Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lidija Jakobek | -- | 3363 | 2024-02-01 14:16:35 | | | |
| 2 | Jason Zhu | -6 word(s) | 3357 | 2024-02-02 02:35:20 | | | | |
| 3 | Jason Zhu | -41 word(s) | 3316 | 2024-03-01 11:31:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jakobek, L.; Matić, P. Phenolic Compounds from Apples. Encyclopedia. Available online: https://encyclopedia.pub/entry/54645 (accessed on 07 February 2026).
Jakobek L, Matić P. Phenolic Compounds from Apples. Encyclopedia. Available at: https://encyclopedia.pub/entry/54645. Accessed February 07, 2026.
Jakobek, Lidija, Petra Matić. "Phenolic Compounds from Apples" Encyclopedia, https://encyclopedia.pub/entry/54645 (accessed February 07, 2026).
Jakobek, L., & Matić, P. (2024, February 01). Phenolic Compounds from Apples. In Encyclopedia. https://encyclopedia.pub/entry/54645
Jakobek, Lidija and Petra Matić. "Phenolic Compounds from Apples." Encyclopedia. Web. 01 February, 2024.
Copy Citation
Conditions in the gastrointestinal tract and microbial metabolism lead to biotransformation of parent, native phenolic compounds from apples into different chemical forms. Phenolic compounds from apples are found in the gastrointestinal tract in a variety of forms: native (flavan-3-ols, phenolic acids, flavonols, dihydrochalcones, and anthocyanins), degradation products, various metabolites, and catabolites. Native forms can show beneficial effects in the stomach and small intestine and during the beginning phase of digestion in the colon. Different products of degradation and phase II metabolites can be found in the small intestine and colon, while catabolites might be important for bioactivities in the colon.
stomach
small intestine
colon
phase II metabolites
1. Introduction
Phenolic compounds are intensively studied for their bioactivities in the human organism [1][2][3]. These studies show their strong potential for various beneficial effects. However, the forms in which they are present in certain parts of the organism are important for their activity.
In the digestive tract, for example, phenolic compounds can be biotransformed due to environmental conditions that include different pH values or the activity of different enzymes, which cause changes in the chemical structures of the phenolic compounds. These biotransformations are important for the beneficial effects of these compounds since the actual forms of phenolic compounds present are the ones that have the potential for bioactivities. Importantly, the digestive tract is the first system that comes in contact with phenolic compounds from food. Bioactivities in the digestive tract can be the basis of their beneficial effects in many diseases. That is why the fate of phenolic compounds in the digestive tract, including their amounts, forms, and bioactivities, should be better known.
It is well known that in the digestive tract, phenolic compounds are found in their native form, as they are in food [4][5][6]. These are parent compounds that are subjected to various different conditions in different phases of digestion (pH) and can change into different degradation products. Naturally present microbiota in the colon can additionally transform phenolic compounds into various catabolites [7]. Phenolic compounds or their catabolites undergo further phase I metabolism (hydrolysis, oxidation, and reduction) and phase II metabolism (glucuronidation, sulfation, and methylation), the result of which are many forms of metabolites [8][9]. These metabolites pass into the liver where additional biotransformation occurs with further such reactions. However, enterohepatic recirculation can send some of the metabolites back into the intestinal lumen [8][9][10]. All these mentioned forms (native phenolic compounds, products of degradation, catabolites, and metabolites) can be found in the digestive tract depending on the phase of digestion. And they have the potential to be active compounds. Their potential bioactive effects are still not completely known.
2. Apples
Apples belong to family Rosaceae, genus Malus, and species Malus domestica. Their origins are in Asia. Apples are consumed worldwide during the whole year which makes them an important source of nutrients. Water takes up the highest portion in apples, with approximately 80% of the whole mass [11]. Other compounds in apples included in their nutritional value are proteins and carbohydrates. As an example, various traditional and exotic apples contain, within 100 g of fresh weight, 0.07–0.08 g of proteins, around 11 g of sugars, and around 3 g of fibers [12]. Similarly, commercial varieties of apples contain 0.2–0.3 g/100 g fw of proteins, 12.9–13.3 g/100 g fw of total sugars, and 2.4–2.6 g/100 g fw of total dietary fibers [11].
