Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexandre Santos Pimenta | -- | 3981 | 2024-02-01 12:33:17 | | | |
| 2 | Fanny Huang | Meta information modification | 3981 | 2024-02-05 03:15:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; Azevedo, T.K.B.D.; Melo, R.R.D.; Andrade, G.S.D. Wood Vinegar as Antimicrobial Agent in Animal Husbandry. Encyclopedia. Available online: https://encyclopedia.pub/entry/54644 (accessed on 12 January 2026).
Gama GSP, Pimenta AS, Feijó FMC, Azevedo TKBD, Melo RRD, Andrade GSD. Wood Vinegar as Antimicrobial Agent in Animal Husbandry. Encyclopedia. Available at: https://encyclopedia.pub/entry/54644. Accessed January 12, 2026.
Gama, Gil Sander Próspero, Alexandre Santos Pimenta, Francisco Marlon Carneiro Feijó, Tatiane Kelly Barbosa De Azevedo, Rafael Rodolfo De Melo, Gabriel Siqueira De Andrade. "Wood Vinegar as Antimicrobial Agent in Animal Husbandry" Encyclopedia, https://encyclopedia.pub/entry/54644 (accessed January 12, 2026).
Gama, G.S.P., Pimenta, A.S., Feijó, F.M.C., Azevedo, T.K.B.D., Melo, R.R.D., & Andrade, G.S.D. (2024, February 01). Wood Vinegar as Antimicrobial Agent in Animal Husbandry. In Encyclopedia. https://encyclopedia.pub/entry/54644
Gama, Gil Sander Próspero, et al. "Wood Vinegar as Antimicrobial Agent in Animal Husbandry." Encyclopedia. Web. 01 February, 2024.
Copy Citation
Wood vinegar (WV), also known as pyroligneous acid, as an alternative antimicrobial with good potential to replace conventional products. Many studies have demonstrated that WV is a promising product. WV is a nontoxic additive widely employed in the food industry to impart a smoked flavor to foods. Studies have shown that, depending on the WV concentration, good results can be achieved using it as an antimicrobial against pathogenic bacteria and fungi and a valuable growth promoter for poultry and pigs.
wood vinegar
pyroligneous acid
animal husbandry
conventional antimicrobials
1. Introduction
To some extent, most of the productive inputs given to animals during their breeding and veterinary treatment can cause environmental contamination. Among these inputs, antimicrobials stand out [1][2][3][4][5], prompting concern about public health and food safety [6]. One of the main impacts is the contamination of arable land, usually when animal excrement is used as organic fertilizer. Residues of antimicrobials remain in the excrement and, through incorporation in soils, spread to the nearby environment, resulting in various types of contamination [7][8][9][10][11].
Environmental contamination caused by antimicrobials has attracted great concern in recent years, mainly because studies have revealed that using them in animal management for growth promotion and disease treatment is more deleterious than human use [12]. In addition to their significant influence on the emergence of microbial resistance to antimicrobial drugs, antimicrobial residues originating from animal production also contaminate soil [13][14], water [8][15], and food products [3]. Furthermore, animal husbandry’s widespread use of antimicrobials interferes with agroecological production [1]. These substances pose noteworthy challenges to human health by increasing microbial resistance to pharmaceuticals [6], thus aggravating the occurrence of severe diseases due to consuming products contaminated with these drugs [16]. Farmers and those producing meat and dairy products face the challenge of maintaining the health of their animals and farms’ profitability, which would be more challenging without conventional antimicrobials [17][18]. Besides antimicrobials, acaricides are also employed extensively in agriculture, in this case to control ectoparasites. These agents can have the same dynamics of contamination as antimicrobials, being able to pollute soil, water, and plant and animal products [19][20][21].
Therefore, developing natural alternatives to these products to eliminate or mitigate environmental contamination is an urgent task. A product that deserves particular attention for this purpose is wood vinegar (WV). WV is a natural product originating from the thermal degradation of wood. It has an extensive range of practical applications, primarily in agriculture [22][23][24][25][26]. WV has some particularities, such as chemical composition and pH, that enable its variable application [24][27][28]. Additionally, recent innovative studies have demonstrated the potential of WV for biomedical applications due to its antioxidant and wound-healing properties [29][30]. Based on previous works with various types of wood and other lignocellulosic raw materials, many researchers have investigated the effectiveness of WV as an antimicrobial agent to compose antiseptics, among other products [25][31][32][33][34].
Although other authors have comprehensively studied environmental contamination by antimicrobials, there is a gap in the literature regarding the possible use of wood vinegar to replace conventional products.
2. Wood Vinegar—Composition, Properties, and Uses
Wood vinegar (WV) is an aqueous liquid originating from the thermal decomposition (pyrolysis) of lignocellulosic materials in the absence of oxygen or under a controlled atmosphere [24][26][35]. During carbonization or slow pyrolysis, charcoal and gaseous products (smoke) are formed [36][37]. Field measures in industrial kilns have shown that roughly 60–65% of the initial bone-dry wood turns into smoke during carbonization [38][39]. When leaving the reaction bed, the smoke from slow pyrolysis can be carried through devices where a portion condenses, generating the raw pyrolysis liquids. These liquids, after settling, separate into two parts, an aqueous one and an oily one. The aqueous fraction is the WV, and the oily one is called vegetable tar [24][40]. In turn, the oily fraction can contain a supernatant consisting of light oils [28][41], depending on the type of raw material, whether hardwood or softwood. Fast pyrolysis processes can produce aqueous liquids from carbonization with properties like WV. The yields and chemical compositions vary according to the tree compartment of origin [42]. Several lignocellulosic raw materials, such as hardwood, softwood, and bamboo, have been investigated as potential sources of WV [24][42][43]. Crop and forest wastes such as cotton stalks, softwood, and bamboo sawdust have also been investigated [44]. As an adjuvant in soil, WV was referred to as an enhancer that could improve the complexation of remediation materials and Pb(II) ions, which is an exciting application [45].
Properties and Chemical Composition
WV is also known as pyroligneous acid or liquid smoke. The latter name is used when the product imparts a smoky flavor to meat products, sauces, and other foods [46]. WV is essentially an additive with a strong smoke odor employed worldwide in the food industry and used by some 80% of manufacturers of smoked products, providing the characteristic flavor and smell [47]. In addition to imparting a smoky flavor, WV can also be applied to preserve food (fish, meat, and sausages) by extending the shelf life [48]. In this regard, WV is a natural chemical product with properties recognized and certified by several renowned international bodies, such as the Chemical Abstracts Service (CAS 8030-97-5), Flavor and Extract Manufacturers Association (FEMA 2967), European Inventory of Existing Chemical Substances (EINECS 232-450-0), Harmonized Commodity Description and Coding Systems (HS 2915.50.5000), and Food and Drug Administration (FDA-21-CFR compliance 172.515).
WV is composed mainly of water (80–85%) and a mixture of at least 200 organic compounds [24][49][50] of different chemical classes, as follows: organic acids; alcohols; esters; furans; ketones; phenolic compounds; pyrans; and other minor compounds [27][32][51][52]. WV has an acidic character, with pH varying between 2.5 and 3.6 [53][54] and density slightly higher than water, ranging from 1.008 to 1.020 g cm−3 [24]. Its color varies from reddish brown or yellowish brown to dark brown [55]. WV has been used in several areas, mainly agriculture, where the product has many applications as a partial or total substitute of pesticides and fungicides [22][25]. Usually, a significant fraction of WV is composed of organic acids, phenolic compounds, and sometimes furfural, depending on the original raw material [23][24][52]. Among the varied chemical components of WV, phenolic compounds are recognized as the agents responsible for the biological effects of WV as an antimicrobial agent and for other uses [22][25][56]. All told, WV contains at least 20 phenolic compounds [24][52].
