Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abraham Abbey Paul | -- | 4136 | 2024-02-01 17:05:16 | | | |
| 2 | Jason Zhu | -1 word(s) | 4135 | 2024-02-02 02:34:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Paul, A.A.; Aladese, A.D.; Marks, R.S. Additive Manufacturing Applications in Biosensors Technologies. Encyclopedia. Available online: https://encyclopedia.pub/entry/54652 (accessed on 07 February 2026).
Paul AA, Aladese AD, Marks RS. Additive Manufacturing Applications in Biosensors Technologies. Encyclopedia. Available at: https://encyclopedia.pub/entry/54652. Accessed February 07, 2026.
Paul, Abraham Abbey, Adedamola D. Aladese, Robert S. Marks. "Additive Manufacturing Applications in Biosensors Technologies" Encyclopedia, https://encyclopedia.pub/entry/54652 (accessed February 07, 2026).
Paul, A.A., Aladese, A.D., & Marks, R.S. (2024, February 01). Additive Manufacturing Applications in Biosensors Technologies. In Encyclopedia. https://encyclopedia.pub/entry/54652
Paul, Abraham Abbey, et al. "Additive Manufacturing Applications in Biosensors Technologies." Encyclopedia. Web. 01 February, 2024.
Copy Citation
Three-dimensional (3D) printing technology, also known as additive manufacturing (AM), has emerged as an attractive state-of-the-art tool for precisely fabricating functional materials with complex geometries, championing several advancements in tissue engineering, regenerative medicine, and therapeutics.
bioinks
polymers
3D (bio)printing
biosensors
additive manufacturing
1. Introduction
The demand to produce sensing devices rapidly and cost-effectively for medical diagnostics, environmental monitoring, and process industries is on the rise. In recent decades, research on biosensor technology has witnessed tremendous progress in response to the increasing spectrum of clinical and environmental analytes, necessitating the need for more rapid and affordable analytical tools. Modern and conventional analytical techniques can offer appreciable accurate and sensitive detection of clinical and environmental analytes. Still, most of these techniques are limited by cost, the need for trained personnel, and impracticability for onsite analysis [1]. Biosensors are seen as the tools of choice to match the rising need for diagnostics and monitoring, as they are inexpensive, seamless to construct, and, most importantly, can be miniaturized into portable formats.
Biosensors are compact analytical devices incorporating a biological sensing element. Biosensors can detect biomolecules in a complex sample by converting the physical or chemical signal into an optical or electrical signal, which can be further processed to yield the analyte concentration, quantitative or semi-quantitatively [2]. Biosensors offer several advantages over conventional analytical methods, such as speed, ease of use, low cost, non-destructive properties, and on-site detection, making them indispensable tools in various fields [3]. They comprise the biological recognition element (BRE), signal transducers, and display units. Biosensors can be classified based on the type of biorecognition element or the nature of transducers used in the device’s development. Based on the kind of biorecognition, biosensors can be classified into catalytic (such as enzymes and catalytically active polynucleotides—DNAzymes) and affinity types such as nucleic acid biosensors and immunosensors). In some whole-cell biosensors, living cells and microorganisms can act as recognition elements (bioreporters) responding to trigger molecules such as inducers) by expressing specific (indicator) genes. The BRE, such as hormones, receptors, antigens/antibodies, enzymes, living cells, nucleic acids, carbohydrates, and tissues, specifically recognize the analytes via catalysis or affinity interactions, which can be further processed to quantify the amount of the analyte in a given sample [4]. Enzyme-based biosensors use specific biochemical recognition and high-efficiency catalysis, leveraging high catalytical power to produce a biosensor with high selectivity and ultra-low detection limits. Other biorecognition molecules such as antigen/antibody, nucleic acid/complementary sequences, and protein/receptor interactions employ a high affinity specific binding interaction as molecular recognition, forming a stable complex. Biosensors can also be classified as optical [5], electrochemical (label-based or label-free), mechanical [6], and conductometric [7] biosensors. Also, based on the signal transducers, biosensors can be classified as thermal, electrochemical, piezoelectric, magnetic, optical, mechanical, or radioactive sensors (Figure 1).
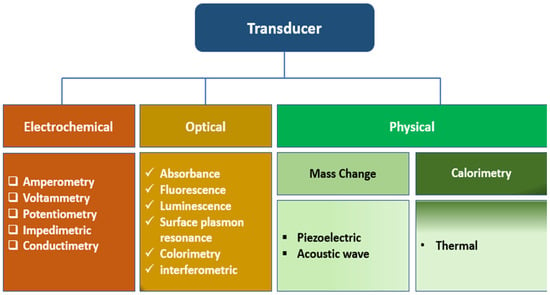
Figure 1. The classification of biosensors based on the types of transducers.