Besides proteins and carbohydrates, apples may contain various vitamins such as vitamin C, vitamins from the B complex, or vitamin E [12]. Traditional and exotic apples contain vitamin C (with averages of 8.88 and 8.28 mg/100 g fw for traditional and exotic varieties, respectively) and vitamin E (with averages of 176 and 141 μg/100 g fw for traditional and exotic varieties, respectively) [12]. Many minerals such as potassium are also common. In old cultivars of apples, the main macroelement was indeed potassium (in peel 104–158 and in flesh 74–93 mg/100 g fw) [13]. Likewise, in the previously mentioned study, potassium was a mineral found in the highest amount (average 101 and 99 mg/100 g fw in traditional and exotic varieties, respectively) [12]. In addition to potassium, apples contain other macroelements such as P, Mg, and Ca [13]. The main microelements in apples are Fe, Al, B, and Na [12][13]. Common organic acids are citric and L-malic acids [12]. The amounts of organic acids and sugars are important because their appropriate ratio gives a pleasant taste to apples, which is essential for consumer acceptance.
3. Phenolic Compounds from Apples
Apples contain five main subgroups of phenolic compounds (Figure 1)—flavan-3-ols (oligomeric procyanidins, procyanidin B1, procyanidin B2, procyanidin B3, procyanidin B4, procyanidin C1, (+)-catechin, and (−)-epicatechin), dihydrochalcones (phloretin-2′-glucoside, and phloretin-2′-xyloglucoside), phenolic acids (chlorogenic acid, neochlorogenic acid, p-coumaroylquinic acid, cryptochlorogenic acid, and protocatechuic acid), flavonols (galactoside, glucoside, rutinoside, xyloside, arabinoside, rhamnoside of quercetin, and kaempferol glycosides), and anthocyanins (cyanidin-3-glucoside, cyanidin-3-galactoside, and others) [12][13][14][15][16][17][18][19][20][21].
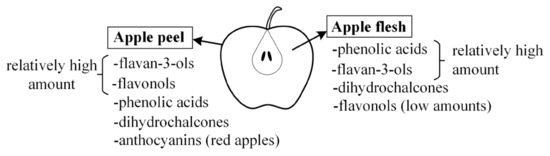
Figure 1. Main subgroups of phenolic compounds from apples.
Phenolic compounds have different distributions of amounts in the peel and in the flesh. In addition to flavan-3-ols and phenolic acids, the peel of red apples usually contains anthocyanins and high amounts of flavonols [13][15]. On the other hand, the flesh usually does not contain anthocyanins, or their content is low [15], except for some varieties that are characterized as having red flesh [22]. Likewise, the amount of flavonols is low in the flesh. However, the flesh has high amounts of phenolic acids [15] and relatively high amounts of flavan-3-ols and dihydrochalcones.
This distribution of phenolic compounds in the flesh or peel can be significant for the beneficial effects of apples. Due to various treatments of apples during their growth, commercial varieties of apples are often eaten without the peel. Accordingly, classes of phenolic compounds from apple flesh such as flavan-3-ols, phenolic acids, or dihydrochalcones can become more important for the potential positive effect in the digestive tract. On the other hand, for apples grown organically, which can be consumed with the peel, phenolic compounds from the peel can be important. In that case, apart from flavan-3-ols, phenolic acids, and dihydrochalcones, flavonols can show the potential for beneficial effects.
4. Phenolic Compounds from Apples in the Digestive Tract
The release and presence of phenolic compounds from apples in the digestive tract is the basis of their beneficial effects. Many in vitro and in vivo studies suggest the presence of relatively high amounts of phenolic compounds from apples in all parts of the digestive tract [5][6][23][24][25][26]; however, these amounts are still lower than the natural amounts in apples.
