techniques dependent on the Ouzo effect (nanoprecipitation) and micelle formation methods [16]. In addition to conventional techniques that have been extensively discussed elsewhere [19][20], novel approaches are under trial like coacervation, ionotropic gelation, and sol-gel encapsulation [4][13]. The choice of method depends on the core substance and encapsulant’s chemical and physical properties, along with the desired product characteristics and morphology [6][15]. Using different methods can result in variations in capsule size, shape, hygroscopicity, lipophilicity, surface tension, and thermal behavior [15]. The primary determinants influencing drug release are the encapsulated drug type, encapsulant characteristics, and drug-to-encapsulant ratio, as well as their interaction [15]. Some key factors to consider in selecting the optimum microencapsulation technique include production scale, cost-effectiveness, reproducibility, and mild processing conditions to preserve the activity of sensitive compounds like curcumin. Evaluating the advantages and limitations of each method based on these factors can help identify the ideal approach for a particular application. The goal is to select a technique that allows for the development of an encapsulated product with the desired properties to effectively deliver curcumin at the target site.
2.1. Spray Drying
Spray drying is a microencapsulation method where the core material is suspended or dissolved in a polymer solution to form a feed solution. The feed solution is then atomized to produce a mist within a chamber. Hot air is added which dries the mist into a powder [6][15]. The resulting powder has a range of particle sizes depending on variables like feed solution properties and operating conditions [15]. As noted by [6][13][15], the key benefits of spray drying are its affordability, flexibility, and suitability for diverse materials. This method has a high encapsulation loading capacity and is easily scalable. The short dryer contact time also enables the handling of labile materials [6]. However, the high temperatures used may degrade active ingredients. For example, hot air was found to increase omega-3 fatty acid powder oxidation susceptibility, shortening shelf life [13][15].
For heat-sensitive compounds like curcumin, the high temperatures involved in spray drying could lead to activity loss. However, measures can be taken to mitigate this, such as using cold water in the atomization process or adding antioxidants. The affordability, scalability, and suitability for fragile substances make spray drying an attractive microencapsulation approach for curcumin. The proper optimization of operating parameters could lead to the production of an encapsulated curcumin powder with enhanced stability and bioavailability compared to unencapsulated curcumin.
2.2. Spray Chilling
Spray chilling, also known as spray cooling, is a microencapsulation method similar to spray drying in processing. The main difference is that cold air is used throughout the chilling process instead of hot air [15]. In spray chilling, the core substance and polymer are atomized together into a mist. Cold air introduction solidifies the microdroplets, producing microencapsulated powder. As stated by [15], spray chilling has tremendous potential for industrial production scale-up. However, research shows that spray-cooled microcapsules are unstable and may expel the core material during storage.
For heat-labile compounds like curcumin, spray chilling offers a significant advantage over spray drying by avoiding high temperatures that could degrade activity. The cold air preserves curcumin’s instability while enabling encapsulation. Spray chilling also shares the scalability benefits of spray drying. However, the storage instability issue reported for some spray-chilled microcapsules needs consideration. Proper polymer selection and the optimization of operating parameters could potentially improve stability. However, evidence of spray-chilled curcumin microcapsule stability during storage would need evaluation. If stable formulations can be developed, spray chilling presents a promising microencapsulation technique for curcumin delivery. The cold-processing temperatures ensure preserved bioactivity, while the industrial scalability facilitates translation and widespread use.
2.3. Fluidized Bed Coating
Fluidized bed coating is a microencapsulation technique where the coating material is sprayed over a fluidized core material [15]. The core is fluidized via air application. The coating can be applied through top spray, bottom spray (most common technique Figure 1), or tangential spray. Coating effectiveness depends on factors like the coating feed rate, atomization pressure, incoming air temperature, and velocity [15]. According to [21], fluidized bed coating offers advantages like easy manageability due to stable conditions and temperature runaway prevention through resistance to rapid temperature changes. It is useful for both large and small scales and enables continuous operation. However, this technique can only accommodate certain particle types and sizes. Its disparate flow patterns are also difficult to predict. Therefore, scaling up from small to industrial scales often poses challenges due to the complex behavior. Particle breakup is frequently observed as well. Particle collisions also cause pipe and vessel wall deterioration [21].
