Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andreas Yallouris | -- | 5660 | 2024-02-01 11:23:02 | | | |
| 2 | Mona Zou | Meta information modification | 5660 | 2024-02-05 08:46:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Filippou, C.; Themistocleous, S.C.; Marangos, G.; Panayiotou, Y.; Fyrilla, M.; Kousparou, C.A.; Pana, Z.; Tsioutis, C.; Johnson, E.O.; Yiallouris, A. Microbial Therapy and Breast Cancer Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/54637 (accessed on 08 February 2026).
Filippou C, Themistocleous SC, Marangos G, Panayiotou Y, Fyrilla M, Kousparou CA, et al. Microbial Therapy and Breast Cancer Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/54637. Accessed February 08, 2026.
Filippou, Charalampos, Sophia C. Themistocleous, Giorgos Marangos, Yiannis Panayiotou, Maria Fyrilla, Christina A. Kousparou, Zoi-Dorothea Pana, Constantinos Tsioutis, Elizabeth O. Johnson, Andreas Yiallouris. "Microbial Therapy and Breast Cancer Management" Encyclopedia, https://encyclopedia.pub/entry/54637 (accessed February 08, 2026).
Filippou, C., Themistocleous, S.C., Marangos, G., Panayiotou, Y., Fyrilla, M., Kousparou, C.A., Pana, Z., Tsioutis, C., Johnson, E.O., & Yiallouris, A. (2024, February 01). Microbial Therapy and Breast Cancer Management. In Encyclopedia. https://encyclopedia.pub/entry/54637
Filippou, Charalampos, et al. "Microbial Therapy and Breast Cancer Management." Encyclopedia. Web. 01 February, 2024.
Copy Citation
Microorganisms play an indirect role in affecting the emergence, natural course, and/or severity of various cancers. The presence of a unique microbiome in breast tissue, previously unacknowledged, has gained recognition through research. Microbiome dissimilarities have been observed between healthy and cancerous breast tissue, implying that cancer may disturb the natural balance of the microbiome in this area. Interestingly, breast tumor tissue showed a decrease in total bacterial DNA, and an inverse relationship was observed between the bacterial DNA load and advanced cancer stages.
microbiome
breast cancer
microbial diversity
microbiota-based cancer therapies
one health
microbiome immunomodulation
1. Microbiome Dynamics in Cancer Management
The dynamic nature of the gut microbiome, combined with tumor heterogeneity, can lead to varied observations across scientific studies. Numerous reports highlight alterations in both the alpha and beta diversity of the gut microbiome across various cancers, including BC.
A study by Aarnoutse et al. (2022) identified a significant reduction in species richness (p = 0.042) within the gut microbiomes of estrogen-receptor-positive BC patients undergoing treatment with (neo)adjuvant chemotherapy drugs [1]. A similar trend was observed in patients undergoing chemotherapy, as documented by Bilenduke et al. (2022), and concentrations returned to baseline post-treatment [2]. Conversely, Horigome et al. (2019) found no notable shifts in gut microbiome composition between chemotherapy-treated BC survivors and non-chemotherapy-treated survivors [3]. This disparity was attributed to the fact that the patients had completed their chemotherapy at least two years before the study’s commencement, suggesting that any chemotherapy-induced dysbiosis may have been reverted. Various other studies, such as the CANTO trial, have reported increased α diversity post-chemotherapy. Wu et al. (2022) made a similar observation for neoadjuvant chemotherapy patients [4].
Depleted microbial richness is linked to intensified depression symptoms, an increased fear of cancer recurrence, and the onset of diarrhea in BC patients undergoing chemotherapy [1][2][5]. Chemotherapy-associated cognitive impairment (CACI) is a widely documented side effect in BC patients, with deficits manifesting in memory retention, processing speed, and visuospatial ability [4][6][7][8]. An array of chemotherapeutic drugs has been implicated in inducing oxidative stress within the brain and promoting inflammation markers within the central nervous system.
Another possible mechanism underlying CACI involves the gut–brain interaction mediated by the peripheral immune system. The “Gut–Immune–Brain Axis” (GBA) theory proposes a communication triad between the intestinal microbiome, the immune system, and the brain. Chemotherapy-induced dysbiosis may disrupt the intestinal barrier, leading to immune cell infiltration and the release of pro-inflammatory cytokines into the bloodstream [4]. These cytokines can penetrate the blood–brain barrier (BBB), inducing neuroinflammation, resulting in cognitive disturbances [9]. Several studies support this theory by identifying a correlation between elevated pro-inflammatory cytokines and CACI in BC patients [7][10].
On a positive note, certain bacteria, known for producing butyrate, demonstrate potential anti-inflammatory and anti-cancer properties. The abundance of such beneficial bacteria, including Coprococcus, Ruminococcus, and Faecalibacterium, was noted in BC patients devoid of neurotoxicity post-chemotherapy, pointing to their possible role in curbing neuroinflammation [4].
1.1. Radiotherapy
Radiation therapy, or radiotherapy, primarily damages the DNA of cancer cells to induce cell death. Typically, it is administered locally but can also be combined with other treatments. Notably, radiation therapy causes gastrointestinal discomfort as a side effect, even if the gastrointestinal region is not the area under treatment. This could be due to microbiome dysbiosis as a side effect [11].
Few studies have delved into the impact of radiotherapy (RT) or chemoradiation (CRT) on cancer patients’ gut microbiomes. Specifically, for BC, clinical studies detailing RT’s influence on the gut microbiome are scant, even though it is a standard treatment for the majority of BC patients. Gynecological cancer patients undergoing RT exhibited variations in gut microbiota richness, with a decrease in beneficial gut commensal Firmicutes and an increase in opportunistic pathogens like Fusobacterium [12]. Several studies corroborate these findings, revealing that reduced alpha diversity and gut microbiome dysbiosis post-RT and CRT often accompany gastrointestinal side effects or fatigue in various cancers.
In cases of RT, it is noteworthy that higher alpha gut diversity has been associated with the increased tumor infiltration of activated CD4+ T-cells, subsequently leading to better recurrence-free and overall survival rates in cancer patients. While animal studies are foundational in understanding the intricate interactions of radiation, bacteria, and fungi, only one study, as of now, has investigated these interactions in a BC mouse model [13]. This study highlighted the importance of both bacteria and fungi in modulating the outcomes of RT.