More specifically, according to in vitro simulated digestion [4][5][6], phenolic compounds are released in the mouth, stomach, and small intestine in lower amounts than the native amounts in apples. In the stomach, total phenolic compounds are released at from 29 to 47 mg/100 g fw (fresh weight), and in the small intestine from 18 to 27 mg/100 g fw, while the native amounts are higher, namely, 36–52 mg/100 g fw in four different apple varieties [5]. Likewise, for in vitro digestion, in a study of the amounts of three phenolic compounds—procyanidins, quercetin-3-rutinoside, and phloretin-2′-glucoside—from the flesh and peel of five apple varieties, before digestion, the amounts of these compounds in apples were found to be up to 79, 44, and 68 mg/100 g fw, respectively [6]. After the digestion process, the released amounts of the same phenolic compounds were as follows: in the mouth, up to 28, 15, and 18; in the stomach, up to 32, 29, and 27; and in the small intestine, up to 44, 21, and 31 mg/100 g fw, respectively [6]. These values are lower than the native amounts.
The study of human subjects also shows that relatively high amounts of phenolic compounds from apples can be found in the digestive tract, but those amounts are still lower than the natural amounts in the fruit [26]. In a study where ileal fluid from ileostomy subjects was analyzed, for subjects who consumed apple juice, 42% of ingested total phenolic compounds was recovered in the ileal fluid. This represents the amount that could be found in the small intestine and could eventually reach the large intestine [26] where these compounds can be degraded by the activity of microbiota into various catabolites. Furthermore, the percentage of catabolites found after colonic fermentation of apples is 29 to 47% of the initial amount in apples [27].
According to the mentioned studies, even though the amounts of released phenolic compounds in the mouth, stomach, small intestine, and colon are lower than the amounts in apples, the amounts are still significant and important for bioactive effects.
4.1. The Forms of Phenolic Compounds in the Digestive Tract
The forms of phenolic compounds from apples that are present in the digestive tract vary according to the different parts of the digestive tract. These differences arise from environmental conditions such as pH. The pH in the stomach is acidic, around 1–2.5 [28][29]. It increases in the proximal small intestine (pH 6.6) and reaches pH 7.5 in the terminal ileum. pH shows a sharp fall to 6.4 in the cecum and increases from the left to the right colon (mean value 7.0) [28]. Similar values of pH or a similar trend were reported in Fallingborg [29] and Nugent et al. [30]. At different pH values, phenolic compounds can be stable or in some cases degrade.
4.1.1. Mouth and Stomach
In the mouth and stomach (Figure 2), phenolic compounds from apples are present in their native form.
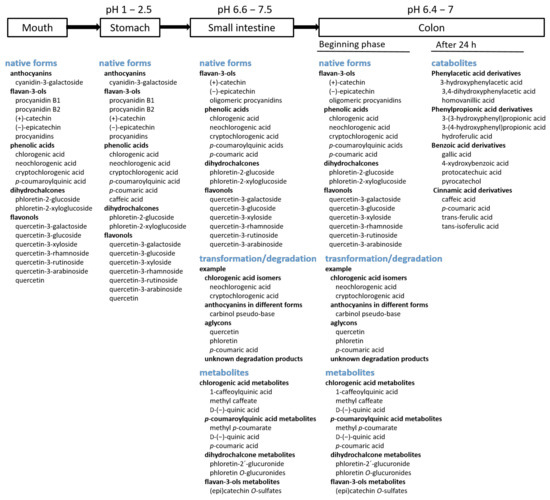
According to the in vitro digestion of apples [5][23][24], flavan-3-ols, phenolic acids, dihydrochalcones, flavonols, and anthocyanins are released in the mouth and stomach from the apple matrix where they stay in their native, unchanged form. Similarly, in the study of Tenore et al. [6], after the in vitro simulated digestion of apple peel and flesh where only procyanidins, quercetin-3-rutinoside, and phloretin-2′-glucoside were studied, phenolic compounds were stable in the gastric phase [6].