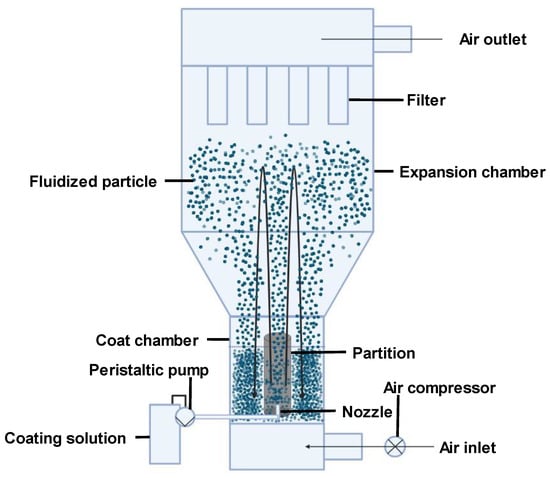
Figure 1. An illustration of the fluidized bed technique (bottom spray).
For heat-sensitive curcumin, fluidized bed coating enables uniform microcapsule coating under mild conditions that preserve bioactivity. Continuous operation and scalability are also beneficial for large-scale production. However, the particle size and type restrictions could limit encapsulation options, and particle collisions may damage curcumin. Proper polymer and operating parameter selection could mitigate these issues. For instance, using a polymer with cushioning properties could protect curcumin from damage during collisions. Fluidized bed coating presents a mild and scalable approach for curcumin microencapsulation, though particle restrictions and the potential for collisions require consideration.
2.4. Coacervation Technique
Coacervation is a physicochemical microencapsulation method involving the formation of a uniform polymeric layer around the core substance. The core and polymer are mixed into an immiscible solution. Changes in pH, temperature, or ionic strength alter the polymer’s physicochemical properties, causing phase separation. Coacervates—small dense polymer droplets—are formed. Enclosing the core in these coacervates creates microcapsules. According to [15], coacervate formation stems from an electrostatic interaction between the two aqueous media. Coacervation is typically used for lipophobic molecule encapsulation. However, its usage is constrained as it functions optimally only within narrow pH ranges, and with certain electrolyte and colloidal solutions [15].
There are two types of coacervation: simple and complex. Simple coacervation uses one polymer like alginate. Sodium alginate is dissolved in water, and then the core material is added to the emulsion formed. This is released as droplets into a gel-forming medium like calcium chloride. The ionic reaction between sodium alginate and calcium chloride forms the insoluble polymer calcium alginate [15]. Complex coacervation involves multiple polymers, for instance, alginate and gelatin. Alginate and gelatin are solubilized in water at basic and acidic pH, respectively, generating negative and positive charges. The core material is added to the alginate solution and homogenized well. The gelatin and alginate phases are thoroughly mixed at increased temperature until a reaction is observed. This produces an insoluble polycationic–polyanionic polymer around the core [15]. For curcumin delivery, coacervation enables mild aqueous encapsulation that maintains stability. Simple coacervation with a suitable polymer could allow for tuned release. However, the constraint to narrow pH/electrolyte ranges may limit applications. Complex coacervation (Figure 2) provides more tailoring options with polymer combinations but needs extensive optimization. Overall, coacervation is a promising approach for stable aqueous curcumin encapsulation if optimal polymers and conditions are identified.
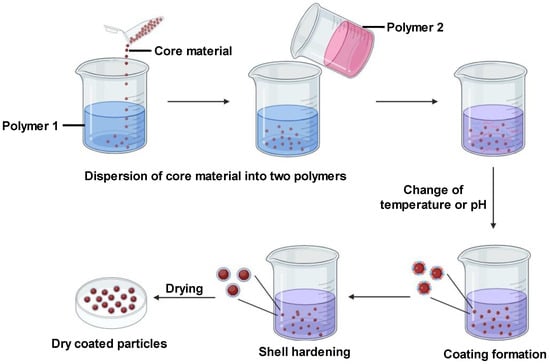
Figure 2. Complex coacervation technique for microencapsulation.