2. Three Categories of Biosensors Based on the Types of Transducers
2.1. Optical Biosensors in Additive Manufacturing Processes
Optical biosensors are widely adapted analytical techniques for the real-time detection of analytes in clinical and environmental samples owing to their high sensitivity and selectivity, ease of deployment, and cost-effectiveness. In this type of biosensor set-up, the information is transduced in the form of photons as absorbance, reflectance, luminescence, or fluorescence emissions over the ultraviolet (UV), visible, or near-infrared regions of the electromagnetic spectrum. By far, fluorescence is the most applied optical detection method and comes in different measurement formats, including fluorescence intensity, quenching efficiency, anisotropy, and decay time, among others.
The easiest way to achieve specificity in an optical biosensor is by using more or less specific biorecognition elements such as enzymes, antibodies, oligonucleotides, or whole cells and tissues [2]. Enzymes catalyze reactions with a high degree of specificity, and the products of these reactions (or of reactants consumed) are monitored directly if they are luminescent or colored or by using optical transducers. The steady-state concentration of detectable species is, thus, related to the concentration of the analyte [2]. A cross-section of an optical enzymatic biosensor based on fiber optic comprising an enzyme layer, an oxygen/pH-sensitive indicator layer. The indicator layer is prepared by either dissolving an indicator dye directly in the polymer matrix or covalently or physically adsorbed onto the surface of microbeads, which can be mixed with the polymer matrix afterward. The indicator layers sense the product’s generation of substrate consumption during an enzymatic reaction.
The enzyme(s) can be immobilized onto the surface of the polymer membrane chemically or physically by entrapment into a polymer network such as hydrogels. a sandwich sensor is mounted on the tip of an optical fiber that transmits excitation light from a light source to the sensor foil and emits (reflected) light back to a photodetector [2]. A variation of the optical sensors that exploit chemiluminescent and bioluminescent reactions is usually more straightforward because no indicator layer is required. They are widely used to monitor highly significant analytes in clinical medicine, food and environmental analysis, and bioprocess monitoring. An affinity-based optical sensor is exemplified using an immunosensors format. In theory, an antigen-antibody binding event is a reversible non-covalent interaction. Still, in practice, most immunoreactions are irreversible due to the significant association constants and very slow dissociation rates [2]. However, efforts have been directed towards the search for reusability of the immunosensors by washing with solutions of high osmolarity, high ionic strength, or low pH, which allows for multiple measurements with a single sensor.
Due to the easy operating principle and quick fabrication of complex 3D models, the additive manufacturing technique has extended to a broad spectrum of biosensing applications, including optical sensors. Three-dimensional (3D) printed structures with integrated sensing components have been widely applied to detect physiological parameters, including blood pressure, heart rate, body motion, respiration rate, brain activity, and skin temperature [8]. Generally, the 3D printed sensors are fabricated by integrating the sensor in the printed platform or directly printing the sensing component.
2.2. Electrochemical Biosensor
In an electrochemical biosensor, biological response is converted into an electrical signal for the detection of specific analytes in a wide range of applications, including pathogen detection, disease diagnosis, food safety, and environmental monitoring [9], by integrating the sensitivity of electroanalytical methods with the biological selectivity of the biological component [10]. Like other types of biosensors, electrochemical biosensor typically consists of a biorecognition element that recognizes its analyte, resulting in a catalytic or binding event that ultimately produces an electrical signal that is proportional to the concentration of the analyte, as monitored by a transduction element.
Electrochemical biosensors fall into two main categories—biocatalytic devices and affinity sensors, depending on the nature of the biological recognition processes. Biocatalytic devices incorporate enzymes, whole cells, or tissue slices that recognize the target analyte and produce electroactive species. Affinity sensors rely on a selective binding interaction between the analyte and a biological component, such as an antibody, nucleic acid, or receptor. Biocatalytic biosensors leverage the catalytic power of enzymes to achieve ultra-low detection limits. They are widely used, but because many biochemical analytes are not amenable to enzymatic detection due to a lack of sufficiently selective enzymes, the analytes and affinity biosensors have appeared as an alternative method. Affinity sensors use the selective and robust binding of biomolecules, such as antibodies (Ab), membrane receptors, or oligonucleotides, with a target analyte to produce a measurable electrical signal [10]. The molecular recognition in affinity biosensors is mainly determined by the complementary size and shape of the binding site to the analyte of interest. The high affinity and specificity of the biomolecule for its ligand make these sensors very sensitive and selective [11].