Furthermore, studies have shown a connection between gut bacteria and the effectiveness of radiation therapy in treating various cancers. Eradicating Gram-positive bacteria with antibiotics improved the radiation’s anti-tumor effects in melanoma, lung, and cervical cancer mouse models. However, reintroducing the metabolite sodium butyrate, produced by these bacteria, eliminated this benefit [14]. The complete removal of gut bacteria reduced radiation’s effectiveness in some cancer models, while gut fungi depletion enhanced it [13]. The greater expression of fungal sensor Dectin-1 correlated with poorer BC survival rates. Mice’s responses to radiation varied based on their microbiota, with Enterococcaceae and Lachnospiraceae being prominent in treatment-responsive mice. Leukemia patients with these bacterial families experienced fewer gastrointestinal symptoms post-radiation. Metabolites from these bacteria were linked to radioprotection. Although research is limited, the role of the gut microbiota in the radiation response emphasizes the need for further study [15].
1.2. Chemotherapy
Chemotherapy primarily involves the use of medicinal drugs to chemically treat cancer by interfering with cell division (mitosis). The effectiveness of chemotherapy differs across various types of cancer. Its main objective is to harm or overwhelm cancer cells to induce programmed cell death (apoptosis), and some chemotherapy drugs can also stimulate immune reactions. Since chemotherapy is administered throughout the body, it may also impact normal cells that undergo rapid division. Due to its systemic administration, chemotherapy affects the gut microbiome, which may in turn also influence the effectiveness of chemotherapeutic treatments [16].
Administering antibiotics to neutralize the gut microbiome decreased the effectiveness of chemotherapy drugs like cisplatin and oxaliplatin in treating lymphoma and colon cancer in mice, attributed to reduced reactive oxygen species (ROS) production. This indicates the necessity of a functional commensal microbiome for the success of these platinum-based treatments. Antibiotics also lowered oxaliplatin’s effectiveness in a specific colon cancer mouse model. Subsequent experiments revealed an association between certain microbes and the drug’s effectiveness. For instance, the presence of Paraprevotella clara was linked to a lack of response for oxaliplatin, while B. fragilis correlated with a positive response. The consequence of administrating cyclophosphamide was the movement of particular gut microbes like Lactobacillus johnsonii and Enterococcus hirae to the spleen and lymph nodes, initiating an immune response, in melanoma and sarcoma mouse models. Further research showed that the existence of E. hirae and Barnesiella intestinihominis in the gut could enhance cyclophosphamide’s activity and rejuvenate its effectiveness post-antibiotic administration.
Additionally, the CANTO trial documented significant changes in the gut microbiome composition throughout chemotherapy [4]. Favorable commensals like Dorea formicigenerans and Methanobrevibacter smithii archaea are commonly found in healthy individuals but increased in abundance in BC patients post-chemotherapy. Coprococcus and members of the Ruminococcaceae and Eubacteriaceae families were positively linked with a good prognosis and the absence of axillary lymph node metastasis. Conversely, harmful bacterial species such as certain Klebsiella and Bacteroides species, along with members of the Lachnospiraceae and Clostridiaceae families, were associated with axillary lymph node invasion and advanced BC stages after chemotherapy. In particular, the presence of B. uniformis was abundant in non-metastatic advanced-stage BC, which was then reduced post-chemotherapy. The high abundance of Bacteroides also correlated with non-responsiveness to trastuzumab treatment in HER2-positive BC patients [17].
A metabolite from the gut microbiome, indole-3-acetic acid, has been observed to enhance the effectiveness of FOLFIRINOX, a combination chemotherapy used in treating metastatic pancreatic ductal adenocarcinoma. This enhancement is due to the accumulation of ROS and a reduction in the cancer cells’ autophagy. The gut microbiome does not solely influence the response to chemotherapy; bacteria in and around the tumor itself also play a significant role. There is growing interest in exploring how these local bacteria impact the effectiveness of chemotherapy and contribute to drug resistance in cancer patients.
Studies have shown that the commensal bacterium E. coli has the ability to modify the effectiveness of various chemotherapy drugs. It increased the toxicity of some drugs, while reducing the toxicity of others, like gemcitabine, doxorubicin, and mitoxantrone. Further experiments involving E. coli and gemcitabine in a mouse model indicated a decrease in the drug’s ability to combat tumors. Likewise, both Mycoplasma hyorhinis and E. coli have been found to metabolize gemcitabine into an inactive form, rendering cancer cells resistant. Similarly, colon cancer cells expressing adequate F. nucleatum exhibited greater drug resistance when exposed to certain chemotherapy drugs. This implies that microbes local to the tumor and its microenvironment can substantially impact chemotherapy’s effectiveness. On the other hand, manipulating the lung microbiota using aerosolized antibiotics and specifically probiotics showed improvements in the efficacy of a chemotherapeutic drug, dacarbazine. Such findings underscore the potential of further exploring microbial interactions in various body regions and within the tumor to enhance the outcomes of chemotherapy treatments.
1.3. Cancer Immunotherapy and Microbiome Immunomodulation
Immunotherapy represents a prominent emerging therapeutic approach for certain hematological and solid malignancies, including BC (Figure 1). Bacterial metabolites directly influence the activities of local immune cells. These effects include the modulation of immunoglobulin secretion, the promotion of lymphocyte differentiation into regulatory T-lymphocytes and T-helper 17 cells, the generation of immunomodulatory cytokines, and even the epigenetic regulation of histone deacetylase enzymes.
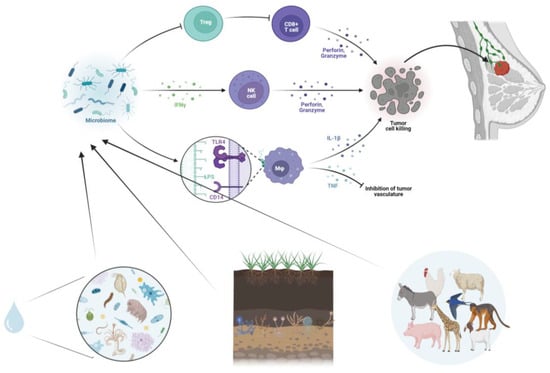
Figure 1. Microbiome and tumor microenvironment interplay. Schematic illustration of the microbiome-mediated modulation of the immune system, including Toll-like receptor activation, cytokine release, anti-tumor responses, inhibition of tumor vasculature, and immune cell activation.
