Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Balu Alagar Venmathi Maran | -- | 2479 | 2024-02-01 07:18:47 | | | |
| 2 | Rita Xu | Meta information modification | 2479 | 2024-02-01 08:34:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Venmathi Maran, B.A.; Jeyachandran, S.; Kimura, M. Electrospinning of Polymer Nanofibers. Encyclopedia. Available online: https://encyclopedia.pub/entry/54625 (accessed on 01 March 2026).
Venmathi Maran BA, Jeyachandran S, Kimura M. Electrospinning of Polymer Nanofibers. Encyclopedia. Available at: https://encyclopedia.pub/entry/54625. Accessed March 01, 2026.
Venmathi Maran, Balu Alagar, Sivakamavalli Jeyachandran, Masanari Kimura. "Electrospinning of Polymer Nanofibers" Encyclopedia, https://encyclopedia.pub/entry/54625 (accessed March 01, 2026).
Venmathi Maran, B.A., Jeyachandran, S., & Kimura, M. (2024, February 01). Electrospinning of Polymer Nanofibers. In Encyclopedia. https://encyclopedia.pub/entry/54625
Venmathi Maran, Balu Alagar, et al. "Electrospinning of Polymer Nanofibers." Encyclopedia. Web. 01 February, 2024.
Copy Citation
Polymeric nanofibers have emerged as a captivating medium for crafting structures with biomedical applications. Spinning methods have garnered substantial attention in the context of medical applications and neural tissue engineering, ultimately leading to the production of polymer fibers. In comparison with polymer microfibers, polymer nanofibers boasting nanometer-scale diameters offer significantly larger surface areas, facilitating enhanced surface functionalization.
electrospinning
polymer
nanofibers
biomedical applications
1. Introduction
Nanotechnology has become a ubiquitous force, impacting a diverse array of fields worldwide, including electronics, medical treatments, industry, military systems, construction materials, and environmental remediation, such as water treatment and air purification [1]. Central to this domain is the manipulation and utilization of materials and structures at molecular, polymer, and nanostructure levels, harnessing distinct properties and phenomena of matter within the 1 to 100 nm size range. Over the past decade, polymer nanofibers, a pivotal class of nanomaterials, have emerged as a focal point of exploration. These nanofibers are defined as fibers with diameters on the order of 100 nm and find application in nanotechnology and nanostructured materials [2]. Notably, sub-micrometer fibers, measuring less than 100 nm in dimension and fabricated with advanced techniques like electrospinning, have also gained prominence. Due to their nanoporosity distribution, high surface area-to-volume ratio, low weight, and customizable surface properties, nanofibers are exceptionally suited for applications such as water and air filtration, membranes, protective clothing, and drug delivery systems [3]. Moreover, nanofibers offer avenues for precise surface modification, enhancing characteristics like water solubility, biocompatibility, and bio-recognition. These attributes position polymer nanofibers at the forefront of healthcare and biotechnology applications [4].
From a biological standpoint, a multitude of natural biomaterials exist in fibrous configurations, including silk, collagen, keratin, and various polysaccharides. These biomaterials display fibrous structures organized hierarchically, extending down to the nanoscale, providing valuable information for biomimicry approaches. Consequently, polymer nanofibers offer a means to emulate and replicate these intricate biosystems [5]. Notably, the research underscores the profound influence of nanoscale surface characteristics and topography, in conjunction with surface chemistry, on cellular behavior, impacting cell attachment, alignment, activation, orientation, and proliferation [6][7]. Cell responses, such as alignment with 66 nm collagen fiber banding, further emphasize the remarkable sensitivity of cells to nanoscale cues, even down to dimensions as small as 5 nm, revealing cellular interactions at scales significantly smaller than the cells themselves [8].