Food stays in the mouth for only a short time period. However, it stays in the stomach for a longer period, so its stability in the stomach has also been studied. Phenolic compounds from apples showed stability in experiments in which they were incubated in an acidic medium similar to that of gastric juice [34] with a certain percentage of hydrolysis. After 4 h in the acidic medium, the percentage of hydrolysis of quercetin glycosides, the most abundant phenolic compounds in apple peel was only 4%, while the hydrolysis of procyanidins was somewhat higher, 27% [34]. In another study, after the incubation of apple peel polyphenols in simulated gastric juice without pepsin, the hydrolysis of flavonol glycosides was 3% and that of procyanidins was 27% [35]. It seems that low pH corresponds well to native forms of phenolic compounds even though a smaller percentage might hydrolyze due to low pH.
4.1.2. Small Intestine
Passing from the stomach to the small intestine, phenolic compounds reach an environment of higher pH. Portions of them are still present in their native forms [5][23][24][26] (Figure 2). However, they can undergo some transformations or degradation due to the increased pH value.
Flavan-3-ols: In studies conducted on ileostomy patients after they consumed apple juice [26] or an apple smoothie [33], 90% [26] or 62% [33] of ingested oligomeric procyanidins were recovered in the ileal fluid, which means that high amounts of native oligomeric procyanidins reach the small intestine. However, their polymerization degree might decrease, and smaller units cleaved from the oligomeric compounds might be absorbed [26][33]. On the other hand, smaller molecule flavan-3-ols such as (+)-catechin and dimeric procyanidins (procyanidin B1 and B2) were not found in the ileal fluid [26], or (+)-catechin and (−)-epicatechin were found in low amounts [33], which suggests that they could have been absorbed [26] or degraded into unknown products [31]. Similarly, in vitro studies suggested that procyanidins and monomeric catechins from apples might degrade in the small intestine [5]. Furthermore, phase II metabolites (epi)catechin O-sulfates found after the consumption of a smoothie were the result of the absorption of monomers by enterocytes and metabolism, which resulted in the appearance of sulphated compounds [33]. According to the mentioned studies, native flavan-3-ols with higher molecular mass are present in the small intestine, however, with a lower degree of polymerization. Smaller molecules might degrade into unknown products, or they can be metabolized into phase II metabolites.
Phenolic acids: Studies of in vitro simulated digestion of apples showed that chlorogenic acid can be present in its native form in the small intestine, but a portion of it can isomerize. This has been shown by the identification of native chlorogenic acid and its isomers (neochlorogenic and cryptochlorogenic acid) after the intestinal phase of simulated digestion of apples [5]. Similarly, after human subjects ingested apple juice [26] or an apple smoothie [33], chlorogenic acids that originate from apples (4-caffeoylquinic acid (cryptochlorogenic acid) and 5-caffeoylquinic acid (chlorogenic acid)) were found in the ileal fluid, together with their isomers (1-caffeoylquinic acid and 3-caffeoylquinic acid (neochlorogenic acid)) [26][33]. The presence of 3-caffeoylquinic acid confirmed the isomerization of native chlorogenic acids from apples in the human small intestine [26] similar to in vitro studies. However, 1-caffeoylquinic acid could be the product of esterase activity of the enterocytes in the small intestine [26]. Native p-coumaroylquinic acids were also detected in the ileal fluid [26][33]. In addition, studies in humans revealed the presence of other metabolites of phenolic acids in the small intestine (d-(−)-quinic acid, methyl caffeate, and methyl p-coumarate) [26]. d-(−)-quinic acid can be the product of the degradation of 5- and 4-caffeoylquinic acids or p-coumaroylquinic acid from apples, a reaction that might be catalyzed by esterase activity [26][33]. Methyl caffeate and methyl p-coumarate might have been created in the liver and transferred to the small intestine via enterohepatic recirculation or they could be the results of the activity of the carboxyl esterase of enterocytes [26]. As the described studies have shown, phenolic acids from apples appear in the small intestine in the native form, in the form of biotransformed compounds such as isomers of chlorogenic acids, and in the form of metabolites.