Many physical and chemical approaches have been studied to immobilize the biomolecules onto the electrochemical transducer to achieve the closest proximity between the biomolecules and the transducer’s surface. A desirable immobilization method would be one that stably retains the structure and function of an immobilized biological entity. Immobilization should be able to achieve enhanced stability, recyclability, and selectivity. Commonly employed immobilization strategies are elucidated by [10].
The most accessible approach has been physically immobilizing the biomolecules on the electrode surface. Physical immobilization does not involve covalent bond formation; as such, the native structure of the biomolecules is preserved. Chemical immobilization usually involves covalent bond formation between the functional groups (NH2, COOH, OH, and SH) biomolecules and the electrodes. Standard enzyme immobilization methods include enzyme entrapment against the electrode using a preformed membrane, encapsulation, inclusion in a gel or electropolymerized film, incorporation in a carbon paste, and using bio-specific interactions such as biotin–avidin binding, adsorption, cross-linking, and covalent attachment [10][12][13].
Electrochemical techniques are generally organized into three main categories of measurement: current, potential, and impedance. The following electrochemical detection methods—voltammetry/amperometry, electrochemical impedance spectroscopy (EIS), conductometry, and potentiometry—have been employed to varying degrees of popularity depending on the analytical needs.
Electrochemical techniques can significantly benefit from using 3D printing technologies in terms of the relatively lower construction costs of custom-made, complex measurement systems and the great flexibility offered by 3D printing technologies [14]. Specifically, 3D printing can be used to create conductive electrodes with unique shapes or compositions. These electrodes can be utilized for redox and catalytic processes that are useful in electrochemical sensors, presenting a promising avenue for developing novel biosensors [15]. This approach enables the integration of bioelectronics with a three-dimensional environment for conducting biological assays, thereby enhancing the sensitivity and accuracy of the sensor [15][16]. The scalability of this approach further contributes to the production of biosensors with improved capabilities for various applications. For instance, Cantù and colleagues reported the realization of miniaturized sensitive electrochemical platforms for protein detection developed through aerosol jet 3D printing [17]. The authors showed the possibility of improving the reliability and repeatability of measurement techniques integrable in several biotechnological applications using 3D printing technologies.
2.3. Physical Sensor
A physical sensor is a device that detects and responds to material inputs, converting them into analog or digital forms. Physical sensors can detect physical quantities based on various physical effects such as force, heat, light, electricity, magnetism, and sound. An example of a physical sensor that involves mass change or calorimetry is the micro-electro-mechanical systems (MEMS) resonant mass sensor. This sensor has been developed to directly measure single adherent cells’ biophysical properties, mass, and growth rate, demonstrating its capability to detect mass changes [18]. An example of a physical sensor is the magnetoelastic sensor. Magnetoelastic sensors have attracted considerable interest within the sensor community as they form an excellent sensor platform that can be used to measure a wide range of environmental parameters, including pressure. Magnetoelastic sensors have been used by applying a mass-changing chemically responsive layer to monitor chemical analyte concentrations, including glucose, carbon dioxide, ammonia, and pH [19]. Coating the magnetoelastic sensor with a mass-changing, chemically responsive layer enables the realization of chemical sensors [19].
Piezoelectric sensors are another class of physical sensors that have found a broad application in biomedical engineering and health monitoring due to their high sensitivity and fast response time. Piezoelectric sensors operate based on the interconversion of electrical and mechanical energies; as such, there has been a growing interest in the use of piezoelectric polymer sensors for energy harvesting and self-powered sensing, leveraging their flexibility, low density, and high piezoelectric constant [20]. This kind of sensor technology demonstrates a high versatility and adaptability towards the fabrication of self-powered and label-free biosensors for the detection of biomarkers, with a wide range of sensing and actuation applications [21][22].
Acoustic wave sensors are among the physical sensors that have emerged as versatile and indispensable tools with applications ranging from biosensing and medical diagnosis to industrial monitoring and safety assurance. These sensors leverage the principles of acoustic wave propagation to detect and analyze physical, chemical, and biological parameters. For instance, surface acoustic wave (SAW) sensors, including Love mode acoustic wave sensors, have been proven to be highly sensitive and reliable for biosensing applications [23][24]. This sensing approach involves the transmission of an acoustic wave across the surface of a device substrate to an interdigitated transducer, where it is converted back into an electric signal through the piezoelectric effect, whereby any alterations to the mechanical wave are reflected in the output electric signal, allowing for the quantification of changes in the surface properties of the device substrate [25][26]. The preceding principle forms the basis for acoustic wave sensors where the SAW is adjustable by adding mass to the surface or by changing the length of the substrate and the spacing between them [25]. Also, film bulk acoustic wave resonators (FBARs) have received specialized attention in electronics and communications for sensing physical parameters such as temperature, pressure, and humidity and for detecting various biochemical substances [27].