Polymer nanofiber processing variables and material structures play crucial roles in the relationship between the structure and characteristics of polymer nanofibers. Surface area, mechanical strength, and porosity undergo significant influence from the nanofiber’s diameter, determined by the electrospinning voltage and solution viscosity. Smaller diameters yield larger surface areas and enhanced mechanical performance. The alignment and positioning of nanofibers are affected by electrospinning conditions like temperature and humidity, contributing to improved mechanical traits in well-aligned nanofibers [9]. Material properties such as biocompatibility, chemical reactivity, and thermal stability are determined by the structure of a material, which is governed by the choice of solvent and polymer. The unique features of various polymers make them suitable for a range of applications. In addition, depending on the requirements of the application, post-processing procedures like cross-linking and functionalization can further customize the structure to improve stability and provide certain functions. For polymer nanofibers to reach their full potential in diverse technical sectors, it is essential to comprehend and optimize a few of their properties [8][9][10].
2. Electrospinning
Spinning methods entail mechanical drawing and solidification, resulting in fibers [11]. Fiber-based materials with varying morphology structure and qualities may be produced using the spinning method, material selection, and processing factors (Figure 1). The most commonly used spinning technologies are electrospinning, microfluidic spinning, centrifugal spinning, and solution blow spinning. All these spinning techniques are dependent on the polymer concentration, voltage, type of solvent, distance between electrodes, temperature, and other factors [12] (Figure 2). Various spinning processes are used to develop nanofibers from a diverse variety of artificial and natural polymers, and also from mixtures [13].