Concerning the connection between the human microbiota and BC, various metabolites have been identified as potential risk factors or modifiers. These include substances such as estrogens, active phytoestrogens, short-chain fatty acids, lithocholic acid, and cadaverine. In particular, the gut microbiota’s production of estrogens, largely driven by the enzyme β-glucuronidase from specific intestinal bacteria, can result in the deconjugation of xenobiotics and sex hormones like estrogens. This process increases the reabsorption of estrogens into the systematic circulation, potentially elevating the risk of hormone-dependent BC in women. Conversely, some metabolites, such as phytoestrogens, lithocholic acid, and cadaverine, have been associated with a protective or risk-reducing influence on BC development [18].
Gut bacteria can also promote BC through the induction of chronic inflammation, which is closely linked to tumorigenesis. These bacteria, via pathogen-associated molecular patterns, can upregulate Toll-like receptors and activate NF-κB, a critical regulator of inflammation and cancer. NF-κB activation leads to the release of several cytokines, including IL-6, IL-12, IL-17, IL-18, and tumor necrosis factor-alpha, contributing to persistent inflammation within the tumor microenvironment [19]. Secondary metabolites released by intestinal bacteria, along with pro-inflammatory molecules reaching the liver via the portal vein, may further promote carcinogenesis. For instance, butyrate, a microbial metabolite, can enhance the anti-tumor cytotoxic CD8 T-cell response by modulating the ID2-dependent IL-12 signaling pathway [20].
The gut microbiome also plays a role in epigenetic deregulation, potentially affecting tumor development. Microorganisms produce bioactive substances with a low molecular weight, such as folates, short-chain fatty acids, and biotin, which can participate in epigenetic processes by altering substrates used for methylation or influencing the activity of epigenetic enzymes [21].
Immune checkpoint inhibitors are a class of therapies that leverage the immune system to combat tumors by blocking inhibitory interactions between T-lymphocyte receptors and ligands on malignant cells. While BC is typically not considered highly immunogenic compared to other malignancies like lung cancer or melanoma, recent data have shown the benefits of immunotherapy, particularly in the triple-negative subtype (ER/PR and HER2 negative).
Clinical trials have demonstrated varying response rates with different immunotherapies, alone or in combination with chemotherapy, in these patients. The KEYNOTE-012 trial investigated a pembrolizumab monotherapy in previously treated triple-negative breast patients, showing an overall response rate (ORR) of 18.5% and a median time to response of 17.9 weeks [22]. The KEYNOTE-086 trial tested pembrolizumab as a first-line therapy for metastatic triple-negative breast cancer, achieving an ORR of 23% [23]. Other trials, such as NCT01375842 and JAVELIN, assessed atezolizumab and avelumab, with observed ORRs of 10% and 5.2%, respectively [24][25].
Combinations of immunotherapy with chemotherapy have also been explored, with atezolizumab combined with nab-paclitaxel yielding an ORR of 67% in first-line treatment [26]. The IMpassion 130 trial examined the combination of atezolizumab and nab-paclitaxel in untreated metastatic triple-negative breast cancer patients, revealing a progression-free survival (PFS) benefit. The ENHANCE-1/KEYNOTE-150 trial evaluated eribulin combined with pembrolizumab, showing a higher ORR in PD-L1-positive BC patients [27]. The KEYNOTE-355 trial combined pembrolizumab with chemotherapy, demonstrating a significant progression-free survival benefit in patients with high PD-L1 values [28]. These trials collectively indicate the potential of immune checkpoint inhibitors in improving outcomes for triple-negative breast patients, particularly in cases with high PD-L1 expression (please see the summary box in Section 3.4).
1.4. Exploring Breast Microbiome Dynamics regarding Cancer Pathogenesis, Early Detection, and Management (Summary Box)
The human microbiome can influence pathways related to cancer initiation, progression, and genetic stability. Studies show that certain bacteria like Propionibacterium and Staphylococcus are abundant in healthy breast tissue but decline in tumors. Lactobacillus, Acetobacter, and others predominantly populate normal breast tissue. The administration of Lactobacillus acidophilus increases lymphocytes and cytokines, promoting anti-tumor immunity. The introduction of Micrococcus luteus in mouse models hinders tumor growth by activating M1 macrophages. Bifidobacterium may help to deliver therapeutic cargo to tumors. The breast microbiome composition differs between tumor subtypes and correlates with prognostic factors. Microbiome profiles linked to lymph node invasion or poorer prognosis involve Bacteroides or Lachnospiraceae. Escherichia coli peptides induce breast cancer cell apoptosis through cell cycle arrest and DNase activity. Colicin E1 increases apoptosis in MCF7 cells more than Colicin A. Brevibacillus sp. Expresses defensin peptides that disrupt breast cancer cell membranes. The gut microbiome’s dynamics influence cancer management. Studies show that chemotherapy reduces species richness and causes dysbiosis, linked to worse depression, cancer recurrence fears, and diarrhea. A possible mechanism for chemotherapy-associated cognitive impairment involves the gut–brain axis; chemotherapy-induced dysbiosis disrupts the intestinal barrier, allowing immune cell infiltration and pro-inflammatory cytokine release into the bloodstream and brain. Beneficial bacteria like Coprococcus may help to curb neuroinflammation. Radiotherapy impacts the gut microbiome, with decreases in Firmicutes associated with side effects. Gut bacteria and fungi modulation alter radiotherapy effectiveness in mouse models. Certain commensals enhance radiation responses. The microbiome influences chemotherapy effectiveness by metabolizing drugs or altering ROS production. Modulating the lung or tumor microbiota impacts chemotherapy responses. Immune checkpoint inhibitors show a benefit for triple-negative breast cancer, linked to microbiome immune modulation.
2. Microbiome-Modulating Interventions
2.1. Bacterial Therapeutics for Tumor Treatment and Immune Modulation
Microbial therapy is also termed oncolytic virotherapy or bacterial therapy. Alterations of certain microbial communities increase the risk of BC as they alter tissue metabolism on multiple levels, including the triggering and progression of cancer in the host [29]. This form of treatment may involve immune regulation, influencing the efficacy of anti-tumor drugs, the targeted therapy of engineered pro- and prebiotic fecal microbiota transplantation, and the administration of anti-tumor drugs [30].