2.5. Ionotropic Gelation Method
Due to its simplicity, ionotropic gelation has received the most attention for microbead creation compared to other techniques [5]. Its flexibility to generate a broad particle size range; medium-to-high drug encapsulation efficacy; and the use of biocompatible, biodegradable polymers also promote its usage [22]. This technique has frequently led to the production of naturally occurring, water-soluble polymeric nanoparticles with great control over bioactive ingredient release through polymer relaxation [22]. This method leverages an ionic polymer’s ability to crosslink with opposing ions to form a sustained-release hydrogel, as noted by [5]. Unlike simple monomeric ions, polyanion–cation interactions cannot be fully explained by electroneutrality. The three-dimensional structure and presence of the other group determine anions’ cation conjugation capacity, and vice versa. Research shows that microbeads prepared with this method have improved bioavailability and lead to controlled oral medication release, reducing dosing frequency [5]. Ionotropic gelation is divided into internal and external gelation based on the crosslinker ion source.
For curcumin, ionotropic gelation offers a simple, mild approach using biocompatible polymers to improve stability and bioavailability. Tunable crosslinking enables sustained release customization. Internal gelation where the crosslinker is present in the initial mixture may better preserve curcumin during encapsulation. The method’s flexibility and control make it well suited for optimizing curcumin delivery through polymeric microbead encapsulation.
In internal gelation, the crosslinker ion is generated “in situ” within the polymer solution. Crosslinker cations come from insoluble metal salts like barium carbonate or calcium carbonate. Lowering the solution pH solubilizes the metal salt, releasing the metal ion internally [5]. In external gelation, crosslinker ions positioned externally come from a metal ion solution. The drug-containing polymer solution is extruded with a needle into the metal ion solution under gentle stirring. Gelation occurs instantly when the polymer drop contacts the metal ions, forming self-sustained beads. The beads are cured in the gelation medium before retrieval and drying. Rapid crosslinking ion diffusion into the partly gelled beads causes external gelation [5].
Ionotropic gelation has several advantages over conventional techniques, chiefly its simplicity and affordability due to the lack of complex machinery, aqueous solvents, and short processing times, as indicated in [22]. Additionally, the reversible electrostatic reaction-induced physical crosslinking prevents potential toxicity and other undesirable biomedical consequences, unlike chemical crosslinking [22]. However, poor mechanical stability is a drawback requiring further improvement [22].
For curcumin encapsulation, ionotropic gelation offers a simple, mild approach with short processing times that maintains stability. Internal gelation may better preserve curcumin by initially incorporating the crosslinker. The mechanical stability issues could be addressed through polymer modifications or gentle downstream processing. Overall, the method’s advantages make it promising for optimized curcumin encapsulation through tunable crosslinked hydrogel microbeads.
2.6. Electrohydrodynamic Atomization (EHDA) Technique
Microencapsulation methods such as extrusion, freeze-drying, and others are commonly used in biomedical applications. However, their use is limited due to the requirement of high temperatures or organic solvents [23]. The electrohydrodynamic atomization (EHDA) technique has emerged as a promising encapsulation alternative that can enhance product fabrication without high temperatures [13]. Also known as electrospraying, EHDA is one of the most notable current microencapsulation techniques [13][24]. Similar to electrospinning, electrospray is a simple and effective method for generating polymeric or fiber-based bioactive nanoparticles. With EHDA, it is possible to overcome the drawbacks of conventional microencapsulation like poor scale-up, low encapsulation efficiency, and particle polydispersity, as indicated in [13]. Furthermore, sustained drug release in the GIT can be readily achieved with the EHDA technique.
Monodispersed particles ranging from microns to hundreds of microns can be produced by EHDA by leveraging electrostatic differences [24][25]. In this method, a syringe is filled with a polymeric solution containing the coating material and core substance and then connected to an infusion pump with a preset regulated flow [24]. A high positive voltage is applied between the needle and a grounded metallic collector, generating an electrostatic field and high voltage on the polymeric solution jet, with negative discharge occurring at the collector [13][25]. Exposure to the electric field deforms the liquid droplet surface at the capillary nozzle tip into a cone shape (Taylor cone) due to internal electrostatic repulsions and external coulombic attraction, from which the jet is ejected. The jets then break into fine droplets due to varicose instability, forming solution jets that are collected as the capsules form [24][25]. Ref. [24] noted that the lower voltage and viscosity encourage highly charged droplets that scatter with solvent evaporation, yielding deposited capsules. A low polymer concentration is needed to destabilize the jet and form tiny droplets. Moreover, low-molecular-weight particles may not provide sufficient viscosity but offer strong inter- and intramolecular forces that must be balanced via surfactant addition [13] (Figure 3).