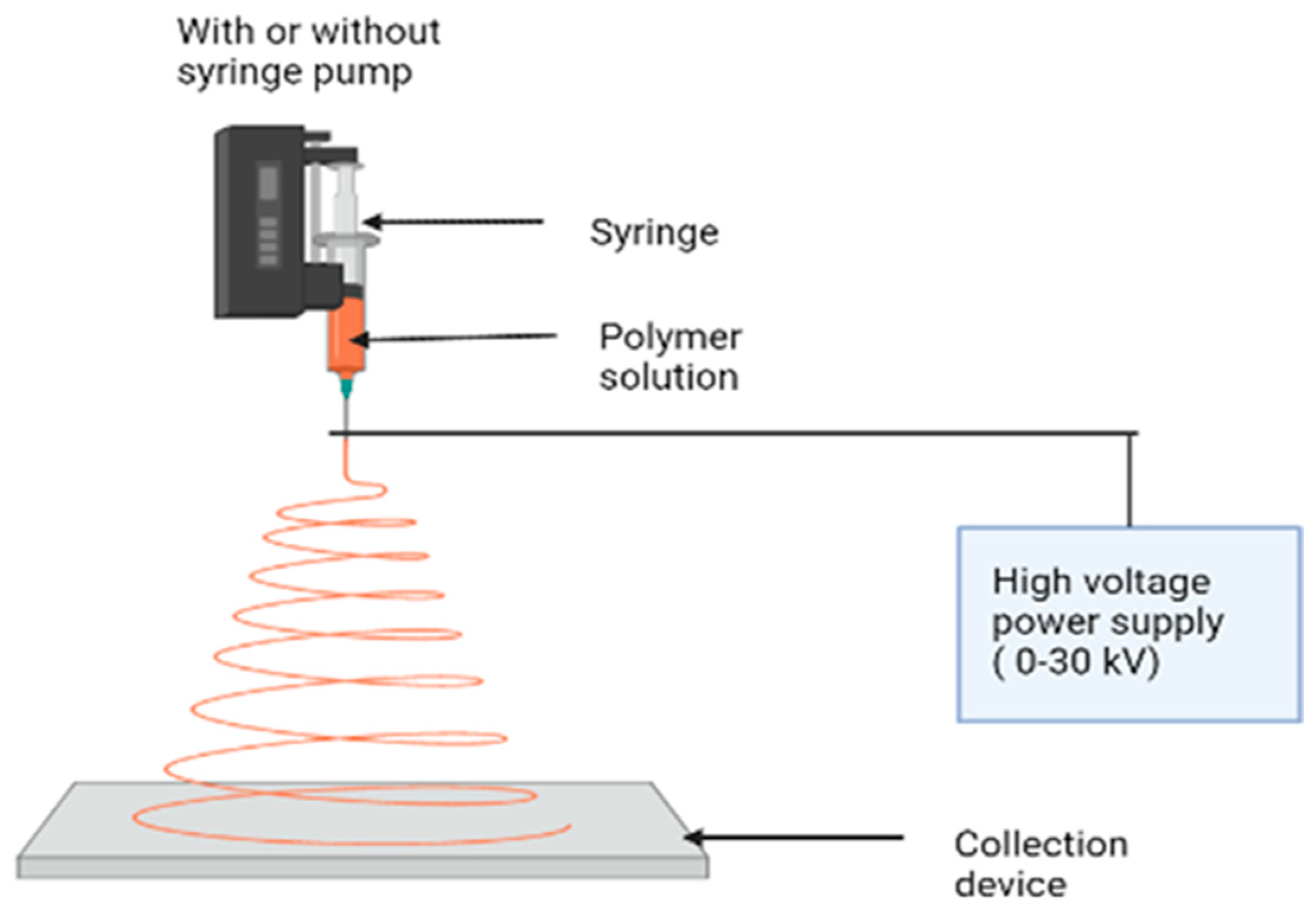
Figure 1. Electrospinning instrument parts and its mechanism for synthesizing nanofibers.
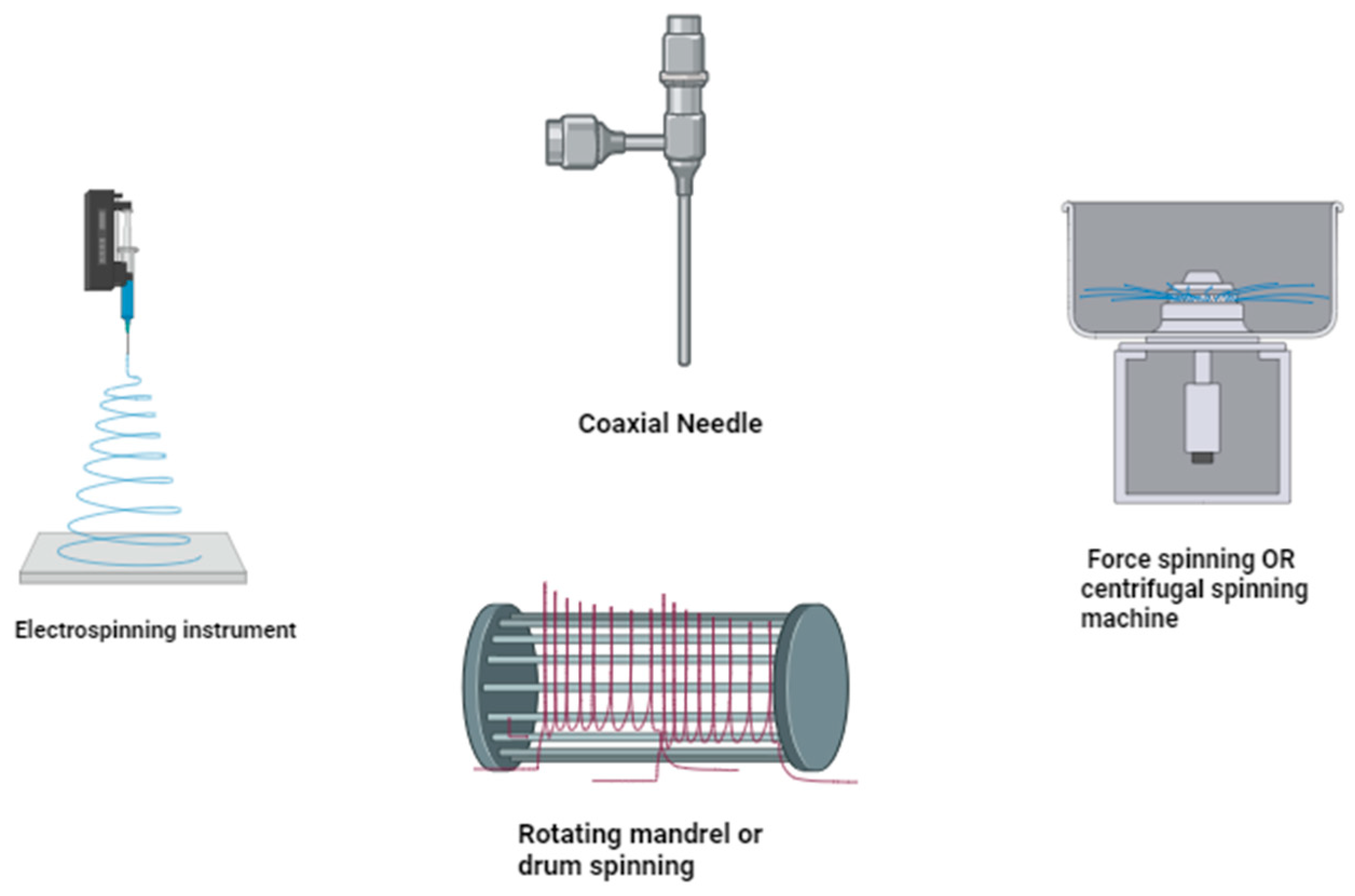
Figure 2. Synthesis of nanofibers using different electrospinning instruments/techniques.
In summary, spinning is the initial phase where the polymer solution is extruded and electrostatic forces are used to form the nanofibers, while drawing refers to the subsequent/*manipulation or elongation of these fibers to achieve specific characteristics in the produced nanofibrous materials. Both spinning and drawing methods are crucial in the electrospinning process for creating nanofibers with tailored properties suitable for various applications.
The following are important aspects of electrospinning:
-
A proper solvent for dissolving the polymer should be provided.
-
The vapor pressure of the solvent needs to be high enough for the fiber to maintain its integrity when it reaches the target but not too high that it hardens before it reaches the nanoscale range.
-
The solvent surface tension and viscosity must be neither too high, to prevent the formation of a jet, nor too low, to enable the solution to drain easily from the pipette.
-
The power source should be sufficient to overcome the viscosity and surface tension of the polymer solution and sustain the jet from the pipette.
-
Maintaining the distance between the pipette and the surface to avoid sparks between the electrodes allows for the evaporation of the solvent to produce fibers.
The most popular fiber-forming technology is electrospinning, which uses electrostatic forces to generate polymer nanofibers [14]. A high voltage is applied to a polymer solution in a syringe. A counter electrode is a plate coated with a sheet of aluminum positioned at a distance of a few cm [15]. Similar charges concentrate in the polymer solution when an electric field is applied, resulting in a strong repulsive force strong