3. Wood Vinegar—An Effective Natural Antimicrobial Agent
Numerous studies have demonstrated the antimicrobial properties of WV against pathogenic microorganisms and its natural, safe, and eco-friendly characteristics [25][28][57][58]. Due to the chemical particularity of the WV originating from each plant species, different products have been tested as antimicrobial agents worldwide [24][28][32][59]. The results have demonstrated that WV is a valuable antimicrobial agent, encouraging its consolidation as an alternative to antimicrobials in practical applications. In in vitro tests, Harada et al. [60], using WV from bamboo (Phyllostachys pubescens), demonstrated inhibition of Escherichia coli, Staphylococcus pseudintermedius, and Pseudomonas aeruginosa. Hou et al. [61] described satisfactory results against several pathogens, including Enterobacter aerogenes. In Canada, using WV from a mixture of wood was active against Salmonella enterica and Lactobacillus acidophilus at concentrations ranging from 0.8 to 3.2% [62]. Other researchers have also tested the antimicrobial activity of WV from different tree species and have stressed their potential to replace conventional antimicrobials [23][25][32][49][57][63]. Some authors have reported WV’s effectiveness as an anti-inflammatory agent [64][65]. Antiviral activity of WV from hardwood, softwood, and bamboo was also reported [43][66].
Besides being a natural product from renewable sources and helping to mitigate atmospheric emissions, since WV is collected from the smoke emitted during the production of charcoal, another advantage of this product is that it poses difficulties to the development of microbial resistance. This is due to the wide variety of bioactive compounds in its chemical composition, which have synergistic antimicrobial actions. As mentioned above, phenolic compounds are recognized as responsible for WV’s biological effects when used as an antimicrobial agent. Since at least 20 phenolic compounds are usually contained in WV, the probability of microorganisms simultaneously developing resistance mechanisms against all these substances is remote [33][62]. In addition to its antibacterial action, WV has high antifungal potential. In Indonesia, WV from cocoa pod shells was found to act against fungal strains, including Candida albicans, with inhibition halos ranging from 6 to 6.12 mm [67]. Other studies have also reported the antifungal action of WV against C. albicans and other fungi [23][32][33][68].
Several mechanisms explain the antimicrobial effects of WV. An assessment of the effects of WV against C. albicans demonstrated the destruction of the cell wall of this yeast [69]. The presence of organic acids in its composition can cause damage to the cell walls of bacteria and fungi, changes in the normal pH and other characteristics of their cytoplasm, and alterations of the microorganisms’ genetic material [32][70]. Changes in pH can result in disequilibrium in the capacity of microorganisms to produce H+ at a regular rate, interfering negatively with the osmotic pressure of both the cytoplasmatic membrane and cell wall, which can result in their distortion and rupture [71][72]. On the other hand, phenolic compounds can thin the cell wall and cause cellular depletion and the dispersion of the ribosomes [72]. Phenolics can also promote the displacement of cytoplasmic membranes, impairing their integrity and hence causing the leakage of cytoplasm components [73].
4. Use of Wood Vinegar in Animal Husbandry
This item highlights the use of WV as an input in livestock management, showing the state of the practice and the results of global studies of WV from different lignocellulosic raw materials or biomass wastes. Several assessments of WV as a growth promoter in animal husbandry have shown its potential to replace conventional antimicrobials in this application. Around the world, research has demonstrated the efficiency of this product in poultry, swine, and cattle. To better illustrate the applications of WV in these fields, Table 1 summarizes the relevant information.
The results of WV addition in livestock diets are relatively scarce. There are many more experimental results of powdered charcoal and biochar on cattle performance than findings regarding WV-only use, as displayed in Table 1. Kook and Kim evaluated the effects of supplemental levels of bamboo WV on the growth performance, serum profile, and meat quality of Hanwoo cows [74] in Korea. Concentrate diets were supplemented with the product with a 3% addition level. This improved the marbling score and crude fat content, decreased the shear strength and cholesterol content, and improved the taste according to sensory evaluation. Adding biochar to cattle feed increases body weight, and using activated charcoal combined with WV has been cited as reducing cryptosporidiosis in goats and cattle. Regarding the assessment of biochar and WV mixtures, O’Reilly et al. [75] investigated the effects of different mixtures on in vitro batch ruminal culture fermentation using other feed substrates as references. Based on their experimental results, they stated that biochar is not effective for methane mitigation in ruminant livestock despite previous works that reported success in CH4 emission reduction.
Concerning pig farming, better growth performance and feed digestibility were found among animals with diets containing variable levels of WV. Some results are shown in Table 1. For instance, Mekbungwan et al. [76] assessed histological intestinal villus alterations in piglets that received a raw pigeon pea diet, including charcoal powder and WV. The results demonstrated that the intestinal features could be atrophied by feeding pigeon pea meal to the animals, resulting in decreased growth performance. In another experiment, Choi et al. [77] assessed the feed value of increasing levels of WV given to weanling pigs until 28 days of age against positive controls with apramycin and a negative one containing feed without any additive. In conclusion, the authors stated further research was necessary for its consolidation as a promising product to replace conventional antimicrobials in pig farming.
In turn, Chu et al. [78] demonstrated that including bamboo charcoal or bamboo WV as an alternate antimicrobial in the diet of fattening pigs led to better growth performance, immune responses, and fecal microflora populations. They measured decreased cortisol levels, higher average daily weight gain, and better feed efficiency with the feed additive. Thus, bamboo charcoal or WV has the potential as an additive in pig production instead of conventional antimicrobials since growth performance, immune response, and fecal microflora populations were improved. Additionally, Wang et al. [79] evaluated the effects of feeding bamboo vinegar combined with an acidifier (a mixture of organic acids) as an antimicrobial substitute on the growth performance and intestinal bacterial communities of weaned piglets. The results indicated that the tested additives could effectively replace antimicrobials in the diets of piglets without adverse effects on production, contributing to a higher diversity of the intestinal bacterial population compared to antimicrobials.
Table 1. Uses of various types of WV in animal management (cattle, swine, and poultry).