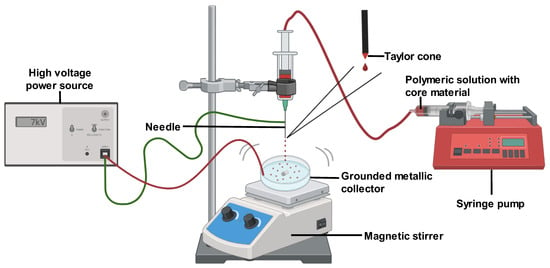
Figure 3. Electrohydrodynamic atomization (EHDA) technique.
Several parameters should be considered when preparing microparticles using the EHDA technique. According to [13], the release kinetics of encapsulated core material are influenced by the shape and size of the electrosprayed particles. These are in turn affected by three main factors: solution properties, operational variables, and environmental conditions. Solution properties include viscosity, surface tension, conductivity, polymer concentration, and molecular weight. Operational variables are the distance between the needle tip and collector, flow rate, and applied voltage. Environmental factors comprise humidity, temperature, and airflow. Ref. [13] found that particles tend to have a more spherical versus irregular shape.
Polymer concentration and molecular weight impact the surface tension and viscosity of the polymeric solution, as [13] discussed. High polymer concentrations are needed to form particles from low-molecular-weight polymers, while high-molecular-weight polymers can yield particles even at low concentrations. Solution viscosity is key for optimizing the process. Surface tension also affects droplet formation, with polymers having low surface tension generating smaller droplets and particles [13]. The polymer’s conductivity and solvent are critical for electrospraying since they influence the electrostatic attraction to the collector. Higher conductivity increases Coulombic repulsion forces that compete with solution viscoelastic forces, untangling polymer chains to form smaller particles. Additionally, the applied voltage is crucial for monodisperse particles. Altering the voltage can modify particle morphology; high voltages result in elongated particles, according to [13].
The EHDA technique is a cost-effective, versatile microencapsulation method with significant potential for drug delivery applications, as described by [25]. It is also a simple, environmentally friendly one-step process that does not require organic solvents and high temperatures [18][24][25]. EHDA can encapsulate both lipophilic and lipophobic substances [24]. As indicated in [14][25] and [13], EHDA allows for the formation of small beads with uniform, narrow size distribution, and greater swelling and diffusion rates. This enhances process performance regarding encapsulation efficacy and formulation flexibility compared to spray drying [13][14][24]. EHDA also achieves higher loading efficiency and particle deposition rates [25]. The technique boosts core material bioavailability and solubility while enhancing protection via improved physical and functional properties [13][25]. Microbeads with larger surface-area-to-volume ratios and more inter/intraparticle pores are also ensured with EHDA [13]. Additional benefits include tailored release profiles and masking undesirable chemicals [13][25], indicating the potential for scale-up. Ref. [18] developed EHDA-enabled small peppermint oil-loaded alginate microbeads with good encapsulation efficiency using a simple apparatus. Ref. [25] reported enhanced release profiles for EHDA-produced PLGA microparticles and 95% encapsulation efficacy for curcumin-loaded PLA microcapsules, which also exhibited potent antibacterial and antioxidant activities. PLA microcapsules developed with EHDA displayed high biocompatibility and minimal cytotoxicity [25]. Encapsulation efficiency around 90% was seen for curcumin-loaded zein–chitosan particles using EHDA [25]. Thus, EHDA is an effective, promising technique for encapsulating bioactive compounds like curcumin.
3. Current Approaches on Encapsulating Curcumin for Drug Delivery
To overcome curcumin limitations resulting in its poor bioavailability and rapid systemic clearance after oral administration, and to fully utilize the therapeutic potential of curcumin, innovative drug delivery systems are required. The encapsulation of curcumin in a protective carrier can enhance its stability, solubility, and absorption while controlling its release rate [26]. Particulate carriers like nanoparticles and microparticles have shown promise for curcumin delivery by increasing surface area, protecting against degradation, bypassing first-pass metabolism, and providing sustained release [7]. While nanoencapsulation has been widely investigated, there are limited studies on formulating curcumin into microbeads using emulsification-based techniques like electrohydrodynamic atomization (EHDA). Compared to nanoparticles, microbeads offer advantages like improved encapsulation efficiency, reduced burst release, and better control over drug release kinetics.