enough to overcome the surface tension and viscous drag force of the polymer solution [16] (Figure 1). As a result, a stream of fluid freely emerges from the needle, subdividing into millions of nano- to submicron-sized jets that deposit as nanofibers on the counter electrode or aluminum foil. This sheet is made up of randomly arranged fibers, with the thickness increasing as the electrospinning period proceeds [17]. This process has been used to produce metal oxide/ceramic nanofibers in recent years, such as titanium oxide, barium titanate, lead zirconate titanate, silica, zirconia, titania, nickel oxide, barium titanate, lead zirconate titanate, and other oxide-containing materials [18]. The majority of fibrous materials derived from natural and synthetic polymers are produced using electrospinning and similar processes (Figure 2) [19].
2.1. Melt Electrospinning
Melt electrospinning (ME) is used to produce fibers using polymers that are difficult to dissolve in solvents prior to electrospinning, examples of which include polyethylene (PE), polypropylene (PP), and polyphenylene sulfide (PPS) [20][21]. Conventional ME equipment includes a supply zone, electrical heating components, a conductive collector, and a high-voltage power supply. During melt electrospinning, a melted polymer is extruded through the spinneret, and a high voltage potential is applied between the collector and spinneret, ejecting a charged jet [22]. The polymer jet is then extended toward the grounded collector, where it swiftly hardens, generating ultrafine solid fibers [23]. The ME process is primarily influenced by polymer flow velocity, polymer structural properties, drawing temperature, and voltage [24]. Melt spinning reduces production costs and enables fine control of fiber deposition. The number of polymers suitable for ME, on the other hand, is substantially smaller than for solution electrospinning. ME has only been tested on a few commercially available polymers [25].