3. Bioprinting Method Applications in Biosensors
Three-dimensional (3D) printing technologies will soon impact the biosensor community at the sensor and sensing layer organization level. Many sensors are intrinsically sophisticated and are often arranged into composite architectures constructed from multiple components [28]. Therefore, new fabrication methods that could be used to create complex sensors rapidly are desired. Many thanks to the rapidly advancing field of additive manufacturing that can enable printing different biomaterials into intricate 2D and 3D architectures, which could be used for sensing.
The convergence of additive manufacturing processes with biomaterials has introduced a new paradigm in the biotechnology engineering community. Indeed, the emergence of new printing materials and a variety of 3D printers for a seamless fabrication of complex hydrogel scaffolds that permit the incorporation of sensing layers within the scaffold with complex geometries have brought new perspectives to most biosensors’ developers [29], whereby 3D bioprinting is now being extended to include critical biotechnological applications such as incorporation of active biomolecular recognition element into the 3D printed objects for (bio)sensing purposes.
Many bioprinting techniques, such as electrodeposition, ink-jet printing, microcontact printing, and extrusion, can be adapted for use in the development of biosensors by a precise, rapid deposition and patterning of the printing material laden with reporter biomolecules. The library of biomolecules that have been bio-printed ranges from biomolecules such as proteins, enzymes [30], nucleic acids, polysaccharides, and bacterial cells to whole cells such as mammalian cells, algae, and bacteria [31].
Three-dimensional (3D) bioprinting of biosensors can benefit from the capability for multiplexing and high-throughput analysis for rapid multianalytes screening. Concerning tissue engineering, the incorporation of sensing capability into 3D printing materials could facilitate a rapid patterning of different sensor molecules over a wide range of concentrations to allow for the detection of threshold levels of biomarkers of cellular responses, thereby allowing for a kind of spatial-temporal monitoring of cellular environment in parallel experiments. The transduction properties of the various biomaterials used as bio-ink are essential when bioprinting is aimed at biosensing applications. The immobilization of BRE is critical in fabricating a sensorized 3D construct. Different fabrication approaches have been employed to date.
4. Approaches of Introducing Biosensors 3D Bio-Printed Biosensors
The ability to rapidly manufacture functional sensors would benefit numerous healthcare and environmental monitoring applications. There are different strategies for introducing sensor units to 3D fabrication. The biomaterials could be functionalized with the BRE during the bio-ink preparation before printing or simultaneously during printing. At the same time, it is also possible to functionalize the 3D construct post-fabrication (Figure 2). the additive manufacturing process offers the unique ability to seamlessly integrate complementary fabrication processes or subcomponents manufactured using traditional methods. This allows for the fabrication of 3D-printed sensors by embedding a sensor unit directly into the printed structures during a process interruption, or the sensors can be entirely printed as an intrinsic feature of the structure [32]. Integrating sensing into customized complex geometries is beneficial for many applications, such as patient-specific 3D biomedical devices, point-of-care diagnostics, and spatial-temporal monitoring of cellular environment in tissue engineering, to name a few.
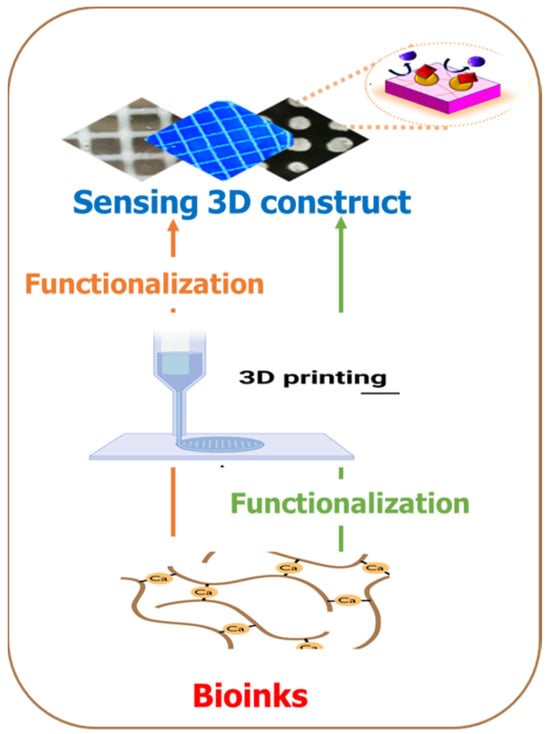
Figure 2. Two strategies for introducing sensing capabilities in additive manufacturing processes. In one approach, the bioink is functionalized with the sensing unit before the 3D printing process, while the second approach entails the functionalization as a kind of post-printing processing.