| Animals | Type of WV | Concentration | Frequency of Use | Effects | Author |
|---|---|---|---|---|---|
| Cattle | Bamboo | 3% | Once a day | Improvement of meat quality (taste and marbling), higher contents of crude fat, less shear strength, and less cholesterol content in meat | Kook & Kim, 2003 [74] |
| Nekka-rich | 10 g (daily dosage) | Included in a milk surrogate for 4 days (every 8 h) | Control of Cryptosporidium parvum in calves | Watarai et al., 2008 [80] | |
| Obionekk | 1.25 g (daily dosage) | Included in a milk surrogate for 14 days (every 8 h) | Control of Cryptosporidium parvum in goats | Parauda et al., 2011 [81] | |
| Swine | Nekka-rich | 3% | Inclusion in feed for 30 days | Improvement of feed conversion and villi height |
Mekbungwan et al., 2008 [76] |
| Commercial WV | 0.3% | Inclusion in feed for 28 days | Improvement of digestibility and control of undesirable coliforms |
Choi et al., 2009 [77] | |
| Bamboo | 0.3% | Inclusion in feed for 42 days | Improvement of performance and stress reduction | Chu et al., 2013 [78] | |
| Bamboo | 0.4% WV + 0.25% acidifier | Inclusion in feed for 25 days | Improvement in intestinal microbiota | Wang et al., 2013 [79] | |
| Acacia auriculiformis wood |
0.3% WV or 0.2% WV + 0.8% biochar | Inclusion in feed twice a day | Control of diarrhea and sulfide hydroxide emissions |
Chao et al., 2016 [82] | |
| Bamboo | 0.5% | Inclusion in feed for 35 days | Regulation of expression levels of mRNA in immune organs | Huo et al., 2016 [83] | |
| Garcinia mangostana | 0.4 to 0.8% | Inclusion in feed for 5 days | Improvement of digestibility | Rodjan et al., 2018 [84] | |
| Not informed | 5.0% | Inclusion in feed for 4 weeks | Improvement in weight gain | Macasait et al., 2021 [85] | |
| Quercus acutissima wood | 0.1% | Inclusion in feed for 16 weeks |
Improvement in weight gain and total digestibility of nutrients |
Sureshkumar et al., 2021 [86] | |
| Poultry | Biochar + WV | 0.5 and 1% | Inclusion in feed | Improvement of egg production and intestinal villi height, less emission of fecal ammonia | Yamauchi et al., 2010 [87] |
| Silicic acid (commercial product) and bamboo vinegar | 0.3% | Inclusion in feed for 112 days | Greater weight gain | Rattanavut et al., 2012 [88] | |
| Silicic acid (commercial product) and bamboo vinegar | 0.2% | Inclusion in feed for 49 days | Improvement of intestinal villi number and height |
Rattanawut & Yamauchi, 2015 [89] | |
| Biochar and bamboo vinegar (8 kg of powder + 3 L of BV) | 1 and 1.5% | Included in daily feed | Improvement of egg quality, digestibility, and control of Escherichia coli and Salmonella sp. | Rattanawut et al., 2017 [90] | |
| Not informed | 0.833% | Inclusion in feed (twice a day) for 84 days | Improvement of laying performance and egg quality |
Nunes, 2019 [91] | |
| WV from Eucalyptus urophylla × Eucalyptus grandis (clone GG100) | 2.5% | Inclusion in feed for 42 days | Improvement of body weight gain, feed conversion, and feed consumption | Diógenes et al., 2019 [92] | |
| WV from the hull of Spina date seed | 0.2% | Inclusion in feed for 50 days | Improvement of egg yolk quality and decrease in n-6 fatty acids |
Zhao et al., 2019 [93] | |
| EP of Myristica fragrans and Acacia confuse | 0.5 or 1% | Added to water twice per day | Improvement of intestinal villi height | Hanchai et al., 2021 [94] |
Nekka-Rich (Cape Cross, Jeffreys Bay, South Africa) and Obionekk (Obione, Charentay, France) are trademarks.
Chao et al. [82] evaluated using charcoal powder and WV to prevent diarrhea and reduce environmental pollution from swine production. The authors determined that the additives could reduce the incidence of diarrhea in pigs and that the concentration of hydrogen sulfide in pig houses decreased in the experimental groups that received a mixture of charcoal and WV in different proportions in the diets. The results demonstrated the undeniable positive effects of charcoal and WV in improving animal health and decreasing environmental pollution from pig breeding. Another study on preventing diarrhea in piglets was conducted by Khai et al. [95], utilizing a WV–activated charcoal mixture. They found positive results in weanling and post-weaned pigs by preventing diarrhea in the rainy and dry seasons. When assessing the effects of mangosteen WV as a potential additive to improve nutrient digestibility in growing pigs, Rodjan et al. [84] found positive results, suggesting that WV can be used as a potential additive in pig farming.
Macasait et al. [85] evaluated the growth performance of pigs and the nutritional and microbial contents of wet and fermented commercial feed containing different levels of WV. Although no differences in microbial and nutritional contents were noted in the fermented feed, a significantly higher profit was determined from pigs fed wet and fermented commercial hog feed containing 5% WV. Also, Sureshkumar et al. [86] assessed the effect of dietary inclusion of WV supplementation on growth performance, nutrient digestibility, and meat quality of grower-finisher pigs, finding enhancement not only of growth performance but also total tract digestibility of nutrients with no effects on lean meat percentage and backfat thickness, claimed to be exciting results.
In an interesting approach, the efficiency of WV was assessed to control offensive odors from piggery wastes [96]. The odorants from piggery wastes were identified as ammonia, methyl sulfide, hydrogen sulfide, butyric acid, and valeric acid. In a laboratory experiment using an air-tight vessel, the WV concentration required for deodorization was 6.6%, with removal efficiency from 70 to 90%. In situ tests at a pig farm showed that the efficiency in removing odorants was similar to that of the laboratory experiments. Besides that, flies were rarely observed, indicating that WV may play a significant role as a repellent.
In poultry farming (see Table 1), the results of experiments have demonstrated the efficiency of using charcoal powder and WV as feed additives in laying hen breeding, resulting in increases in egg production and eggshell thickness and decrease in fecal ammonia concentration and damaged egg rate [87]. Furthermore, the use of WV in feeding these animals increased weight gain of the animals and maximized cell mitoses, resulting in better intestinal health and egg quality and reduced pathogenic populations of Escherichia coli and Salmonella sp. [88][89][90]. Furthermore, Watarai and Tana [97] assessed the protective efficacy of activated charcoal containing WV (Nekka-Rich) against intestinal infection caused by Salmonella enterica serovar Enteritidis in domestic fowls. In a study by Watarai and Tana [97], the adsorption effectiveness of Nekka-Rich against S. Enteritidis and normal bacterial flora in the intestine, Enterococcus faecium, was evaluated. According to the authors, significantly less fecal excretion of S. Enteritidis was observed in chickens fed Nekka-Rich for ten days after the challenge. On day 15 after the challenge, S. Enteritidis was no longer isolated from fecal samples. On the other hand, immunization of chickens with the S. Enteritidis vaccine did not fully inhibit bacterial growth. Therefore, the experimental results indicated that Nekka-Rich may effectively eliminate the presence of S. Enteritidis in domestic fowls.
Also, Sittiya et al. [98] evaluated the effects of a wood charcoal powder and wood vinegar solution on Escherichia coli, ammoniacal nitrogen, vitamin C, and the productive performance of laying hens. They found that at 72 weeks of age, the performance parameters showed the highest values in the mixture dosage of 2%, while the yolk color and Haugh units were the highest in the 3% dietary group. According to the authors, the fecal E. coli concentration increased at the early stage of 66 weeks in the 1% mixture group without any change in ammoniacal nitrogen. They attributed the results to the reduced power of the mixture itself and the vitamin C increase through its action. These results were found with 2 to 3% supplementation levels, which were considered most suitable for tropical climates. In another study, Hanchai et al. [94] found no effect of pure WV supplemented in drinking water on growth performance, intestinal morphology, and gut microorganisms of broilers but determined improvement in villi number and height.