2.2. Near-Field Electrospinning
Near-field electrospinning (NF-ES) is a simple approach for accurately controlling the locations of deposited fibers that need organized or pre-designed nanoscale fibrous structures [26]. During the NF-ES technique, the probe tip is used to dip the liquid polymer solution or is typically placed near the collector. Using molten polymer from a distance reduces the required voltage and instability in bending during spinning, enabling the precise deposition of fibers with excellent spatial definition on a 3D motion platform [27]. The ability of NF-ES to build (2D) and (3D) structures has been proven, expanding the potential use of fibers in domains such as energy harvesting and tissue engineering [28]. Indeed, NF-ES has several benefits over conventional electrospinning, including a lower applied voltage and material usage, as well as position-controlled fiber deposition in the three axes, namely, the X, Y, and Z axes [20]. Additionally, the fibers produced with NF-ES are often thicker than those produced with standard electrospinning [29].
2.3. Coaxial Electrospinning
For coaxial electrospinning, the setup is mostly the same as for electrospinning with one exception: the use of a coaxial needle, which consists of two concentric aligned hollow needles [30]. Using two syringe pumps, two polymeric solutions are injected independently through the outer and inner needles. Nanofibers with regulated core and sheath layer compositions may now be made using coaxial electrospinning [31]. Many substances, including proteins, oils, and medications, have been electrospun as the main structure for core–sheath nanofibers (Figure 2) [32]. Coaxial electrospinning is another method for producing hollow nanofibers [33]. One of the most important techniques for creating core–sheath nanofibers is emulsion electrophoresis [34]. This improved electrospinning technology reduces the branching and splitting of spinning jets by injecting a magnetic field that balances out the forces that induce instability, leading to more uniform nanofibers and regulated deposition [35]. Furthermore, when the jet reaches the substrate, the magnetic field increases its velocity as well as the internal alignment of the polymer network, resulting in a reduction in fiber diameter. These are suitable for producing nanofibers in parallel along magnetic field lines for biomedical purposes [36].
2.4. Solution Blow Spinning
Solution blow spinning (SBS), a method rooted in gas-assisted fiber manufacturing technology, has garnered significant attention in recent years due to its high productivity and adaptability [37]. This approach proves particularly advantageous for spinning fibers from materials possessing low electrical conductivity, which poses challenges in the electrospinning process. In the literature, SBS has several names including air spinning, solution blowing, pressure-driven spinning jet spraying, and solution spraying [38]. Its fundamental setup comprises concentrated nozzles, a compressed gas supply (such as air, nitrogen, synthetic air, or oxygen), a fiber collection system, and an infusion pump regulating the polymer ejection rate in a whirling configuration (i.e., similar to a thread-milling motion).
The polymer solution is driven through the inner nozzle with SBS, resulting in the production of a droplet at the inner nozzle’s tip, which is stretched by the high-pressure compressed gas stream flowing through the outer nozzle [39]. The pressurized air exiting the nozzle shapes the drop’s surface into a cone, similar to Taylor’s cone in electrospinning. When the critical air pressure is attained, surface tension forces are overcome, and a jet blasting from the cone’s tip is driven toward the collecting target. As the jets traverse the environment (across the working distance), the polymer liquid undergoes stretching while the solvent rapidly evaporates. This process results in the formation of a web comprising micro- and nanofibers that are collectible on various surfaces, including living tissue, sheets, membranes, liquid surfaces, and rotating drums [40] (Figure 2 and Figure 3).