In 2018, Trampe et al. demonstrated the possibility of combining 3D printing with incorporated sensor particles into the bio-ink by functionalizing the bioink with luminescent oxygen sensors [29]. The authors developed a simple method to functionalize an alginate-based bioink with luminescent O2-sensing nanoparticles, showing excellent printability and biocompatibility when fabricated microalgae and/or mammalian cell-laden scaffolds. The 3D-printed optical sensor was applicable for spatiotemporal imaging of oxygen within the printed—construct to facilitate a rapid evaluation of cell activity in printed constructs as a function of structural complexity, metabolic interactions in mixed-species bio-prints, and response to external incubation conditions. However, successfully printing the scaffold with active biomolecular recognition requires optimized temperature and aqueous environment conditions, especially when the recognition molecules of interest are enzymes, antibodies, or other structurally complex macromolecules [33].
The O2-sensitive nanoparticle-functionalized bioinks were subjected to printability and viability tests, after which the 3Dprinted construct was calibrated. This extensive calibration experiment showed that the ratio of the red and green channels in the acquired images could describe the O2 dependence of the nanoparticle luminescence in the matrix. Furthermore, the authors observed the absence of nanoparticle leakage from the printed hydrogel scaffold into the surrounding medium during several days of incubation. Moreover, no significant photobleaching was detected under the experimental irradiance levels employed [29].
Interestingly, the work of Trampe et al. demonstrated the possibility of mapping local differences in O2 concentrations due to different metabolic activities in hydrogel compartments with mammalian and microalgal cells. More complex 3D-printed hydrogel scaffolds comprising sensor nanoparticle-laden hydrogel strands with either microalgae, mammalian cells, or without cells were analyzed to demonstrate the ability to map local differences in O2 concentrations due to different metabolic activities in hydrogel compartments with mammalian and microalgal cells.
The approach is a landmark and opens a window of opportunities to design unique scaffolds enabling optimal O2 supply to mammalian cells growing in the 3D constructs. The ability to conduct online imaging of dynamic changes in O2 concentration as a proxy for metabolic activity is a powerful tool in tissue engineering. The use of sensor-functionalized bioinks has a wide range of applications in 3D bioprinting and additive manufacturing, as it enables simple, rapid, and noninvasive mapping of the chemical microenvironment and activity of embedded cells in printed scaffolds [29]. Another sensor molecule, such as a pH-sensitive molecule, can be incorporated in hydrogel-based bioinks, individually or in combination with O2 nanoparticles, in an attempt to create multiparameter measurements and mapping of chemical microenvironments, concentration gradients, and dynamics in 3D-bioprinted constructs with living cells [34].
Due to the detrimental effects of excessive exposure to ultraviolet (UV) radiation on human health, an affordable, simple sensor for monitoring UV radiation is desirable. In 2020, Finny et al. [35] prepared a hydrogel-based 3D printing ultraviolet (UV) sensor to quantify exposure. In their study, a color-changing hydrogel ink containing alginate, gelatine, photoactive titanium dioxide nanoparticles, and dyes (methyl orange, methylene blue, and malachite green) was first developed and 3D printed [35]. The nanoparticles initiate photocatalytic degradation of dyes, leading to dye discoloration. The authors show that TiO2 nanoparticles conserve their photocatalytic properties inside transparent hydrogels, retaining their stability upon exposure to UV light.
Attempts were made to assess the photo discoloration rate under outdoor exposure conditions and determine the correlation between discoloration and UV exposure. The sensors prepared from the three dyes were exposed to outdoor sun under the recorded weather conditions and UV index.
The viscosity and composition of the ink were optimized to achieve printability, which resulted in a one-step fabrication approach. The resulting sensors are inexpensive, stable, extremely robust, biodegradable, and easy to use [35]. The hydrogel, comprising alginate and gelatine biopolymers, provided an excellent medium for stabilizing the nanoparticles and the dyes. The hydrogel composition was optimized for room temperature gelation and to facilitate 3D printing of mechanically stable, robust, and reproducible constructs. The ink’s tunability, biocompatibility, and printability offer excellent potential for developing advanced 3D printing methods that, in addition to UV sensors, can be applied more broadly to fabricate other sensing technologies for various other applications.