Supplemental feeding of laying hens with varied concentrations of WV from spina date seeds was assessed by Zhao et al. [93], aiming to decrease the n-6 to n-3 fatty acids ratio. In an experiment with broiler quails (Coturnix coturnix) fed with increasing concentrations of WV, Diógenes et al. [92] observed increased weight gain and feed consumption and decreased feed conversion as the additive concentration increased. Another experiment with laying quails was carried out by supplementing WV in the diet and assessing the animals’ performance and egg quality according to increasing additive levels [91]. The experiment also employed control groups where animals received feed containing conventional additives such as enramycin, probiotics, prebiotics, and essential oils. The results showed that 1.2% WV could replace the antimicrobial, probiotics, prebiotics, and plant-essential oil additives with statistically significant effects on the parameters of feed consumption, laying rate, average egg weight, egg mass, feed conversion per egg mass and dozen eggs, the relative and absolute weight of yolk, albumen, and shell, in addition to the Haugh units. Among these parameters, feed consumption and conversion were better than those determined for the negative and positive groups.
5. Wood Vinegar Refining
WV has been used mainly in agriculture, where the product has many applications as a partial or total substitute for pesticides and fungicides [22][25]. Among many other uses, WV from the carbonization of different types of woody biomass is effectively used worldwide as a safe food additive for human consumption. As cited previously, international regulations approve this type of use [46][47]. International standards require refining so that the WV reaches food and pharmaceutical grade, depending on the product type generated with this input. WV must undergo purification processes to reach the appropriate quality due to contaminants in its chemical composition. The acute toxicity and genotoxicity of chemical components of liquids and tars from wood pyrolysis and other lignocellulosic raw materials are comprehensively presented in the literature [99][100][101][102]. Therefore, depending on the targeted application of WV, it must be subjected to refining processes to remove contaminants. Most likely, raw WV’s main class of harmful compounds is polycyclic aromatic hydrocarbons (PAHs) [101][103]. PAHs are proven carcinogens and are classified as primary pollutants by international health agencies [42].
In the refining phase, PAHs and other contaminants are removed, along with tar residues and remaining soluble tar [100][101][104][105]. Several processes are recommended to purify WV, with decantation and filtration being the most common. Other options for refining include combining filtration with ultrafiltration, centrifugation, and distillation. However, depending on the refining method, traces of tar may remain in the final product, carrying contaminants in their structure. Vacuum refining is considered the most reliable and efficient process to refine WV and pyrolysis oils for food uses such as liquid smoke, generating a high-quality final product [40][42][52][106][107]. Even for agricultural applications, refining WV is recommended to remove soluble and insoluble tar [40]. The WV must undergo more pronounced refinement for more restrictive applications, such as research in health areas. The raw product must be subjected to a single distillation process to ensure its purity [23][32][108].
After refining, the WV is usually investigated to determine the main components, especially those with greater biological effects according to the final use. When studying the chemical composition of WV from Eucalyptus wood, 63 main components were found [52], with the methoxyphenol group having the highest representation. The most abundant components were 2,6-dimethoxy-phenol (syringol), 1,2,3-trimethoxybenzene, 2-methoxy-4-methylphenol, guaiacol, and 5-tert-butylpyrogallol. Yang et al. [28] reported the same chemical profile for WV from Litchi chinensis wood. Other studies have identified other compounds in WV from different forest species [24][59][62][109]. The phenolic compounds, for instance, occur in all types of WV, with concentrations that depend on the woody material from which the product originated.
Several other researchers have determined similar profiles, unsurprising since most phenolic compounds come from lignin thermal decomposition [24][35][101]. Lignin is a polymer in the chemical composition of all types of woody biomass. Therefore, WV from most of these wood sources has similar biological properties. This characteristic of various chemical compounds in WV explains its wide applications, such as in human and veterinary medicine, animal husbandry, agriculture, sustainable development, etc. [22][26][32][58][63][110]. Other contaminants, such as the heavy metals lead, cadmium, arsenic, and mercury, are also removed from WV by refining [40]. Figure 1 depicts WV cleanliness after sequential refining steps. After one month of settling, the raw product has a significant concentration of wood tar and heavy oils with which PAHs are associated [100][101]. After refining, the contaminants are entirely removed, and the final product reaches high crystallinity and suitable refractive index.
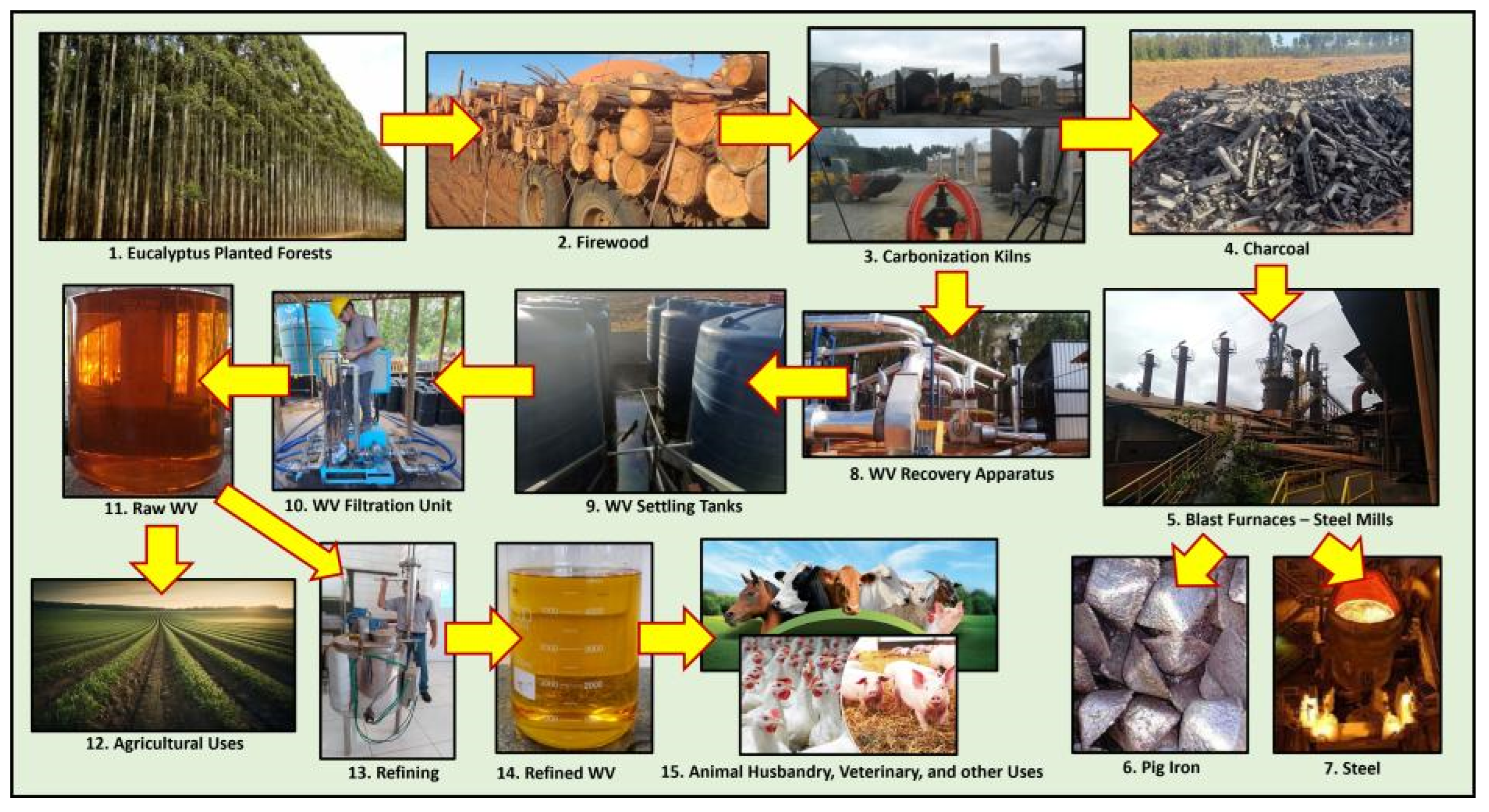
Figure 1. Charcoal and WV production chain of a pig iron and steel producer in Brazil with an additional step of WV refining.