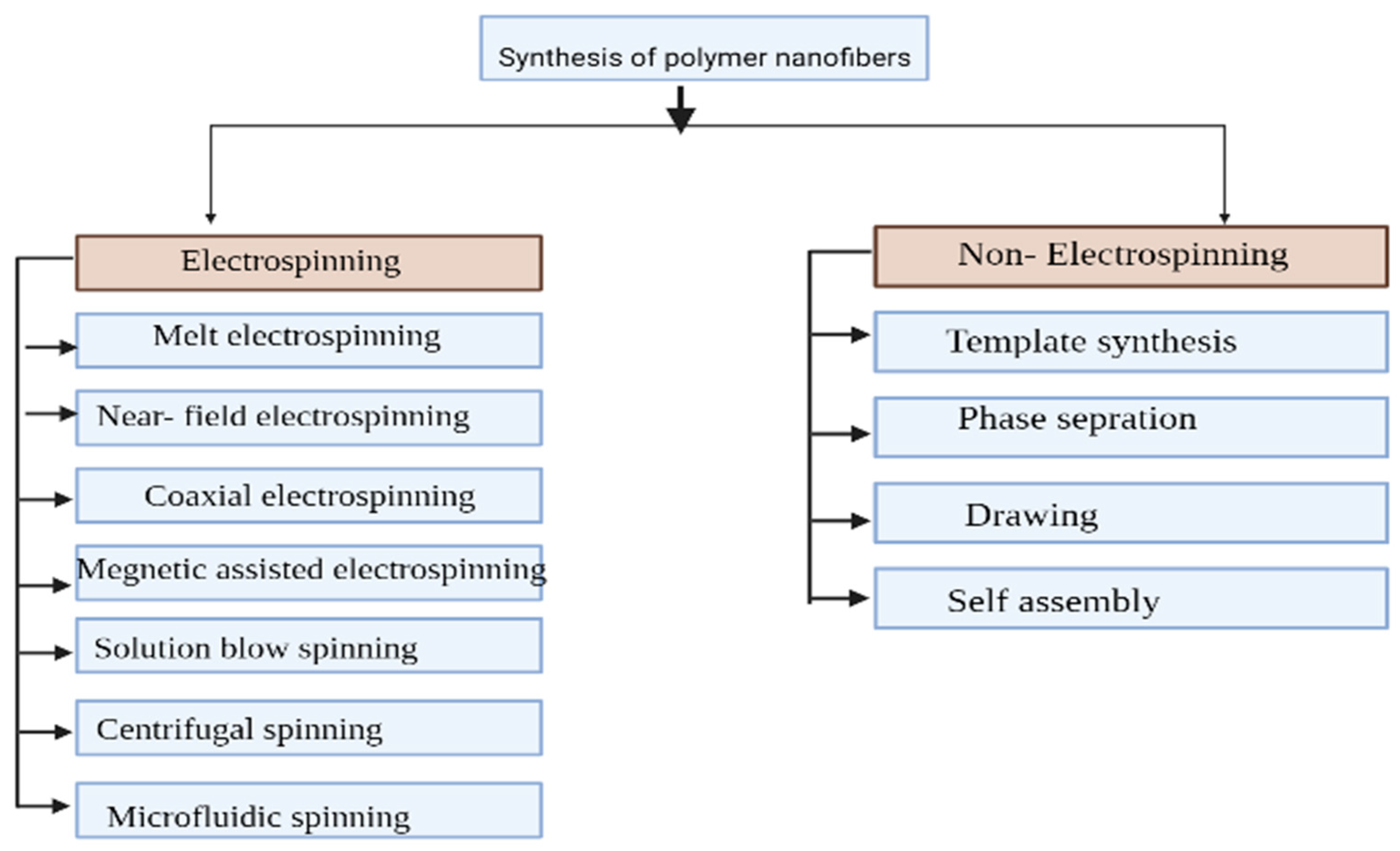
Figure 3. Synthesis of polymer nanofibers showing electrospinning and non-electrospinning methods.