Worthy of mention is the work of Liu and Li [36], who reported a 3D printing-based strain sensor using an ultra-stretchable and self-healing double-network hydrogel. Leveraging the thermoreversible sol–gel transition behavior of κ-carrageenan in water, a double-network (DN) hydrogel was prepared by combining an ionically cross-linked κ-carrageenan network with a covalently cross-linked polyacrylamide (PAAm) network, showing an enhanced self-healing feature and excellent recoverability. The warm pre-gel solution of the dual network was 3D printable.
Also, a printable hydrogel microarray-based drug-screening platform capable of unambiguously differentiating true enzyme inhibitors from false inhibitors has been developed, by immobilizing the enzyme through entrapment within the hydrogel [37]. A drop-on-demand syringe solenoid printer was used to print hydrazide (POH) and aldehyde sequentially (POA) functionalized poly(oligoethylene glycol methacrylate) (PO) precursor polymers, previously shown to rapidly gel upon mixing via hydrazone bond formation [38] on a nitrocellulose substrate. To demonstrate the potential of printable hydrogel-enzyme thin films for practical biosensing applications, TEM-1 β-lactamase was printed in a POA/POH hydrogel array onto the microzones of a 96-well nitrocellulose wax-printed template. This process created a microplate mimic adaptable to current high-throughput screening protocols [37]. Inhibitor solutions and nitrocefin (a colorimetric β-lac substrate) were deposited on the microzones at different concentrations using a high-throughput dispensing robot. The resulting colorimetric read-out of β-lac activity was quantified via image analysis [37]. This report elucidates a novel technological advancement in drug discovery that employs a printed hydrogel screening assay instead of the conventional microplate assay platforms. This innovative method circumvents the primary limitations of the existing microplate assay platforms by substantially reducing reagent volumes, negating the costs associated with microtiter plates, and augmenting the assay’s sensitivity.
Recently, a gelatine methacrylamide-based hydrogel harboring a sugar-sensitive fluorophore has been printed as a 3D sugar-sensing hydrogel [39]. This was based on the possibility of fluorescently monitoring the reversible binding ability of boronic acids (BAs) with diols such as sugars. The author reported an extrusion-based 3D-printed sugar-sensing hydrogels by incorporating a boronic acid–fluorophore (BA-fluorophore) pair in a gelatin methacrylamide-based matrix. The principle behind the sensing system is based on the intermolecular interaction between BA and fluorophore, resulting in a quenched state of fluorescence [39]. But in the presence of sugar, cyclic BA-ester forms, which can induce a structural change around boron, thus weakening the interaction between the BA unit and fluorophore, and thereby restoring the fluorescence. [39]. In so doing, saccharide concentration can quantitatively modulate the fluorescence response.
Accurate and reproducible fluorescent detection of saccharides over physiologically relevant concentration ranges (up to 40 mM) has been demonstrated. The hydrogel fluorescence emission increases linearly in the presence of glucose (1.72-fold) or fructose (2.65-fold) up to 100 mM.
Mandon et al., 3D printed objects with entrapped sequential enzymatic reactions (glucose oxidase and peroxidase) and entrapped the antibody for a sandwich immunoassay to detect brain natriuretic peptide [33].
Most recently, Leggett and colleagues [40] 3D printed pH-indicating filaments of poly-lactic acid by using a fused filament fabrication (FFF) approach. polylactic acid (PLA) and poly-(ethylene glycol) (PEG) were blended with pH indicator powder to prepare filaments with environmental sensing functionalities. The novel PLA-PEG-indicator sensor filament was robust, with characteristic color changes in different pH conditions tested, thermally stable, and biodegradable. The fabrication approach entailed pre-mixing the components—PLA, PEG, and the indicators (bromothymol blue, phenolphthalein, and thymol blue) before extrusion. A particular type of extrusion-based 3D printing—the direct ink-write technique—was employed to additively manufacture complex geometrical structures with an embedded wireless temperature and relative humidity (RH) sensor during the 3D-printing process [41]. The printed sensor object could read up to 65 RH and temperatures of up to 85 °F from a maximum distance of 141.7 m.
A reagent-less additively manufactured sensor for multi-analytes has been developed by Finny and colleagues [42]. The hydrogel-based (bio)sensors with incorporated receptor molecules and transduction interfaces were 3D printed by extruding the bioink formulation comprising enzymes and catalytic and photoactive properties. The 3D-printed biosensors were a lactate sensor for measuring physiological activity in the sweat and a UV sensor for quantifying harmful UV radiation exposure.