The recent scientific discourse has highlighted the significance of the microbiota in mitigating BC via its anti-tumor activities [31]. Contemporary studies assert that specific intestinal bacteria can obstruct oncogenesis and promote tumor regression. Nonetheless, the indiscriminate modulation of the gut microbiota can manifest unforeseen consequences. The precise targeting of tumor-associated bacteria is thus crucial in ensuring the safety and effectiveness of therapeutic modalities [32][33]. Current research underscores the imperative of regulating specific tumor-inducing bacteria through methodologies such as bacteria-mediated tumor therapy (BMTT), fecal bacterial and bacteriophage transplantation, prebiotic enhancement, and the utilization of bacterial toxins and enzymes [34].
However, a salient concern in harnessing bacterial agents for cancer therapeutics pertains to their potential cytotoxicity and pathogenic manifestations. To mitigate these risks, the scientific community has explored genetic engineering as a solution, allowing the excision of virulence-inducing genes while preserving therapeutic features [35]. However, the selection of bacteria is paramount: an ideal bacterial candidate should effectively permeate tumors, possess minimal infectiousness, and remain amenable to antibiotic-mediated elimination post-intervention. Numerous bacterial candidates await rigorous clinical evaluations, leaving certain aspects of their metabolic functions ambiguous. Challenges encompass inadvertent cytotoxic effects on healthy cells, incomplete tumor eradication, and unpredictable bacterial genome mutations. The transient existence of bacterial peptides in the human body further complicates their therapeutic potential. As such, there is an emergent demand for exhaustive clinical trials to elucidate these bacteria–tumor cell dynamics and the potential ramifications of such interventions [36].
Conventional cancer treatments, primarily radiotherapy and chemotherapy, remain central to BC intervention paradigms. Despite their dominance, the associated adverse effects and non-selective toxicity of these treatments necessitate the exploration of more targeted therapeutic options. In this context, genetically engineered bacteria, which exhibit a selective affinity for cancer cells while sparing healthy tissue, have emerged as promising agents in cancer research [37].
These bacteria inherently possess the ability to produce and excrete proteinaceous toxins that can inhibit specific cellular functions, a property that may be harnessed for therapeutic purposes. These agents can target cancer cells, thereby minimizing damage to surrounding healthy cells. Studies have suggested a potential symbiotic relationship between certain bacterial strains and tumor regression, observed in animal models. Nevertheless, there is an unequivocal need for additional research to validate and understand these associations further [38].
While the exclusive use of bacterial agents may not secure complete tumor elimination, their combination with conventional drugs presents a promising avenue for cancer treatment. This integrated approach—incorporating drug-laden bacteria—appears especially propitious in overcoming the limitations of existing treatments, primarily their inability to effectively penetrate the tumor microenvironment [39][40].
The utilization of bacteria, particularly Listeria, Clostridium, and Salmonella, offers a solution to this challenge as they can navigate and infiltrate the tumor’s environment. For instance, Salmonella typhimurium has demonstrated remarkable anti-tumor properties, showing efficiency in invading and eliminating various cancer cells in in vivo studies. This strain has yielded significant results as a monotherapy against pancreatic, prostate, and BC in animal models [41][42].
Furthermore, Clostridium perfringens has been acknowledged for producing an enterotoxin that, upon interaction with specific transmembrane proteins, can induce tumor regression. The FDA, recognizing the potential of BMTT, approved the use of Bacillus Calmette–Guerin, a weakened strain of Mycobacterium bovis, for a specific bladder cancer treatment in the late 1970s, a testament to the enduring clinical relevance of this approach [43][44].
Despite these promising advances, bacterial-mediated therapies are not without challenges. Concerns like potential antibiotic resistance and infection risks associated with the use of live bacteria in treatments need careful consideration and resolution. Hence, while the paradigm of BMTT is not new in oncology, its widespread application and implementation remain the subjects of ongoing debate and investigation. The preliminary findings, although promising, underscore the importance of continued and expanded research to fully comprehend and validate the potential symbiosis between bacterial strains and tumor regression.
2.2. Probiotics and Prebiotics
Emerging data suggest a nexus between microbial dysbiosis in the breast and gut regions and the onset and progression of BC [45]. BC pathogenesis is frequently linked to sustained inflammation, incited by intestinal bacteria that activate NF-κB, subsequently releasing pro-inflammatory cytokines such as TNF-alpha [46][47][48]. Variations in the gut microbiome have been observed across different BC stages [49][50]. These alterations can influence the therapeutic efficacy and potential toxicity. Interventions aimed at modifying the gut microbiome, employing probiotics and prebiotics, could be instrumental in attenuating systemic inflammation and alleviating treatment-associated toxicity. Specific strains such as Bifidobacterium and Lactobacillus have shown promise due to their immunomodulatory and antigenotoxic characteristics [51]. Recent research by Yazdi et al. (2012) revealed that the prophylactic administration of Lactobacillus plantarum augmented with selenium nanoparticles led to a significant reduction in tumor volume and elevated survival rates in a murine model of advanced human BC [52]. Furthermore, milk fermented with Lactobacillus casei CRL 431 administered in the same model demonstrated a marked decrease in tumor invasiveness and metastatic potential [53].
In addition, there have been a multitude of clinical trials in the oncology field investigating the efficacy of probiotics alongside standard anti-cancer regimens. These studies predominantly indicate a beneficial reduction in the gastrointestinal side effects that often arise from conventional cancer therapies [54][55][56][57][58][59]. A significant finding was obtained in RCC patients who received a bacterial supplement, CBM588, alongside immunotherapy, reporting improved progression-free survival rates and response outcomes [60]. Probiotics play a crucial role in enhancing the immune system, significantly elevating the immunoglobulin A (IgA) levels in the gut, which is vital for immune function. Specific probiotic bacteria, including L. casei and Sphingomonas yanoikuyae, have been noted to bolster the production of natural killer (NK) cells, playing a pivotal role in regulating cancer progression by actively participating in the body’s defense against cancer. Moreover, probiotics not only foster immune defenses but also produce compounds instrumental in protecting against DNA damage and breaking down carcinogens, thereby potentially preventing cancer. For example, L. casei strain Shirota (BLS) has been studied for its probable cancer-preventive properties, showing an inverse correlation between its consumption and the incidence of BC [61]. Furthermore, probiotics have shown promise in reducing chemotherapy-related cognitive impairment (CRCI) in BC patients and alleviating various other chemotherapy-induced side effects [62][63].
Species like L. casei Shirota and Bifidobacterium Bb12 have exhibited antigenotoxic activity, a crucial component in preventing the genetic mutations that may lead to cancer, although the degree of this effect varies between bacterial species and is dependent on long-term exposure. Furthermore, the effectiveness of immune cell activation by probiotics varies and is based on the dosage and bacterial strain.