References
- Franklin, A.M.; Aga, D.S.; Cytryn, E.; Durso, L.M.; McLain, J.E.; Pruden, A.; Roberts, M.C.; Rothrock Júnior, M.J.; Snow, D.D.; Watson, J.E.; et al. Antimicrobials in agroecosystems: Introduction to the special section. J. Environ. Qual. 2016, 45, 377–393.
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.R.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the veterinary antimicrobials enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581–582, 359–368.
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antimicrobial Use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795.
- Low, C.X.; Tan, L.T.H.; Mutalib, N.S.A.B.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the impact of antimicrobials and alternative methods for animal husbandry: A review. Antibiotics 2021, 10, 578.
- Kulik, K.; Lenart-Borón, A.; Wyrzykowska, K. Impact of antimicrobial pollution on the bacterial population within surface water with special focus on mountain rivers. Water 2023, 15, 975.
- WHO—World Health Organization. Stop Using Antimicrobials in Healthy Animals to Prevent the Spread of Antimicrobial Resistance. 2017. Available online: https://www.who.int/news/item/07-11-2017-stop-using-antimicrobials-in-healthy-animals-to-prevent-the-spread-of-antimicrobial-resistance (accessed on 28 September 2023).
- Trombete, F.M.; Santos, R.R.; Souza, A.L.R. Antimicrobial residues in Brazilian milk: A review of studies published in recent years. Rev. Chil. Nutr. 2014, 41, 191–197.
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 2020, 13, 189.
- Huygens, J.; Daeseleire, E.; Mahillon, J.; Elst, D.V.; Decrop, J.; Meirlaen, J.; Dewulf, J.; Heyndrickx, M.; Rasschaert, G. Presence of antimicrobial residues and antimicrobial resistant bacteria in cattle manure intended for fertilization of agricultural fields: A one health perspective. Antibiotics 2021, 10, 410.
- Zeng, H.; Li, J.; Zhao, W.; Xu, J.; Xu, H.; Li, D.; Zhang, J. The current status and prevention of antimicrobial pollution in groundwater in China. Int. J. Environ. Res. Public Health 2022, 19, 11256.
- Chandrakar, C.; Sakya, S.; Patyal, A.; Bhonsle, D.; Pandey, A.K. Detection of antimicrobial residues in chicken meat from different agro-climatic zones of Chhattisgarh, India by HPLC-PDA and human exposure assessment and risk characterization. Food Control 2023, 148, 109667.
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antimicrobial pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180.
- Li, C.; Chen, J.; Wang, J.; Ma, Z.; Han, P.; Luan, Y.; Lu, A. Occurrence of antimicrobials in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521–522, 101–107.
- Agga, G.; Cook, L.; Netthisinghe, A.M.P.; Gilfillen, R.A.; Woosley, P.B.; Sistani, K.R. Persistence of antimicrobial resistance genes in beef cattle backgrounding environment over two years after cessation of operation. PLoS ONE 2019, 14, e0212510.
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An investigation of anticoccidial veterinary drugs as emerging organic contaminants in groundwater. Sci. Total Environ. 2020, 746, 141116.
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The public health issue of antimicrobial residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671.
- Zigo, F.; Sasáková, N.; Gregová, G.; Výrostková, J.; Ondrašovicová, S. Effects of using an alternative bedding composition on the Levels of indicator microorganisms and mammary health in dairy farm conditions. Agriculture 2020, 10, 245.
- Zigo, F.; Farkašová, Z.; Výrostková, J.; Regecová, I.; Ondrašovicov, S.; Vargová, M.; Sasáková, N.; Pecka-Kielb, E.; Bursová, Š.; Kiss, D.S. Dairy cows’ udder pathogens and occurrence of virulence factors in Staphylococci. Animals 2022, 12, 470.
- Meneghi, D.; Stachurski, F.; Adakal, H. Experiences in tick control by acaricide in the traditional cattle sector in Zambia and Burkina Faso: Possible environmental and public health implications. Frontiers 2016, 4, 239.
- Reich, H.; Triacchini, G.A. Occurrence of residues of fipronil and other acaricides in chicken eggs and poultry muscle/fat. EFSA J. 2018, 16, e05164.
- Adum, A.N.; Gibson, G.; Chimbevo, L.M.; Oshule, P.S.; Essuman, S.; Asamba, M.N. Detection and quantification of chlorpyrifos in soil, milk, dip wash, and spray race residues using high-performance liquid chromatography in selected dairy farms in Kenya. J. Anal. Chem. 2021, 9, 88–95.
- Tiilikkala, K.; Fagernas, L.; Tiilikkala, J. History and Use of Wood Pyrolysis Liquids as Biocide and Plant Protection Product. Open Agric. J. 2010, 4, 111–118.
- Araújo, E.S.; Pimenta, A.S.; Feijó, F.M.C.; Castro, R.V.O.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2017, 124, 85–96.
- Pimenta, A.S.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Chemical composition of pyroligneous acid obtained from Eucalyptus GG100 clone. Molecules 2018, 23, 426.
- Souza, J.L.S.; Guimarães, V.B.S.; Campos, A.D.; Lund, R.G. Antimicrobial potential of pyroligneous extracts—A systematic review and technological prospecting. Braz. J. Microbiol. 2018, 49, 128–139.
- Aguirre, J.L.; Baena, J.; Martín, M.T.; Nozal, L.; González, S.; Manjón, J.L.; Peinado, M. Composition, aging and herbicidal properties of wood vinegar obtained through fast biomass pyrolysis. Energies 2020, 13, 2418.
- Schnitzer, J.A.; Su, M.J.; Ventura, M.U.; Faria, R.T. Doses de extrato pirolenhoso no cultivo de orquídea. Rev. Ceres 2015, 62, 101–106.
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 2016, 21, 1150.
- Hamzah, M.A.A.M.; Hasham, R.; Malek, N.A.N.N.; Hashim, Z.; Yahayu, M.; Razak, F.I.A.; Zakaria, Z.A. Beyond conventional biomass valorisation: Pyrolysis-derived products for biomedical applications. Biofuel Res. J. 2022, 35, 1648–1658.
- Theapparat, Y.; Khongthong, S.; Roekngam, N.; Suwandecha, T.; Sririyajan, S.; Faroongsarng, D. Wound healing activity: A novel benefit of pyroligneous extract derived from pyrolytic palm kernel shell wood vinegar. Ind. Crops Prod. 2023, 192, 115994.
- Feijó, F.M.C.; Pimenta, A.S.; Pereira, A.F.; Soares, W.N.C.; Benicio, L.D.M.; Silva Junior, E.C.; Ribeiro, Y.S.R.; Santos, C.S.; Praxedes, D.A.C.; Sousa, E.M.M.; et al. Use of eucalyptus wood vinegar as antiseptic in goats. In Goat Science-from Keeping to Precision Production; Kukovics, S., Ed.; IntechOpen Limited: London, UK, 2023.