Alginate, a naturally occurring anionic polysaccharide, is an attractive polymer for fabricating drug-loaded microbeads owing to its biocompatibility, low cost, and entrapment efficiency [27]. By optimizing process parameters like voltage, flow rate, and polymer concentration, the size, morphology, drug loading, and release characteristics of alginate microbeads can be tailored using the EHDA technique. Site-specific delivery to the colon can also be achieved by exploiting the colonic microflora-triggered degradation of alginate. Therefore, the proposed research aims to develop curcumin-loaded alginate microbeads using EHDA as an oral delivery system, with enhanced stability, bioavailability, and colon-targeted release intended for therapeutic applications like colon cancer treatment. The systematic optimization and characterization of the microbeads will provide fundamental insights into designing efficacious curcumin delivery systems.
Various drug delivery systems have been explored to overcome the poor water solubility, chemical instability, rapid metabolism, and low bioavailability of curcumin. Encapsulation protects curcumin from degradation, improves its solubility and absorption, and provides controlled release at target sites [28][29]. For instance, the authors of [28] encapsulated curcumin in iron oxide nanoparticles using coprecipitation. The nanocarrier improved curcumin’s solubility and achieved high encapsulation efficiency. The pH-sensitive sustained release of curcumin was demonstrated, with more release at lower pH. This nanoformulation shows promise for targeted curcumin delivery. In another study [29], the authors prepared curcumin-loaded complex coacervates using gum arabic and whey protein nanofibrils. An exceptionally high encapsulation efficiency of 99% was attained, along with enhanced antioxidant activity, versus free curcumin. The coacervates displayed sustained release with more curcumin release under simulated gastric conditions due to weakened electrostatic interactions.
Beyond nanoparticles, polymeric microcapsules also offer advantages like high encapsulation efficiency, tunable drug release profiles, and improved stability. Mai et al. (2017) [30] encapsulated curcumin in poly(lactic acid) (PLA) microcapsules fabricated via electrospraying. Encouragingly, encapsulation efficiency exceeded 95%, and sustained release was maintained for 200 h after a minimal initial burst. Cytotoxicity assays confirmed that microcapsules were nontoxic and highly biocompatible. Silk fibroin nanoparticles developed by [31] delivered encapsulated curcumin in a time-dependent manner to colon cancer cells with enhanced permeability. The nanoparticles provided a slow, sustained release to amplify anticancer effects while reducing side effects. Chitosan–zein nanoparticle carriers synthesized by [32] exhibited a high encapsulation efficiency of 92% and potent cytotoxic activity against neuroblastoma cells. Collectively, these studies demonstrate that nano- and microencapsulation are promising strategies to improve the efficacy and pharmacological properties of curcumin for oral, intravenous, and site-specific delivery. The systematic optimization of formulation parameters and thorough in vitro/in vivo characterization will be imperative to translate these innovative curcumin delivery systems into viable clinical therapeutics.
In addition to the nano- and microencapsulation approaches already discussed, researchers have also explored other techniques like ionotropic gelation to improve the efficacy of curcumin as a therapeutic agent. For example, the authors of [33] encapsulated curcumin in nanoparticles using ionotropic gelation with sodium alginate, achieving high encapsulation efficiency of up to 95%. The nanoparticles displayed minimal dissolution in simulated gastric and intestinal fluid but readily dissolved in simulated colonic fluid. The oral bioavailability of nanoencapsulated curcumin was enhanced five-fold compared to free curcumin. Thus, this formulation shows promise for the targeted delivery and improved efficacy of curcumin for treating colon diseases.
The authors of [34] developed another nanoformulation by encapsulating curcumin in alginate oligosaccharide nanoparticles. The controlled release of curcumin was demonstrated, with 28.9% and 67.5% cumulative release under neutral and acidic conditions, respectively. An excellent trapping efficiency of 91% was also reported. Compared to free curcumin, the nanoparticles showed enhanced absorption by colon cancer cells and improved tumor cell targeting. This highlights the potential of nanoencapsulation to improve the selectivity and anticancer efficacy of curcumin.
References
- Yashika, U.; Manoj, K.S.; Kriti, D. Formulation and evaluation of microbeads for colon targeted drug delivery using natural polymer. World J. Pharm. Med. Res. 2019, 5, 153–163.
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374.
- Wang, Y.; Tang, Q.; Duan, P.; Yang, L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2018, 40, 476–482.
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers 2022, 14, 3834.
- Mullaicharam, B.; Alka, A.J.; Sushama, P. Formulation of micro beads: A review. Int. J. Pharm. Sci. Res. 2021, 12, 95–103.