Dihydrochalcones: Native dihydrochalcones (phloretin-2′-glucoside, phloretin-2′-xyloglucoside) are stable in the small intestine according to in vitro studies [5][31][36]. The previously mentioned study in humans supported the stability of dihydrochalcones since phloretin-2′-xyloglucoside was found in the ileal fluid after subjects consumed apple juice [26][33]. However, phloretin-2′-glucoside was not found in the ileal fluid, but its phase II metabolite phloretin-2′-glucuronide was [26][33]. It was suggested that phloretin-2′-glucoside in the digestive system can be cleaved into phloretin and glucose. An aglycon phloretin can then be absorbed. Its glucuronidation takes place in the liver after which phloretin-2′-glucuronide is created. A metabolite, phloretin-2′-glucuronide can reach the small intestine through the enterohepatic recirculation, or glucuronidation could take place in the enterocytes of the small intestine [26]. An aglycon phloretin has been found in the ileal fluid after the consumption of an apple smoothie [33]. As suggested, dihydrochalcones from apples can be present in the small intestine in their native form and in the form of phase II metabolites, and even aglycons can be present in a low amount.
Anthocyanins: In vitro studies suggest that anthocyanins from apples change form when passing from the stomach and low pH, in which they have the form of flavylium cation, into the small intestine and higher pH [4][5][23][24]. At the higher pH in the small intestine, they change into a colorless carbinol pseudo-base [37]. In addition to the influence of pH, the presence of initial low amounts of anthocyanins in the consumed food contributes to their loss in the small intestine. Since anthocyanins change form in the small intestine and their initial amounts in whole apples is usually low, they are not identified in their native form in the small intestine [23][24]. Similarly, in the study in humans who consumed apple juice, anthocyanins were not found in either the apple juice or the ileal fluid after the consumption of juice [26]. Accordingly, it can be suggested that due to their usually low amount in whole apples and the influence of a higher pH on the change in their native form, anthocyanins from apples will usually not be found in the small intestine in their native form.
Flavonols: Flavonols are found in the small intestine in their native form according to in vitro simulated digestion of apples [5][23][24][31]. However, they were not found in the ileal fluid after human subjects consumed apple juice [26], which might be the result of their low amount in the consumed juice. In contrast, after the human subjects consumed an apple smoothie, native flavonols were present in the ileal fluid [33] due to the higher amount of flavonols in the smoothie. It can be suggested that flavonols can be present in the native form in the small intestine. However, this amount depends on the amount in the consumed apple or apple product. Usually, apple peel contains higher amounts of flavonols. If the apple with the peel is consumed, that can lead to potentially higher detectable amounts of native flavonols in the small intestine, as it has been shown in earlier studies [5][6][23][24][25][31][32][33].
4.1.3. Colon
The mixture of all forms of phenolic compounds in the small intestine mentioned earlier, such as native phenolic compounds, transformed compounds, and metabolites, reaches the colon and is present in the colon at least at the beginning phase (Figure 2). Furthermore, native phenolic compounds can be additionally released in the colon. This has been shown in an in vitro study [25]: insoluble material, which remained after in vitro oral, gastric, and duodenal phases of digestion, was subjected to the action of bacterial protease (suitable to hydrolyze insoluble material) and a cellulolytic enzyme mixture (to completely disrupt the whole-fruit matrix). This in vitro experiment simulated the phase of the digestion in the colon. In that experiment, amongst different native phenolic compounds released from whole apples or apple flesh, hydroxycinnamic acids were released in the highest amount. In the case of the colonic digestion of apple peel, flavonols were released in the highest amount [25]. However, during their passage through the colon, most of them were degraded into various catabolites due to fermentation by the present microbiota [27][38]. After 6 h of colonic fermentation of apple polyphenol extracts, a small percentage of native phenolic compounds were detected, only 0.4–0.5% of the initial amount. The amounts of catabolites were much higher [38]. Similarly, after the 24 h colonic fermentation of apple polyphenols, small amounts of native phenolic compounds from apples were found, but the amounts of catabolites were higher [27].