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; Santos, C.S.; Fernandes, B.C.C.; Oliveira, M.F.; Souza, E.C.; Monteiro, T.V.C.; Fasciotti, M.; Azevedo, T.K.B.; et al. Antimicrobial activity and chemical profile of wood vinegar from eucalyptus (Eucalyptus urophylla × Eucalyptus grandis—Clone I144) and bamboo (Bambusa vulgaris). World J. Microbiol. Biotechnol. 2023, 39, 186.
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; Santos, C.S.; Castro, R.V.O.; Azevedo, T.K.B.; Medeiros, L.C.D. Effect of pH on the antibacterial and antifungal activity of wood vinegar (pyroligneous extract) from eucalyptus. Rev. Árvore 2023, 47, e4711.
- Silva, B.A.; Feijó, F.M.C.; Alves, N.D.; Pimenta, A.S.; Benicio, L.D.M.; Silva Junior, E.C.; Santos, C.S.; Pereira, A.F.; Moura, Y.B.F.; Gama, G.S.P.; et al. Use of a product based on wood vinegar of Eucalyptus clone I144 in the control of bovine mastitis. Vet. Microbiol. 2023, 279, 109670.
- Medeiros, L.C.D.; Pimenta, A.S.; Braga, R.M.; Carnaval, T.K.A.; Medeiros Neto, P.N.; Melo, D.M.A. Effect of pyrolysis heating rate on the chemical composition of wood vinegar from Eucalyptus urograndis and Mimosa tenuiflora. Rev. Árvore 2019, 43, e430408.
- Diniz, J. Conservação Térmica de Casca de Arroz a Baixa Temperatura: Produção de Bioóleo e Resíduos Silíciocarbono adsorvente. Ph.D. Thesis, Graduate Program in Chemistry, Universidade Federal de Santa Maria, Santa Maria, RS, Brazil, 2005.
- Fackovcova, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117.
- Silva, S.I.S.; Pimenta, A.S.; Iranda, N.O.; Lourença, Y.B.C.; Souza, E.C. Wood vinegar inhibits emergence and initial growth of Leucaena (Leucaena leucocephala/Lam./de Wit) seedlings. Agric. Conspec. Sci. 2020, 85, 153–158. Available online: https://hrcak.srce.hr/237843 (accessed on 20 September 2023).
- Meira, A.M.; Nolasco, A.M.; Klingenberg, D.; Souza, E.C.; Dias Júnior, A.F. Insights into the reuse of urban forestry wood waste for charcoal production. Clean. Technol. Environ. Policy 2021, 23, 2777–2787.
- Campos, A.D. Técnicas para produção de extrato rodução pirolenhoso para uso agricola. Pelotas: Empresa Brasileira de Pesquisa Agropecuária, EMBRAPA Clima Temperado. Circ. Técnica 2007, 65, 243. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/30826/1/Circular-65.pdf (accessed on 20 September 2023).
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28.
- Pimenta, A.S.; Monteiro, T.V.C.; Fasciotti, M.; Braga, R.M.; Souza, E.C.; Lima, K.M.G. Fast pyrolysis of trunk wood and stump wood from a Brazilian Eucalyptus clone. Ind. Crops Prod. 2018, 125, 630–638.
- Ruibo, L.; Narita, R.; Nishimura, H.; Marumoto, S.; Yamamoto, S.P.; Ouda, R.; Yatagai, M.; Fujita, T.; Watanabe, T. Antiviral activity of phenolic derivatives in pyroligneous acid from hardwood, softwood, and bamboo. ACS Sustain. Chem. Eng. 2018, 6, 119–126.
- Wu, Q.; Zhang, S.; Hou, B.; Zheng, H.; Deng, W.; Liu, D.; Tang, W. Study on the preparation of wood vinegar from biomass residues by carbonization process. Bioresour. Technol. 2015, 179, 98–103.
- Zhu, J.; Gao, W.; Zhao, W.; Ge, L.; Zhu, T.; Zhang, G.; Niu, Y. Wood vinegar enhances humic acid-based remediation material to solidify Pb(II) for metal-contaminated soil. Environ. Sci. Pollut. Res. 2020, 28, 12648–12658.
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010; 2162p.
- Montazeri, N.; Oliveira, A.C.M.; Himelbloom, B.H.; Leigh, M.B.; Crapo, C.A. Chemical characterization of commercial liquid smoke products. Food Sci. Nutr. 2013, 1, 102–115.
- Achmadi, S.S.; Mubarik, N.R.; Nursyamsi, R.; Septiaji, P. Characterization of redistilled liquid smoke of oil-palm shells and its application as fish preservatives. J. Appl. Sci. 2013, 13, 401–408.
- Suresh, G.; Pakdel, H.; Roussi, T.; Brar, S.K.; Fliss, I.; Roy, C. In vitro evaluation of antimicrobial efficacy of pyroligneous acid from softwood mixture. Biotechnol. Res. E Innov. 2019, 3, 47–53.
- Rocha, F.T.; Cruz, I.V.; Leite, H.M.F.; França Neto, A.C.; Ferreira, E. Extrato pirolenhoso na germinação de sementes forrageiras. Conjecturas 2022, 22, 1657–5830.
- Yatagai, M.; Nishimoto, M.; Hori, K.; Ohira, T.; Shibata, A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 2002, 48, 338–342.
- Souza, J.B.G.; Ré-Poppi, N.; Raposo Júnior, J.L. Characterization of pyroligneous acid used in agriculture by gas chromatography-mass spectrometry. J. Braz. Chem. Soc. 2012, 23, 610–617.
- Aubin, H.; Roy, C. Study on the corrosiveness of wood pyrolysis oils. Fuel Sci. Technol. Int. 1990, 8, 77–86.
- Rahmat, B.; Pangesti, D.; Natawijaya, D.; Sufyadi, D. Generation of wood-waste vinegar and Its effectiveness as a plant growth regulator and pest insect repellent. Bioresources 2014, 9, 6350.
- Petchpoung, K.; Siklom, S.; Siri-Anusornsak, W.; Khlangsap, N.; Tarac, A.; Maneeboon, T. Predicting antioxidant activity of wood vinegar using color and spectrophotometric parameters. MethodsX 2020, 7, 100783.
- Theapparat, Y.; Chandumpai, A.; Leelasuphakul, W.; Faroongsarng, D. Physicochemistry and Utilization of Wood Vinegar from Carbonization of Tropical Biomass Waste; Chapter 8; Open Access Books; IntechOpen: London, UK, 2018.
- Ariffin, S.J.; Yahayo, M.; El-Enshasy, H.; Malek, R.A.; Aziz, A.A.; Hashim, N.M.; Zakaria, Z.A. Optimization of pyroligneous acid production from Palm kernel shell and its potential antimicrobial and antibiofilm activities. Indian J. Exp. Biol. 2017, 55, 427–435.
- Feijó, F.M.C.; Fernandes, F.C.; Alves, N.D.; Pimenta, A.S.; Santos, C.S.; Rodrigues, G.S.O.; Pereira, A.F.; Benicio, L.D.M.; Moura, I.B.F. Efficiency of pyroligneous extract from jurema preta (Mimosa tenuiflora Poiret) as an antiseptic in cats (Felis catus) subjected to ovariosalpingohysterectomy. Animals 2022, 12, 2325.