The facile integration of chemical sensing technologies into 3D-fabricated manifolds, which can garner quantitative, measurable responses to the local environment, remains an unmet need of tissue engineering. For example, the incorporation of sensing units into a hydrogel for glucose detection in cancer cells [43] and tissue culture [44][45] has been reported. Glucose detection in solution has been well documented using boronic acid (BA) that binds reversibly to diols of glucose and fructose, leading to a quantitative fluorescent response. A transition from solution-based methodologies to a solid, insoluble platform must be made for the practical realization of this technology. Therefore, Bruen and colleagues [39] researched how to create a 3D-printed hydrogel-based sugar sensor. BA–fluorophore pair was incorporated into a gelatin methacrylamide-based matrix and fabricated by extrusion-assisted 3D printing. The resulting extruded structured porous hydrogels displayed a measurable and reproducible linear fluorescence response to glucose and fructose up to 100 mM. This is a landmark attempt to generate a 3D-printed structure with chemical sensing capability, and as such, could provide a viable route for applying spatiotemporal sensing capabilities to emerging 3D cell culturing environments [39].
References
- Garg, M.; Mehrotra, S. Biosensors. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2017; pp. 341–363.
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461.
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364.
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628.
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948.
- Arlett, J.; Myers, E.; Roukes, M. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215.
- Gundogdu, A.; Gazoglu, G.; Kahraman, E.; Yildiz, E.; Candir, G.; Yalcin, D.; Koç, A.; Şen, F. Biosensors: Types, applications, and future advantages. J. Sci. Rep. 2023, 52, 457–481.
- Llandro, J.; Palfreyman, J.; Ionescu, A.; Barnes, C.H.M. Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput. 2010, 48, 977–998.
- Zhang, C.; Kong, J.; Wu, D.; Guan, Z.; Ding, B.; Chen, F. Wearable sensor: An emerging data collection tool for plant phenotyping. Plant Phenomics 2023, 5, 0051.
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763.
- Eggins, B.R. Chemical Sensors and Biosensors; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Lojou, E.; Bianco, P. Application of the electrochemical concepts and techniques to amperometric biosensor devices. J. Electroceramics 2006, 16, 79–91.
- Cosnier, S. Affinity biosensors based on electropolymerized films. Electroanalysis 2005, 17, 1701–1715.
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755.
- Ragones, H.; Schreiber, D.; Inberg, A.; Berkh, O.; Kósa, G.; Freeman, A.; Shacham-Diamand, Y.J. Disposable electrochemical sensor prepared using 3D printing for cell and tissue diagnostics. Sens. Actuators B Chem. 2015, 216, 434–442.
- Yang, H.; Rahman, M.T.; Du, D.; Panat, R.; Lin, Y. 3-D printed adjustable microelectrode arrays for electrochemical sensing and biosensing. Sens. Actuators B Chem. 2016, 230, 600–606.
- Cantù, E.; Tonello, S.; Abate, G.; Uberti, D.; Sardini, E.; Serpelloni, M. Aerosol jet printed 3D electrochemical sensors for protein detection. Sensors 2018, 18, 3719.
- Park, K.; Millet, L.J.; Kim, N.; Li, H.; Jin, X.; Popescu, G.; Aluru, N.; Hsia, K.J.; Bashir, R. Measurement of adherent cell mass and growth. Proc. Natl. Acad. Sci. USA 2010, 107, 20691–20696.
- Grimes, C.A.; Mungle, C.S.; Zeng, K.; Jain, M.K.; Dreschel, W.R.; Paulose, M.; Ong, K.G. Wireless magnetoelastic resonance sensors: A critical review. Sensors 2002, 2, 294–313.
- Li, L.; Peng, F.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Electrospun Core–Sheath PVDF Piezoelectric Fiber for Sensing Application. ACS Appl. Mater. Interfaces 2023, 15, 15938–15945.
- Liu, Q.; Wang, X.-X.; Song, W.-Z.; Qiu, H.-J.; Zhang, J.; Fan, Z.; Yu, M.; Long, Y.-Z. Wireless single-electrode self-powered piezoelectric sensor for monitoring. ACS Appl. Mater. Interfaces 2020, 12, 8288–8295.
- Lee, Y.; Seo, H.; Jeon, S.; Moon, W. Piezoelectric micro cantilever sensor for non-labeling detection of biomarker. In Proceedings of the SENSORS, 2008 IEEE, Lecce, Italy, 26–29 October 2008; pp. 250–253.
- Liu, Q.; Flewitt, A.J. On-chip temperature-compensated Love mode surface acoustic wave device for gravimetric sensing. Appl. Phys. Lett. 2014, 105, 213511.