Various catabolites can be found after colonic fermentation. When three cultivars of commercial apples were fermented in vitro using a colonic fermentation experiment, catabolites belonging to phenylacetic acid derivatives, phenylpropionic acid derivatives, benzoic acid derivatives, and cinnamic acid derivatives [11] were found. The degradation of precursor native polyphenols started at 5 h of the fermentation and was completed after 24 h [11]. Similar catabolites belonging to the same groups of derivatives were reported after the colonic fermentation of the insoluble fraction remained after the in vitro gastrointestinal digestion of apple peel [32]. Examples of some catabolites are shown in Figure 2.
Chlorogenic acid, which is the most prevalent phenolic acid in apples, has relatively low absorption in the stomach and small intestine (33%) and passes to the colon at a high percentage [39][40]. Accordingly, its degradation in the colon might be important. Chlorogenic acid is hydrolyzed into caffeic and quinic acid by the activity of bacterial cinnamoyl esterase [39][41][42] and then to other catabolites. Its product caffeic acid has the potential for beneficial effects in the colon itself. The hydrolyzation with bacterial cinnamoyl esterase and the presence of caffeic acid in the colon gives additional positive value to chlorogenic acid from apples.
According to these studies, it seems that the most diverse mixture of phenolic compounds from apples could be present in the colon at the beginning of the passage through the colon (native compounds, metabolites, degradation products, and catabolites). Some of them can be absorbed. All these forms can have the potential for positive influence in the colon since they are present there. However, the time of passage through the colon eventually leads to biotransformation into catabolites. That is why special attention needs to be paid to catabolites. Since one of the major compounds in apples is chlorogenic acid, the presence of chlorogenic acid, its product caffeic acid, and the enzymes that hydrolyze chlorogenic acid, such as cinnamoyl esterase, might all be important and confer beneficial effects in the colon.
References
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420.
- Liu, D.; Ji, Y.; Guo, Y.; Wang, H.; Wu, Z.; Li, H.; Wang, H. Dietary supplementation of apple phlorizin attenuates the redox state related to gut microbiota homeostasis in C57BL/6J mice fed with a high-fat diet. J. Agric. Food Chem. 2021, 69, 198–211.
- Qian, Q.; Qiu, D.; Wu, Z.; Yang, H.; Xie, Y.; Li, S.; Yin, Y.; Li, X. Apple polyphenol extract alleviates DSS-induced ulcerative colitis and linked behavioral disorders via regulating the gut-brain axis. Food Biosci. 2023, 53, 102720.
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21.
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472.
- Tenore, G.C.; Campiglia, P.; Ritieni, A.; Novellino, E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 141, 3519–3524.
- Pereira-Caro, G.; Clifford, M.N.; Polyviou, T.; Ludwig, I.A.; Alfheeaid, H.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Plasma pharmacokinetics of (poly)phenol metabolites and catabolites after ingestion of orange juice by endurance trained men. Free Radical Biol. Med. 2020, 160, 784–795.
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225.
- Van Duynhoven, J.; van Velzen, E.; Jacobs, D.M. Nutrikinetic assessment of polyphenol exposure. Curr. Opin. Food Sci. 2017, 16, 88–95.
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334.
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533.
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1.—Nutritional, phytochemical and sensory evaluation. J. Funct. Food 2010, 2, 35–45.
- Šavikin, K.; Živković, J.; Zdunić, G.; Gođevac, D.; Ðorđević, B.; Dojčinović, B.; Ðorđević, N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. J. Food Compos. Anal. 2014, 35, 101–111.
- Cao, X.; Wang, C.; Pei, H.; Sun, B. Separation and identification of polyphenols in apple pomace by high-speed counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2009, 1216, 4268–4274.
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 45.