- Furtado, C.M.; Stols, A.S.; Pinto, F.L.; Moura, A.B.D.; Morisso, F.D.P.; Pitarelo, A.P.; Ramos, L.P.; Mühlen, C.V.; Riegel-Vidotti, I.C. Pyroligneous liquor produced from Acacia mearnsii de wild wood under controlled conditions as a renewable source of chemicals. Química Nova 2015, 38, 1068–1074.
- Harada, K.; Iguchi, A.; Yamada, M.; Hasegawa, K.; Nakata, T.; Hikasa, Y. Determination of maximum inhibitory dilutions of bamboo pyroligneous acid against pathogenic bacteria from companion animals: An in vitro study. J. Vet. Adv. 2013, 3, 300–305.
- Hou, X.; Qiu, L.; Luo, S.; Kang, K.; Zhu, M.; Yao, Y. Chemical constituents and antimicrobial activity of wood vinegars at different pyrolysis temperature ranges obtained from Eucommia ulmoides Olivers branches. RSC Adv. 2018, 8, 40941–40949.
- Suresh, G.; Pakdel, H.; Roussi, T.; Brar, S.K.; Diarra, M.; Roy, C. Evaluation of pyroligneous acid as a therapeutic agent against Salmonella in a simulated gastrointestinal tract of poultry. Braz. J. Microbiol. 2020, 51, 1309–1316.
- Soares, W.N.C.; Lira, G.P.O.; Santos, C.S.; Dias, G.N.; Pimenta, A.S.; Pereira, A.F.; Benicio, L.D.M.; Rodrigues, G.S.O.; Amora, S.S.A.; Alves, N.D.; et al. Pyroligneous acid from Mimosa tenuiflora and Eucalyptus urograndis as an antimicrobial in dairy goats. J. Appl. Microbiol. 2020, 131, 604–614.
- Ho, C.L.; Lin, C.Y.; Ka, S.M.; Chen, A.; Tasi, Y.L.; Liu, M.L.; Chiu, Y.C.; Hua, K.F. Bamboo vinegar decreases inflammatory mediator expression and NLRP3 inflammasome activation by inhibiting reactive oxygen species generation and protein kinase C-a/d activation. PLoS ONE 2013, 8, e75738. Available online: www.plosone.org (accessed on 18 October 2023).
- Yildizli, G.; Coral, G.; Ayaz, F. Anti-bacterial, antifungal, and anti-inflammatory activities of wood vinegar: O potential remedy for major plant diseases and inflammatory reactions. Biomass Convers. Biorefin. 2022, 1–10.
- Ruibo, L.; Narita, R.; Ouda, R.; Kimura, C.; Nishimura, H.; Yatagai, M.; Fujita, T.; Watanabe, T. Structure-dependent antiviral activity of catechol derivatives in pyroligneous acid against the encephalomycarditis virus. RSC Adv. 2018, 8, 35888–35896.
- Desvita, H.; Faisal, M.; Mahidin, S. Antimicrobial potential of wood vinegar from cocoa pod shells (Theobroma cacao L.) against Candida albicans and Aspergillus niger. Mater. Today Proc. 2022, 63, 2214–7853.
- Teo, C.L. Evaluating the effect of pyroligneous extract as natural antimicrobial agent under different contact times. J. Teknol. 2022, 84, 83–92.
- Ibrahim, D.; Kassim, J.; Sheh-Hong, L.; Rusli, W. Efficacy of pyroligneous acid from Rhizophora apiculata on pathogenic Candida albicans. J. Appl. Pharm. Sci. 2013, 3, 7–13.
- Wang, Y.; Dai, A.; Huang, S.; Kuo, S.; Shu, M.; Tapia, C.; Yu, J.; Two, A.; Zhang, H.; Gallo, R.; et al. Propionic acid and its esterified derivative suppress the growth of methicillinresistant Staphylococcus aureus USA30. Benef. Microbes 2014, 5, 161–168.
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370.
- Alshuniaber, M.A.; Krishnamoorthy, R.; Alqhtani, W.H. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464.
- Albano, M.; Alves, F.; Andrade, B.; Barbosa, L.; Pereira, A.; Cunha, M.; Rall, V.; Fernandes Júnior, A. Antibacterial and anti-staphylococcal enterotoxin activities of phenolic compounds. Innov. Food Sci. Emerg. Technol. 2016, 38, 83–90.
- Kook, K.; Kim, K.H. The effects of supplemental levels of bamboo vinegar on growth performance, serum profile and meat quality in fattening Hanwoo cow. J. Anim. Sci. Technol. 2003, 45, 57–68.
- O’Reilly, G.C.; Huo, Y.; Meale, S.J.; Chaves, A.V. Dose response of biochar and wood vinegar on in vitro batch culture ruminal fermentation using contrasting feed substrates. Transl. Anim. Sci. 2021, 5, txab107.
- Mekbungwan, A.; Yamauchi, K.; Sakaida, T.; Buwjoom, T. Effects of a charcoal powder–wood vinegar compound solution in piglets for raw pigeon pea seed meal. Animal 2008, 2–3, 366–374.
- Choi, J.Y.; Shinde, P.L.; Kwon, I.K.; Song, Y.H.; Chae, B.J. Effect of wood vinegar on the performance, nutrient digestibility and intestinal microflora in weanling pigs. Asian Aust. J. Anim. Sci. 2009, 22, 267–274. Available online: www.ajas.info (accessed on 18 October 2023).
- Wang, H.F.; Gao, K.; Wang, C.; Zhang, W.M.; Liu, J.X. Effects of feeding bamboo vinegar and acidifier as an antimicrobial substitute on the growth performance and intestinal bacterial communities of weaned piglets. Acta Agric. Scand. Sect. A 2013, 63, 143–150.
- Chu, G.M.; Jung, C.K.; Kim, H.Y.; Ha, J.H.; Kim, J.H.; Jung, M.S.; Lee, S.J.; Song, Y.; Ibrahim, R.I.H.; Cho, J.H.; et al. Effects of bamboo charcoal and bamboo vinegar as antimicrobial alternatives on growth performance, immune responses and fecal microflora population in fattening pigs. Anim. Sci. J. 2013, 84, 113–120.
- Watarai, S.; Tana; Koiwa, M. Feeding activated charcoal from bark containing wood vinegar liquid (Nekka-rich) is effective as treatment for cryptosporidiosis in calves. J. Dairy Sci. 2008, 91, 1458–1463.
- Parauda, C.; Pors, I.; Journal, J.P.; Besnier, P.; Reisdorffer, L.; Chartier, C. Control of cryptosporidiosis in neonatal goat kids: Efficacy of a product containing activated charcoal and wood vinegar liquid (Obionekk) in field conditions. Vet. Parasitol. 2011, 180, 354–357.
- Chao, N.V.; Thong, H.T.; QuynhChau, H.L.; Tam, V.T.; Rui, Z. Effects of charcoal and wood vinegar dietary supplementation to diarrhea Incidence and fecal hydrogen sulfide emissions in pigs. Int. J. Sci. Res. Publ. 2016, 6, 707–713.
- Huo, Y.; Liu, Z.; Xuan, H.; Lu, C.; Yu, L.; Bao, W.; Zhao, G. Effects of bamboo vinegar powder on growth performance and mRNA expression levels of interleukin-10, interleukin-22, and interleukin-25 in immune organs of weaned piglets. Anim. Nutr. 2016, 2, 111–118.
- Rodjan, P.; Theapparat, Y.; Khongthong, S.; Jeenkeawpieam, J. Effects of mangosteen wood vinegar as a potential additive on nutrient digestibility in growing pigs. Songklanakarin J. Sci. Technol. 2018, 40, 1002–1008.