2.5. Force Spinning
Force spinning (FS), also known as rotary spinning or rotating jet spinning, has advanced significantly. Centrifugal spinning is a simple and controlled procedure for producing fibers by utilizing centrifugal forces (Figure 2) [41]. Centrifugal forces overcome the surface tension and viscosity of a molten polymer or polymer solution injected via the valve in this approach, resulting in the ejection of a jet, which is then expanded and stretched by the air vortices [42]. The solvent evaporates and the filament hardens during this phase, resulting in the formation of fibers that are placed on the collector’s desk. Fluid viscoelasticity and the size of the nozzle, its rotational speed, its length, its construction, and the distance between it and the collector are all important factors and may all be adjusted to alter the shape and diameter of centrifugally spun fibers [43]. Centrifugal spinning, similar to electrospinning, uses a diverse set of polymers and solvents. Also, high voltage is not required, and the method has significant industrial scaling potential. One corporation has produced industrial centrifugal spinning equipment that is speedier than electrospinning-based industrial setups [44]. Given the fact that centrifugal spinning and SBS provide greater fiber yields, using these techniques to prepare nanofibers with a range, such as core–sheath or designed nanofibers, offers a challenge [45].
2.6. Microfluidic Spinning
Microfluidic spinning is a cost-effective approach for producing fibers with varied shapes and compositions at the micro- and nanoscale, which has been used over the last decade [46]. In this technique, two different fluids—the fluid, which helps in fiber extrusion, and the core fluid, a polymer precursor solution—are equally injected into microscale channels through different input ports, resulting in a 3D coaxial flow at the channel crossover. Using photopolymerization, chemical or ionic crosslinking techniques, etc., the core fluid is solidified to create fibers [47].
The microfluidic spinning of nanofibers involves the use of microfluidic devices to precisely control the fabrication of nanofibers with diameters on the nanometer scale. This innovative technique harnesses the principles of microfluidics, a field focusing on manipulating small amounts of fluids within microscale channels, to produce high-quality and tailored nanofibers. The process typically involves the controlled flow of polymer solutions or melts through microchannels, where the fluid encounters shearing forces, electrostatic fields, or other relevant mechanisms. By manipulating the flow rates, compositions, and channel geometries within the microfluidic device, it becomes possible to precisely regulate the size, structure, and composition of the resulting nanofibers. Microfluidic devices enable precise control over the flow of materials, allowing for the creation of nanofibers with specific diameters, morphologies, and functionalities. The technology can be scaled up for mass production while maintaining uniformity and quality in nanofiber products [48]). Various polymers, nanoparticles, and functional materials can be used in microfluidic spinning, providing versatility in producing nanofibers for diverse applications, including biomedical, filtration, tissue engineering, and more. Microfluidic platforms can facilitate the creation of composite or multicomponent nanofibers by mixing different materials within the microchannels, offering enhanced functionalities and tailored properties. Microfluidic techniques allow for precise control over the alignment, patterning, and spatial arrangement of nanofibers, enabling the creation of complex structures and architectures.
The microfluidic spinning of nanofibers holds significant promise in advancing various fields due to its ability to produce nanofibers with controlled characteristics and tailored properties, fostering innovation in numerous applications across industries such as healthcare, textiles, filtration, and beyond.
3. Modification of Polymer Nanofibers
Although the opacity of polymer nanofibers is beneficial, some pure polymers still have limitations in the adsorption process due to their higher opacity [49]. Polyacrylonitrile (PAN) [5] and nylon are examples of materials that have little adsorption capacity for the removal of pollutants; PVA, polyacrylic acid (PAA), and polyvinyl pyrrolidone (PVP) are examples of materials that are unstable in aqueous solutions; and chitosan is an example of a material with subpar mechanical properties [21][49][50]. Scientists have put a lot of effort into improving the properties of nanofibers with surface modification to solve these problems. Surface modification aims to improve the mechanical characteristics, hydrophilicity, and wettability of nanofibers in aqueous solutions, as well as their adsorption sites on the surface [51]. Nanocomposites and blends, which are one-step treatments applied during electrospinning and post-treatments applied after electrospinning, are two ways to modify the surface of nanofibers.
References
- Roco, M.C.; Mirkin, C.A.; Hersam, M.C. Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook; Springer Science & Business Media: Berlin, Germany, 2011; Volume 1.