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; Charles, M.T.; Rupasinghe, H.P.V. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J. Food Compos. Anal. 2008, 21, 396–401.
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Food 2019, 56, 127–135.
- Lo Piccolo, E.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient apple cultivars from Garfagnana (Tuscany, Italy): A potential sourcefor ‘nutrafruit’ production. Food Chem. 2019, 294, 518–525.
- Stracke, B.A.; Rűfer, C.E.; Weibel, F.P.; Bub, A.; Watzl, B. Three-year comparison of the polyphenol contents and antioxidant capacities in organically and conventionally produced apples (Malus domestica Bork. Cultivar Golden Delicious). J. Agric. Food Chem. 2009, 57, 4598–4605.
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530.
- Zhao, T.; Sun, L.; Wang, Z.; Nisar, T.; Gong, T.; Li, D.; Niu, P.; Guo, Y. The antioxidant property and α-amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT-Food Sci. Technol. 2019, 111, 252–259.
- Faramarzi, S.; Pacifico, S.Y.A.; Lettieri, A.; Nocera, P.; Piccolella, S. Red-fleshed apples: Old autochthonous fruits as a novel source of anthocyanin antioxidants. Plant Food Hum. Nutr. 2015, 70, 324–330.
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food Chem. 2021, 363, 130283.
- Jakobek, L.; Ištuk, J.; Barron, A.; Matić, P. Bioactive phenolic compounds from apples during simulated in vitro gastrointestinal digestion: Kinetics of their release. Appl. Sci. 2023, 13, 8434.
- Graziani, G.; Gaspari, A.; Di Vaio, C.; Cirillo, A.; Ronca, C.L.; Grosso, M.; Ritieni, A. Assessment of in vitro bioaccessibility of polyphenols from Annurca, Limoncella, Red Delicious, and Golden Delicious apples using a sequential enzymatic digestion model. Antioxidants 2021, 10, 541.
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J. Agric. Food Chem. 2007, 55, 10605–10614.
- Veeriah, S.; Hofmann, T.; Glei, M.; Dietrich, H.; Will, F.; Schreier, P.; Knaup, S.; Pool-Zobel, B.L. Apple polyphenols and products formed in the gut differently inhibit survival of human cell lines derived from colon adenoma (LT97) and carcinoma (HT29). J. Agric. Food Chem. 2007, 55, 2892–2900.
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041.
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196.
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577.
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. 2020, 66, 102486.
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514.
- Hagl, S.; Deusser, H.; Soyalan, B.; Janzowski, C.; Will, F.; Dietrich, H.; Albert, F.W.; Rohner, S.; Richling, E. Colonic availability of polyphenols and D-(-)-quinic acid after apple smoothie consumption. Mol. Nutr. Food Res. 2011, 55, 368–377.
- Carrasco-Pozo, C.; Speisky, H.; Brunser, O.; Pastene, E.; Gotteland, M. Apple peel polyphenols protect against gastrointestinal mucosa alterations induced by indomethacin in rats. J. Agric. Food Chem. 2011, 59, 6459–6466.
- Pastene, E.; Spiesky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In vitro and in vivo effects of apple polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2010, 58, 7172–7179.
- Pollini, L.; Juan-García, A.; Blasi, F.; Mañes, J.; Cossignani, L.; Juan, C. Assessing bioaccessibility and bioavailability in vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS. Food Biosci. 2022, 48, 101799.
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Fritzsche Rodrigues, R.; Maróstica Junior, M.R.; de Souza Fonseca, B.; Ragagnin de Menezes, C.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Food 2020, 65, 103714.
- Bellion, P.; Hofmann, T.; Pool-Zebel, B.L.; Will, F.; Dietrich, H.; Knaup, B.; Richling, E.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line Caco-2. J. Agric. Food Chem. 2008, 56, 6310–6317.
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197.
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71.
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684.
- Guglielmetti, S.; De Noni, I.; Caracciolo, F.; Molinari, F.; Parini, C.; Mora, D. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 2008, 74, 1284–1288.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