However, despite the optimistic preliminary findings, it is essential to acknowledge that the effects of probiotics tend to diminish over time. This diminishing effect underscores the need for ongoing research to understand the long-term role and potential benefits of probiotics in both cancer prevention and treatment, and in mitigating the side effects associated with cancer therapies. Given the initial promising results, predominantly based on in vivo experiments, there is a clear need for enhanced, comprehensive research, including in vitro exploration, to overcome the challenges observed in existing studies, such as inefficient mucosal adhesion and reduced gastrointestinal activity.
Current clinical trials are sparse, especially those focusing on probiotics specific to BC. Nevertheless, the initial findings are promising, and the results from ongoing clinical trials are anticipated to provide a clearer understanding of the role that probiotics play in potentially enhancing BC treatment outcomes and in cancer prevention more broadly.
2.3. Bacteriotherapy Approaches
Bacteriotherapy, an evolving field in the domain of anti-cancer therapies, employs a diverse range of bacterial forms, inclusive of genetically modified organisms (GMOs), in both their living and attenuated states. These bacterial entities function via promoting apoptosis or disrupting cell membranes, primarily targeting cancer cells through a range of anti-cancer agents, including bacteriocins, spores, and bacterial peptides [64].
Historical records indicate the rudimentary utilization of bacteriotherapy as far back as 1550 BC. A seminal advancement in cancer immunotherapy can be attributed to Dr. William Coley, an American orthopedic surgeon. His pioneering work involved the development of a heat-inactivated concoction of Streptococcus pyogenes and Serratia marcescens, subsequently referred to as “Coley’s toxin”. Administering this therapeutic concoction to patients with inoperable cutaneous carcinoma yielded substantial clinical outcomes, notably tumor regression and complete remission in a significant proportion of patients [65].
Various bacterial strains, encompassing Vibrio, Shigella, Salmonella, Listeria, and Bifidobacteria, have shown profound efficacy in tumor invasion, colonization, and subsequent eradication. However, the relationship between bacteria and cancer remains multifaceted. Certain bacterial strains possess the potential to instigate carcinogenesis or induce malignancies. A case in point is Helicobacter pylori, which, through the secretion of specific cytokines and chemokines, can incite chronic inflammatory responses with detrimental cellular implications. Notably, the cytotoxin-associated gene A (cagA) has emerged as a pivotal bacterial protein with profound implications in oncogenesis, primarily by compromising the function of the tumor suppressor protein p53 [66][67].
Although, historically, malignancies were often associated with pathogenic bacterial infections, contemporary research is increasingly highlighting the anticarcinogenic potential of certain bacteria. This paradigm shift can be epitomized by the nuanced understanding of how tumor cells proliferate by subverting host immunologic defenses. Recent studies highlight the anticarcinogenic activity of Salmonella, which appears to operate through a multifaceted mechanism involving both adaptive and innate immune response activation.
Bacteriocins, intricate peptides or proteins synthesized ribosomally, first garnered attention in 1920, courtesy of the seminal work by the Belgian scientist André Gratia and the discovery of colicin (the first bacteriocin) from E. coli [68]. Today, their applications transcend the clinical domain, with their antimicrobial properties finding utility in food preservation as well. Based on their molecular weight, they are categorized into four classes. Significant bacteriocins include Bovicin HC5 from S. bovis and Nisin A from Lactococcus lactis. Some research indicates that the enterotoxin (TcdA) and cytotoxin (TcdB) produced by Clostridium difficile could be pivotal in treating colorectal cancer [69].
Bacteria, in their myriad forms, are increasingly being recognized as potent immunotherapeutic agents. Their ability to modulate tumor antigenicity holds promise in augmenting immune responses. The novel insights into bacterial interactions, particularly infections with Clostridium novyi, underscore the profound potential in harnessing bacteria for therapeutic advancements in oncological properties. Therefore, bacteria are hailed as promising immunotherapeutic agents due to their ability to amplify the antigenicity of tumor cells, thus bolstering immune responses. By using bacterial cancer immunotherapy, tumor cells are identified as infected cells rather than mere cancer cells, elevating the likelihood of their elimination. For instance, infections with C. novyi can trigger the formation of heat shock proteins like Hsp70, promoting the maturation of professional dendritic and antigen-presenting cells, leading to potent antigen-specific immune responses [70][71].
2.4. Fungal Microbial Polysaccharides
Microbial polysaccharides (MPs) play a role in various cellular processes, including signal transduction and immune response modulation [72]. Glycans and Basidiomycetes-derived MPs, like krestin, schizophyllan, and lentinan, exhibit anti-cancer properties through immunostimulation, the downregulation of NF-κB responses, and the induction of tumor cell apoptosis [73][74].
Polysaccharide peptides from the mushroom YunZhi (Coriolus versicolor/Trametes versicolor) have been used in combination with chemotherapy to treat BC patients in Asian countries for decades. These peptides exhibit anti-proliferative properties, as they significantly reduce BC cell (MDA-MB-231) proliferation by upregulating the p21 gene expression [75]. Moreover, a meta-analysis by L.Y. Eliza et al. (2012) concluded that Coriolus versicolor can increase survival rates in cancer patients, including those with BC [76].
Additional beneficial MPs include Levan, which induces apoptotic cell death in MCF-7 BC cells, and the proteoglucan D-Fraction from Grifola frondosa, which reduces mammary tumor cell migration and lung metastases [77][78]. A clinical trial on D-Fraction in breast and lung cancer patients (stage II–IV) found that it hindered cancer progression and metastasis and increased NK cell activity [79]. This suggests MPs’ potential in enhancing anti-tumor immunity in BC patients without significant toxicity. However, there is a need for additional clinical trials to study D-Fraction’s efficacy in combination with chemotherapeutics in a larger patient population.
2.5. Oncolytic Virotherapy
Oncolytic virotherapy (OV) employs either naturally occurring or genetically engineered viruses with an affinity for tumor cells, leading to their selective targeting and replication. This process culminates in tumor regression, attributed not only to direct cytotoxicity but also the stimulation of anti-tumor immune responses. Remarkably, this occurs without negative effects on healthy cells and tissue [80].