- Garg, A.; Chhipa, K.; Kumar, L. Microencapsulation techniques in pharmaceutical formulation. Eur. J. Pharm. Med. Res. 2018, 5, 199–206.
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619.
- Rathore, S.; Mukim, M.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A review for health benefits. Int. J. Res. Rev. 2020, 7, 273–290.
- Prabhuraj, R.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Selection of superior targeting ligands using PEGylated PLGA nanoparticles for delivery of curcumin in the treatment of triple-negative breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101722.
- Chen, Y.; Chen, C.; Zhang, X.; He, C.; Zhao, P.; Li, M.; Fan, T.; Yan, R.; Lu, Y.; Lee, R.J. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm. Sin. B 2020, 10, 1106–1121.
- Lin, X.; Wang, Q.; Du, S.; Guan, Y.; Qiu, J.; Chen, X.; Yuan, D.; Chen, T. Nanoparticles for co-delivery of paclitaxel and curcumin to overcome chemoresistance against breast cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 104050.
- da Silva Feltrin, F.; Agner, T.; Sayer, C.; Lona, L.M.F. Curcumin encapsulation in functional PLGA nanoparticles: A promising strategy for cancer therapies. Adv. Colloid Interface Sci. 2022, 300, 102582.
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850.
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a ph-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219.
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 426–442.
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284.
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985.
- Azad, A.K.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875.
- Dhivya, S.; Rajalakshmi, A. A Review on the preparation methods of Curcumin Nanoparticles. Pharma Tutor 2018, 6, 6–10.
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100.
- Patil, P.; Karnavat, D.; Chavan, S. Review on fluidized bed granulation and coating using fluidized bed coater. IAJPR 2018, 8, 1794–1809.
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic gelation of chitosan flat structures and potential applications. Molecules 2021, 26, 660.
- Niamah, A.K.; Al-Sahlany, S.T.G.; Ibrahim, S.A.; Verma, D.K.; Thakur, M.; Singh, S.; Patel, A.R.; Aguilar, C.N.; Utama, G.L. Electro-hydrodynamic processing for encapsulation of probiotics: A review on recent trends, technological development, challenges and future prospect. Food Biosci. 2021, 44, 101458.
- Radünz, M.; Camargo, T.M.; dos Santos Hackbart, H.C.; Blank, J.P.; Hoffmann, J.F.; Stefanello, F.M.; da Rosa Zavareze, E. Encapsulation of broccoli extract by electrospraying: Influence of in vitro simulated digestion on phenolic and glucosinolate contents, and on antioxidant and antihyperglycemic activities. Food Chem. 2021, 339, 128075.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762.
- Ahmad, R.S.; Hussain, M.B.; Sultan, M.T.; Arshad, M.S.; Waheed, M.; Shariati, M.A.; Plygun, S.; Hashempur, M.H. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid. Based Complement. Altern. Med. 2020, 2020, 7656919.
- Lopresti, A.L. The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018, 9, 41–50.
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.N.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38.
- Mohammadian, M.; Salami, M.; Alavi, F.; Momen, S.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Fabrication and characterization of curcumin-loaded complex coacervates made of gum Arabic and whey protein nanofibrils. Food Biophys. 2019, 14, 425–436.
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. 2017, 7, 1724–1734.
- Xie, M.; Fan, D.; Li, Y.; He, X.; Chen, X.; Chen, Y.; Zhu, J.; Xu, G.; Wu, X.; Lan, P. Supercritical carbon dioxide-developed silk fibroin nanoplatform for smart colon cancer therapy. Int. J. Nanomed. 2017, 12, 7751–7761.
- Baspinar, Y.; Ustundas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334.
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.; Joshi, H.K.; Divakar, G. Enhanced water dispersibility of curcumin encapsulated in alginate-polysorbate 80 nano particles and bioavailability in healthy human volunteers. Pharm. Nanotechnol. 2019, 7, 39–56.
- Liu, C.; Jiang, F.; Xing, Z.; Fan, L.; Li, Y.; Wang, S.; Ling, J.; Ouyang, X.-K. Efficient delivery of curcumin by alginate oligosaccharide coated aminated mesoporous silica nanoparticles and in vitro anticancer activity against colon cancer cells. Pharmaceutics 2022, 14, 1166.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
04 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