- Macasait, D.R.; Roylo, B.B.; Espina, D.M. Growth performance of grower pigs (Sus scrofa domesticus L.), nutritional and microbial contents of wet and fermented commercial hog ration with different levels of wood vinegar. Asian J. Dairy Food Res. 2021, 40, 220–224.
- Sureshkumar, S.; Sampath, V.; Kim, I.H. The influence of dietary inclusion of wood vinegar supplementation on growth performance, nutrient digestibility, and meat quality in grower-finisher pigs. Acta Biochim. Pol. 2021, 68, 287–292.
- Yamauchi, K.; Ruttanavut, J.; Takenoyama, S. Effects of dietary bamboo charcoal powder including vinegar liquid on chicken performance and histological alterations of intestine. J. Anim. Feed Sci. 2010, 19, 257–268.
- Rattanavut, J.; Matsumoto, Y.; Yamauchi, K. fluorescence-based demonstration of intestinal villi and epithelial cell in chickens fed dietary silicic acid powder including bamboo vinegar compound liquid. Histol. Histopathol. 2012, 27, 1333–1342.
- Rattanawut, J.; Yamauchi, K. Growth performance, carcass traits and histological changes in the intestinal villi of male broiler chickens fed dietary silicic acid powder containing bamboo vinegar liquid. J. Anim. Feed Sci. 2015, 24, 48–52.
- Rattanawut, J.; Todsadee, A.; Yamauchi, K. Effects of bamboo charcoal powder including vinegar supplementation on performance, eggshell quality, alterations of intestinal villi and intestinal pathogenic bacteria populations of aged laying hens. Ital. J. Anim. Sci. 2017, 16, 259–265.
- Nunes, T.S. Pyroligneous Extract in Japanese Quail Feed. Master’s Thesis, Graduate Program in Veterinary Sciences, Center for Agricultural Sciences and Engineering, Universidade Federal do Espírito Santo—UFES, Alegre, ES, Brazil, 2019.
- Diógenes, G.V.; Teixeira, E.N.M.; Pimenta, A.S.; Souza, J.G.; Moreira, J.A.; Marinho, A.L.; Veras, A.; Chemane, I.A. Wood Vinegar from Eucalyptus as an Additive in Broiler Quail Feed. Int. J. Plant Anim. Environ. Sci. 2019, 9, 3.
- Zhao, N.; Xin, H.; Li, Z.; Wang, Z.; Zhang, L. Supplemental feeding of laying hens with wood vinegar to decrease the ratio of n-6 to n-3 fatty acids in eggs. Chem. Res. Chin. Univ. 2019, 35, 983–989.
- Hanchai, K.; Trairatapiwan, T.; Lertpatarakomol, R. Drinking water supplemented with wood vinegar on growth performance, intestinal morphology, and gut microbial of broiler chickens. Vet. World 2021, 14, 92–96. Available online: www.veterinaryworld.org/Vol.14/January-2021/12.pdf (accessed on 20 October 2023).
- Khai, L.T.L.; Nghia, N.T.; Hayashidani, H. Study on effectiveness of activated charcoal and wood vinegar on prevention of piglet diarrhea. Can. Tho Univ. J. Sci. 2019, 11, 9–15.
- Takahara, Y.; Katoh, K.; Inaba, R.; Iwata, H. Study on odor control using wood vinegars. Application of wood vinegars to piggery wastes. Nihoh Koshu Eisei Zasshi 1994, 41, 147–156.
- Watarai, S.T. Eliminating the carriage of Salmonella enterica serovar Enteritides in domestic fowls by feeding activated charcoal from bark containing wood vinegar liquid (Nekka-Rich). Poult. Sci. 2005, 84, 515–521.
- Sittiya, J.; Yamauchi, K.; Yamauchi, K. Bark charcoal powder containing wood vinegar liquid can shorten the time to shipping of broilers raised in tropical areas by activating performance and intestinal function. Can. J. Anim. Sci. 2021, 101, 735–744.
- Mezerette, C.; Girard, P. Environmental aspects of gaseous emissions from wood carbonization and pyrolysis processes. In Biomass Pyrolysis Liquids: Upgrading and Utilisation; Bridgwater, A.V., Grassi, G., Eds.; Elsevier Applied Science: London, UK, 1991; pp. 263–287.
- Pakdel, H.; Roy, C. Hydrocarbon content of liquid products and tar from pyrolysis and gasification of wood. Energy Fuels 1991, 5, 427–436.
- Pimenta, A.S.; Bayona, J.M.; García, M.T.; Solanas, A.M. Evaluation of acute toxicity and genotoxicity of liquid products from pyrolysis of Eucalyptus grandis wood. Arch. Environ. Contam. Toxicol. 2000, 38, 169–175.
- Zhang, Z.; Ning, S.; Li, Q.; Sun, M.; Lin, J.; Wang, X. Levels and risk assessment of polycyclic aromatic hydrocarbons in wood vinegars from pyrolysis of biomass. Chemosphere 2021, 278, 130453.
- Pimenta, A.S.; Vital, B.R.; Bayona, J.M.; Alzaga, R. Characterisation of polycyclic aromatic hydrocarbons in liquid products from pyrolysis of Eucalyptus grandis wood by supercritical fluid extraction and GC/MS determination. Fuel 1998, 77, 1133–1139.
- Barbosa, J.M.S.; Ré-Poppi, N.; Santiago-Silva, M. Polycyclic aromatic hydrocarbons from wood pyrolysis in charcoal production furnaces. Environ. Res. 2006, 101, 304–311.
- Xue, R.; Zhang, W.; Wang, Z.-P.; Zhu, M.-Q. Refining of Eucommia ulmoides Oliver derived wood vinegar for excellent preservation of the typical berries. LWT Food Sci. Technol. 2023, 174, 114415.
- Higashino, T.; Shibata, A. Basic study for establishing specifications for wood vinegar by distillation. Mokuzai Gakkaishi 2005, 51, 180–188.
- Silveira, C.M. Influência do Extrato Pirolenhoso no Desenvolvimento e Crescimento de Plantas de Milho. Ph.D. Thesis, Faculdade de Ciências Agrárias e Veterinárias, Graduate Program in Agronomy, Faculdade de Ciências Agrárias e Veterinárias, Jaboticabal, SP, Brazil, 2010; 93p.
- Li, Z.; Zhang, L.; Chen, G.; Wu, L.; Liu, B.; Li, Y.; Sun, S.; Zhang, H.; Zhang, Z.; Wang, Z. A new method for comprehensive utilization of wood vinegar by distillation and liquid−liquid extraction. Process Biochem. 2018, 75, 194–201.
- Lourenço, Y.B.C.; Pimenta, A.S.; de Paiva, L.L.; Feijó, F.M.C.; Fasciotti, M.; Castro, R.V.O.; de Souza, E.C. Eucalyptus wood vinegar: Chemical profiling, evaluation of acute toxicity to Artemia salina, and effect on the hatching of Betta splendens eggs. Ens. E Ciência 2022, 25, 776–782.
- Ho, C.L.; Lin, C.S.; Li, L.H.; Hua, K.F.; Ju, T.C. Inhibition of pro-inflammatory mediator expression in macrophages using wood vinegar from Griffith’s ash. Chin. J. Physiol. 2021, 64, 232–243.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
13 Jan 2025
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