In both in vitro and in vivo preclinical investigations, the third-generation oncolytic herpes simplex virus-1 (HSV-1) vector, G47Δ, exhibited amplified cytotoxic effects across multiple BC cell lines, inclusive of MCF-7, MDA-MB-468, and the tamoxifen-resistant variant MCF-7/TAM-R [81][82][83]. Notably, a synergistic cytotoxic effect was observed in BC cells when G47Δ was combined with the chemotherapeutic agent paclitaxel. This potentiated the anti-tumor efficacy of paclitaxel, resulting in a five-fold dosage reduction to achieve an equivalent tumor reduction in vivo [83], a shift that could minimize chemotherapy-associated adverse effects.
In 2015, the U.S. Food and Drug Administration (FDA) granted approval for talimogene laherparepvec (T-VEC) as the inaugural oncolytic agent for melanoma treatment. Derived from the genetically modified HSV-1, its utility was further highlighted in a phase II clinical trial, which revealed that, in conjunction with neoadjuvant chemotherapy (NAC), it enhanced pathological complete response rates in triple-negative breast cancer (TNBC) patients, resulting in an 89% 2-year disease-free rate [84]. It warrants mention that the observed adverse effects, though generally mild, ranged from injection site pain and headaches to low-grade fevers. This was notably in contrast to the more severe immune-mediated toxicity associated with TNBC treatment involving both chemotherapy and pembrolizumab [85].
GLV-1h68, an oncolytic vaccinia virus, has been engineered to selectively target and destroy cancer cells. Studies found that this virus replicated more efficiently in ALDEFLUOR-positive BC cells, which are known for their resistance to chemotherapy and radiation and their higher expression of cancer stem cell markers. These cells also demonstrated greater migration and invasion abilities. In mouse models, tumors derived from these cells responded more robustly to GLV-1h68 treatment, showing earlier fluorescence detection and faster regression. The virus also showed preferential replication in another cancer stem-like population, CD44+CD24+ESA+ cells. This efficient infection and destruction of BC stem-like cells by GLV-1h68 highlight its potential as a promising agent against tumor initiation, recurrence, and metastasis [86].
Similarly, the measle virus secretory form of NAP (MV-s-NAP), an oncolytic measles virus, has been engineered to express secretory neutrophil-activating protein (s-NAP) from H. pylori, which is an immunostimulatory bacterial protein. This virus selectively targets and destroys cancer cells, particularly cancer stem-like cells. It also triggers both local and systemic anti-tumor immunity. s-NAP attracts immune cells like neutrophils and macrophages to the site of infection and induces the expression of pro-inflammatory cytokines and chemokines. This activates both the innate and adaptive immune responses against the infected tumor cells. In preclinical studies, MV-s-NAP demonstrated improved efficacy over the MV alone, doubling the survival time in mouse models of BC and increasing the levels of certain cytokines like TNFα, IL-6, and IL-12/23 in the pleural effusion. The virus was well tolerated in transgenic mice, with no adverse effects observed, supporting its favorable preclinical safety profile. These promising results have led to the initiation of a first-in-human phase I clinical trial for metastatic BC [87].
2.6. Phage-Based Immunotherapy
Likewise, phage therapy is emerging as a promising strategy in BC immunotherapies. It utilizes the ability of phages to invoke anti-tumor immune responses. Specifically, in phage display immunotherapy, antigens (proteins or peptides), fused to phage coat proteins, function as protective vaccines against cancer. Certain peptides, including E75, AE37, and GP2, have shown potential in BC tests on BALB/c mice. Phages offer two main vaccine delivery techniques: (i) showcasing immunogenic peptides through modified phage coat proteins and (ii) acting as delivery media for DNA vaccines by inserting a eukaryotic promoter-driven vaccine gene within their genome. These vaccines, presenting numerous antigen copies on immunogenic phage particles, incite strong immune reactions. They are also stable, cost-effective, and potent. Experiments have validated their effectiveness in mice and rabbits. Vaccines against human papilloma viruses (HPV), such as Gardasil-9, demonstrate applications beyond BC. Another anti-breast cancer development is a phage-based anti-HER2 vaccine, designed to bypass immune tolerance. Other studies have developed vaccines for prostate cancer and utilized inovirus-associated vector vaccines for antibody production. Furthermore, a dual anthrax-plague vaccine and a lambda phage-based vaccine for hepatocellular carcinoma underscore the expansive potential of phage-based therapies.
Additionally, in a recent study by Catala and colleagues (2021), protein-lipid particles (PLPs) have been innovatively crafted using bacteriophage lambda to display a fluorescent probe and the therapeutic antibody trastuzumab (Trz), leading to the formation of Trz-PLPs [88]. These are designed to target HER2-positive BC cells. By increasing Trz’s density on PLPs, the more prolonged inhibition of cell growth is achieved compared to using free Trz. Trz-PLPs have impacts on numerous cellular pathways, influencing amino acid metabolism, mitochondrial function, and more. They modulate the phosphorylation of key signaling proteins, such as Akt and mTOR, influencing the vital PI3K/Akt/mTOR signaling pathway in cancer cells [88]. Dong and colleagues (2022) have also spotlighted the potential of the M13 phage-based vaccines [89]. By combining an M13 phage with a cationic polymer, PEI, a hybrid platform (M13@PEI) was designed, capable of efficiently absorbing negatively charged antigens. This resulted in the MPO vaccine, combined with the Ovalbumin (OVA) antigen, which improved the maturation of antigen-presenting cells (APCs) and boosted antigen presentation. The M13 phage genome’s CpG regions and PEI’s role as a TLR5 agonist facilitated this. Enhanced antigen processing and uptake were further confirmed through in vitro assays, emphasizing the robust cytotoxic T-lymphocyte (CTL) response. In vivo studies also confirmed the MPO vaccine’s ability to deliver antigens effectively and enhance antigen-specific T-cell-mediated responses. When combined with α-PD1 treatment, the vaccine showcased powerful anti-tumor effects, improved survival rates, and enhanced immune memory responses, proving the significant potential of M13 phage-based vaccines in anti-tumor immunotherapy [89].