- Li, C.; Zhang, J.; Xie, H.; Luo, J.; Fu, C.; Tao, R.; Li, H.; Fu, Y. Highly Sensitive Love Mode Acoustic Wave Platform with SiO2 Wave-Guiding Layer and Gold Nanoparticles for Detection of Carcinoembryonic Antigens. Biosensors 2022, 12, 536.
- Edmonson, P.J.; Campbell, C.K.; Hunt, W.D. Surface Acoustic Wave Sensor or Identification Device with Biosensing Capability. U.S. Patent 7053524, 30 May 2006.
- Hao, D.; Kenney, M.G.; Cumming, D.R. Plasmonic gold nanodiscs using piezoelectric substrate birefringence for liquid sensing. Appl. Phys. Lett. 2016, 108, 251601.
- He, X.; Garcia-Gancedo, L.; Jin, P.; Zhou, J.; Wang, W.; Dong, S.; Luo, J.; Flewitt, A.; Milne, W. Film bulk acoustic resonator pressure sensor with self temperature reference. J. Micromech. Microeng. 2012, 22, 125005.
- Kumar, A.J. Methods and materials for smart manufacturing: Additive manufacturing, internet of things, flexible sensors and soft robotics. Manuf. Lett. 2018, 15, 122–125.
- Trampe, E.; Koren, K.; Akkineni, A.R.; Senwitz, C.; Krujatz, F.; Lode, A.; Gelinsky, M.; Kühl, M. Functionalized bioink with optical sensor nanoparticles for O2 imaging in 3D-bioprinted constructs. Adv. Funct. Mater. 2018, 28, 1804411.
- Ammam, M.; Fransaer, J. Two-enzyme lactose biosensor based on β-galactosidase and glucose oxidase deposited by AC-electrophoresis: Characteristics and performance for lactose determination in milk. Sens. Actuators B Chem. 2010, 148, 583–589.
- Poortinga, A.T.; Bos, R.; Busscher, H.J. Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. Biotechnol. Bioeng. 2000, 67, 117–120.
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, aaf2093.
- Mandon, C.l.A.; Blum, L.J.; Marquette, C. Adding biomolecular recognition capability to 3D printed objects. Procedia Technol. 2016, 88, 10767–10772.
- Brodersen, K.; Koren, K.; Lichtenberg, M.; Kühl, M. Nanoparticle-based measurements of pH and O2 dynamics in the 2 rhizosphere of Zostera marina L. Plant Cell Environ. 2016, 39, 1619–1630.
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D printed hydrogel-based sensors for quantifying UV exposure. ACS Appl. Mater. Interfaces 2020, 12, 43911–43920.
- Liu, S.; Li, L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437.
- Mateen, R.; Ali, M.M.; Hoare, T. A printable hydrogel microarray for drug screening avoids false positives associated with promiscuous aggregating inhibitors. Nat. Commun. 2018, 9, 602.
- Smeets, N.M.; Bakaic, E.; Patenaude, M.; Hoare, T. Injectable and tunable poly (ethylene glycol) analogue hydrogels based on poly (oligoethylene glycol methacrylate). Chem. Commun. 2014, 50, 3306–3309.
- Bruen, D.; Delaney, C.; Chung, J.; Ruberu, K.; Wallace, G.G.; Diamond, D.; Florea, L. 3D Printed Sugar-Sensing Hydrogels. Macromol. Rapid Commun. 2020, 41, 1900610.
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J.J.P. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3d printing. Polymers 2021, 13, 598.
- Marquez, C.; Mata, J.J.; Renteria, A.; Gonzalez, D.; Gomez, S.G.; Lopez, A.; Baca, A.N.; Nuñez, A.; Hassan, M.S.; Burke, V.; et al. Direct Ink-Write Printing of Ceramic Clay with an Embedded Wireless Temperature and Relative Humidity Sensor. Sensors 2023, 23, 3352.
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D printed hydrogel-based biosensors for wearable applications. ECS Meet. Abstr. 2020, MA2020-01, 1973.
- Hietala, M.; Rautio, T.; Mäkikangas, J.; Järvenpää, A. Mechanical properties of the laser powder deposition and laser powder bed fusion printed 316L. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1280, 012018.
- Deckard, C.R. Method and Apparatus for Producing Parts by Selective Sintering. W.O. Patent 1988002677A2, 5 March 1991.
- Yan, X.; Gu, P. A review of rapid prototyping technologies and systems. Comput. -Aided Des. 1996, 28, 307–318.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
592
Revisions:
2 times
(View History)
Update Date:
02 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