2.7. Microbiome-Modulating Interventions (Summary Box)
Bacterial therapeutics show promise for tumor treatment and immune modulation. Specific gut bacteria can prevent cancer development, while others promote it, so precisely targeting tumor-associated bacteria is important. Approaches include bacteria-mediated tumor therapy, fecal transplants, bacteriophages, and using bacterial toxins/enzymes. However, risks include cytotoxicity and pathogenicity, which can be mitigated through genetic engineering. Ideal bacterial candidates effectively reach tumors with low infectivity and can be eliminated with antibiotics. Probiotics and prebiotics also show potential by reducing inflammation and treatment side effects. Lactobacillus plantarum and Lactobacillus casei have demonstrated anti-tumor effects in mouse models. Clinical trials show that probiotics can reduce chemotherapy side effects. Specific strains like L. casei and Sphingomonas increase natural killer cells and immune defenses against cancer. However, the effects tend to diminish over time, underscoring the need for long-term research. Bacteriotherapy employs living or attenuated bacteria to promote apoptosis, disrupt cell membranes, or deliver drugs specifically to cancer cells. Approaches include genetically modified organisms, bacteriocins, spores, and peptides. Historical examples include Coley’s toxin, which induced remissions. Salmonella, Listeria, and Clostridium can penetrate tumors. However, concerns include antibiotic resistance, infection risks, and uncertain metabolic functions. Fungal polysaccharides also exhibit anti-cancer properties through immunostimulation, inflammation reduction, and apoptosis induction. Mushroom extracts have shown activity against breast cancer in Asian studies and mouse models. Oncolytic virotherapy employs viruses, like HSV-1, to selectively target and replicate in tumors, inducing direct killing and immune responses against cancer cells. Several viruses are under clinical investigation for breast and other cancers. Phage display vaccines also show potential by presenting tumor antigens on phage coat proteins to stimulate immune responses.
References
- Aarnoutse, R.; Ziemons, J.; Hillege, L.E.; de Vos-Geelen, J.; de Boer, M.; Bisschop, S.M.P.; Vriens, B.E.P.J.; Vincent, J.; van de Wouw, A.J.; Le, G.N.; et al. Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo)adjuvant chemotherapy. NPJ Breast Cancer 2022, 8, 89.
- Bilenduke, E.; Sterrett, J.D.; Ranby, K.W.; Borges, V.F.; Grigsby, J.; Carr, A.L.; Kilbourn, K.; Lowry, C.A. Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci. Rep. 2022, 12, 19547.
- Horigome, A.; Okubo, R.; Hamazaki, K.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y. Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Benef. Microbes 2019, 10, 751–758.
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M.; et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021, 28, 2778–2796.
- Okubo, R.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain Behav. Immun. 2019, 85, 186–191.
- Martín, B.R.; Rodríguez, E.J.F.; Galve, M.I.R.; Hernández, J.J.C. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896.
- Williams, L.J.; Fletcher, E.; Douglas, A.; Anderson, E.D.C.; McCallum, A.; Simpson, C.R.; Smith, J.; Moger, T.A.; Peltola, M.; Mihalicza, P.; et al. Retrospective cohort study of breast cancer incidence, health service use and outcomes in Europe: A study of feasibility. Eur. J. Public Health 2017, 28, 327–332.
- Jim, H.S.; Phillips, K.M.; Chait, S.; Faul, L.A.; Popa, M.A.; Lee, Y.-H.; Hussin, M.G.; Jacobsen, P.B.; Small, B.J. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated with Standard-Dose Chemotherapy. J. Clin. Oncol. 2012, 30, 3578–3587.
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217.
- Cheung, Y.T.; Ng, T.; Shwe, M.; Ho, H.K.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Fan, G.; Tan, Y.P.; Yong, W.S.; et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 2015, 26, 1446–1451.
- Shadad, A.K. Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. World J. Gastroenterol. 2013, 19, 185.
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.-W. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE 2013, 8, e82659.
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6.
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-De-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2019, 130, 466–479.
- Guo, H.; Chou, W.-C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097.
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365.
- Di Modica, M.; Gargari, G.; Regondi, V.; Bonizzi, A.; Arioli, S.; Belmonte, B.; De Cecco, L.; Fasano, E.; Bianchi, F.; Bertolotti, A.; et al. Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Res. 2021, 81, 2195–2206.
- Costa, D.A.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer—Is There any Relevant Link?—A Literature Review and New Horizons toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332.
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465.
- Wu, M.; Bai, J.; Ma, C.; Wei, J.; Du, X. The Role of Gut Microbiota in Tumor Immunotherapy. J. Immunol. Res. 2021, 2021, 5061570.
- Haque, S.; Raina, R.; Afroze, N.; Hussain, A.; Alsulimani, A.; Singh, V.; Mishra, B.N.; Kaul, S.; Kharwar, R.N. Microbial dysbiosis and epigenetics modulation in cancer development—A chemopreventive approach. Semin. Cancer Biol. 2022, 86, 666–681.
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467.
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404.
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686.
- AACR Annual Meeting 2017 Online Proceedings and Itinerary Planner|Presentation. Available online: https://www.abstractsonline.com/pp8/#!/4292/presentation/1296 (accessed on 1 November 2023).
- Emens, L.A.; Adams, S.; Loi, S.; Schneeweiss, A.; Rugo, H.S.; Winer, E.P.; Barrios, C.H.; Dieras, V.; de la Haba-Rodriguez, J.; Gianni, L.; et al. IMpassion130: A Phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). J. Clin. Oncol. 2016, 34, TPS1104.
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.; Kaufman, P.A.; Wilks, S.; Andreopoulou, E.; et al. A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). J. Clin. Oncol. 2020, 38, 1015.
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828.
- Liu, M.; Jia, S.; Dong, T.; Zhao, F.; Xu, T.; Yang, Q.; Gong, J.; Fang, M. Metabolomic and Transcriptomic Analysis of MCF-7 Cells Exposed to 23 Chemicals at Human-Relevant Levels: Estimation of Individual Chemical Contribution to Effects. Environ. Health Perspect. 2020, 128, 127008.
- Xu, J.-Y.; Liu, M.-T.; Tao, T.; Zhu, X.; Fei, F.-Q. The role of gut microbiota in tumorigenesis and treatment. Biomed. Pharmacother. 2021, 138, 111444.
- Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells 2023, 12, 1945.
- Zhu, R.; Lang, T.; Yan, W.; Zhu, X.; Huang, X.; Yin, Q.; Li, Y. Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Adv. Sci. 2021, 8, 2003542.
- Mendes, I.; Vale, N. How Can the Microbiome Induce Carcinogenesis and Modulate Drug Resistance in Cancer Therapy? Int. J. Mol. Sci. 2023, 24, 11855.
- Trivanović, D.; Pavelić, K.; Peršurić, Ž. Fighting Cancer with Bacteria and Their Toxins. Int. J. Mol. Sci. 2021, 22, 12980.
- Khoshnood, S.; Fathizadeh, H.; Neamati, F.; Negahdari, B.; Baindara, P.; Abdullah, M.A.; Haddadi, M.H. Bacteria-derived chimeric toxins as potential anticancer agents. Front. Oncol. 2022, 12, 953678.
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047.
- Patyar, S.; Joshi, R.; Byrav, D.P.; Prakash, A.; Medhi, B.; Das, B. Bacteria in cancer therapy: A novel experimental strategy. J. Biomed. Sci. 2010, 17, 21.
- Nandi, D.; Parida, S.; Sharma, D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes 2023, 15, 2221452.
- Allemailem, K.S. Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management. Int. J. Nanomed. 2021, 16, 8159–8184.
- Liang, S.; Wang, C.; Shao, Y.; Wang, Y.; Xing, D.; Geng, Z. Recent advances in bacteria-mediated cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1026248.
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15.
- Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Recent Advances in Bacteria-Based Cancer Treatment. Cancers 2022, 14, 4945.
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins 2016, 8, 73.
- Cardillo, F.; Bonfim, M.; Sousa, P.d.S.V.; Mengel, J.; Castello-Branco, L.R.R.; Pinho, R.T. Bacillus Calmette–Guérin Immunotherapy for Cancer. Vaccines 2021, 9, 439.
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727.
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell 2014, 27, 27–40.
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812.
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86.
- Eslami-S, Z.; Majidzadeh-A, K.; Halvaei, S.; Babapirali, F.; Esmaeili, R. Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 2020, 10, 120.
- Luu, T.H.; Michel, C.; Bard, J.-M.; Dravet, F.; Nazih, H.; Bobin-Dubigeon, C. Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutr. Cancer 2017, 69, 267–275.
- Malik, S.S.; Saeed, A.; Baig, M.; Asif, N.; Masood, N.; Yasmin, A. Anticarcinogenecity of microbiota and probiotics in breast cancer. Int. J. Food Prop. 2018, 21, 655–666.
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The Preventive Oral Supplementation of a Selenium Nanoparticle-enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittelforschung 2012, 62, 525–531.
- Aragón, F.; Carino, S.; Perdigón, G.; LeBlanc, A.d.M.d. Inhibition of Growth and Metastasis of Breast Cancer in Mice by Milk Fermented with Lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196.
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31.
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362.
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145.
- Theodoropoulos, G.E.; Memos, N.A.; Peitsidou, K.; Karantanos, T.; Spyropoulos, B.G.; Zografos, G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann. Gastroenterol. 2016, 29, 56–62.
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767.
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034.
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712.
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200.
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2021, 161, 10–22.
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339.
- Nallar, S.C.; Xu, D.-Q.; Kalvakolanu, D.V. Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine 2017, 89, 160–172.
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158.
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794.
- Hatakeyama, M. Helicobacter pylori CagA and Gastric Cancer: A Paradigm for Hit-and-Run Carcinogenesis. Cell Host Microbe 2014, 15, 306–316.
- Felgner, S.; Kocijancic, D.; Frahm, M.; Heise, U.; Rohde, M.; Zimmermann, K.; Falk, C.; Erhardt, M.; Weiss, S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. OncoImmunology 2017, 7, e1382791.
- Field, D.; Cotter, P.D.; Ross, R.P.; Hill, C. Bioengineering of the model lantibiotic nisin. Bioengineered 2015, 6, 187–192.
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.-Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi -NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111.
- Bhardwaj, N.; Gnjatic, S.; Sawhney, N.B. TLR AGONISTS: Are They Good Adjuvants? Cancer J. 2010, 16, 382–391.
- Shanmugam, M.; Abirami, R. Microbial Polysaccharides—Chemistry and Applications. J. Biol. Act. Prod. Nat. 2019, 9, 73–78.
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304.
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 4, 285–289.
- Chow, L.W.; Lo, C.S.; Loo, W.T.; Hu, X.-C.; Sham, J.S.T. Polysaccharide Peptide Mediates Apoptosis by Up-regulating p21 Gene and Down-regulating Cyclin D1 Gene. Am. J. Chin. Med. 2003, 31, 1–9.
- Eliza, W.L.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87.
- Queiroz, E.A.; Fortes, Z.B.; da Cunha, M.A.; Sarilmiser, H.K.; Dekker, A.M.B.; Öner, E.T.; Dekker, R.F.; Khaper, N. Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol. 2017, 102, 565–570.
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget 2018, 9, 23396–23412.
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-Fraction on the Activation of NK Cells in Cancer Patients. J. Med. Food 2003, 6, 371–377.
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2016, 15, 660.
- Fan, J.; Jiang, H.; Cheng, L.; Liu, R. The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncol. Rep. 2016, 35, 1741–1749.
- Wang, L.-J.; Han, S.-X.; Bai, E.; Zhou, X.; Li, M.; Jing, G.-H.; Zhao, J.; Yang, A.-G.; Zhu, Q. Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncol. Rep. 2013, 29, 1563–1569.
- Zeng, W.-G.; Li, J.-J.; Hu, P.; Lei, L.; Wang, J.-N.; Liu, R.-B. An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncol. Rep. 2013, 29, 2355–2361.
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial. Nat. Med. 2023, 29, 450–457.
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567.
- Wang, H.; Chen, N.G.; Minev, B.R.; Szalay, A.A. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J. Transl. Med. 2012, 10, 167.
- Viker, K.B.; Steele, M.B.; Iankov, I.D.; Concilio, S.C.; Ammayappan, A.; Bolon, B.; Jenks, N.J.; Goetz, M.P.; Panagioti, E.; Federspiel, M.J.; et al. Preclinical safety assessment of MV-s-NAP, a novel oncolytic measles virus strain armed with an H. pylori immunostimulatory bacterial transgene. Mol. Ther.-Methods Clin. Dev. 2022, 26, 532–546.
- Catala, A.; Dzieciatkowska, M.; Wang, G.; Gutierrez-Hartmann, A.; Simberg, D.; Hansen, K.C.; D’alessandro, A.; Catalano, C.E. Targeted Intracellular Delivery of Trastuzumab Using Designer Phage Lambda Nanoparticles Alters Cellular Programs in Human Breast Cancer Cells. ACS Nano 2021, 15, 11789–11805.
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 bacteriophage-based vaccine platform for personalized cancer immunotherapy. Biomaterials 2022, 289, 121763.
More
Information
Subjects:
Microbiology; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
424
Revisions:
2 times
(View History)
Update Date:
05 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