- Camposeo, A.; Moffa, M.; Persano, L. Electrospun fluorescent nanofibers and their application in optical sensing. In Electrospinning for High Performance Sensors; Springer: Cham, Switzerland, 2015; pp. 129–155.
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2017, 302, 1600353.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347.
- Goyal, R.; Vega, M.E.; Pastino, A.K.; Singh, S.; Guvendiren, M.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J. Biomed. Mater. Res. Part A 2017, 105, 2162–2170.
- Chen, C.; Hu, L. Nanoscale Ion Regulation in Wood-Based Structures and Their Device Applications. Adv. Mater. 2021, 33, 2002890.
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian cell behaviour on hydrophobic substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48.
- Liu, Y.; Liu, S.; Luo, D.; Xue, Z.; Yang, X.; Gu, L.; Zhou, Y.; Wang, T. Hierarchically staggered nanostructure of mineralized collagen as a bone-grafting scaffold. Adv. Mater. 2016, 28, 8740–8748.
- Chiang, Y.-C.; Huang, C.-C.; Chin, W.-T. Carbon dioxide adsorption on Carbon nanofibers with different porous structures. Appl. Sci. 2021, 11, 7724.
- Zhang, X.; Lu, Y. Centrifugal spinning: An alternative approach to fabricate nanofibers at high speed and low cost. Polym. Rev. 2014, 54, 677–701.
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166.
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975.
- Sencadas, V.; Correia, D.M.; Areias, A.; Botelho, G.; Fonseca, A.M.; Neves, I.C.; Ribelles, J.G.; Mendez, S.L. Determination of the parameters affecting electrospun chitosan fiber size distribution and morphology. Carbohydr. Polym. 2012, 87, 1295–1301.
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520.
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389.
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2023, 308, 2200502.
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805.
- Mokhtari, F.; Cheng, Z.; Raad, R.; Xi, J.; Foroughi, J. Piezofibers to smart textiles: A review on recent advances and future outlook for wearable technology. J. Mater. Chem. A 2020, 8, 9496–9522.
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes 2018, 8, 62.
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456.
- Dos Santos, A.E.A.; Dos Santos, F.V.; Freitas, K.M.; Pimenta, L.P.S.; de Oliveira Andrade, L.; Marinho, T.A.; de Avelar, G.F.; da Silva, A.B.; Ferreira, R.V. Cellulose acetate nanofibers loaded with crude annatto extract: Preparation, characterization, and in vivo evaluation for potential wound healing applications. Mater. Sci. Eng. C 2021, 118, 111322.
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313.
- Nemati, S.; Kim, S.J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36.
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647.
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35.
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113.
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369.
- Hashimdeen, S.H. A Prototype for 3D Electrohydrodynamic Printing. Doctoral Dissertation, UCL (University College London), London, UK, 2016.
- Di Camillo, D.; Fasano, V.; Ruggieri, F.; Santucci, S.; Lozzi, L.; Camposeo, A.; Pisignano, D. Near-field electrospinning of conjugated polymer light-emitting nanofibers. arXiv 2013, arXiv:1310.5101.
- Torres-Giner, S. Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: New Delhi, India, 2011; pp. 108–125.
- Pant, B.; Park, M.; Park, S.J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305.
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci. 2013, 3, 121.
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087.
- George, D.; Garcia, A.; Pham, Q.; Perez, M.R.; Deng, J.; Nguyen, M.T.; Madou, M. Fabrication of patterned graphitized carbon wires using low voltage near-field electrospinning, pyrolysis, electrodeposition, and chemical vapor deposition. Microsyst. Nanoeng. 2020, 6, 7.
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789.
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired nanocomposite hydrogels with highly ordered structures. Adv. Mater. 2017, 29, 1703045.
- Chakrapani, G.; Ramakrishna, S.; Zare, M. Functionalization of electrospun nanofiber for biomedical application. J. Appl. Polym. Sci. 2023, 140, e53906.
- Dias, F.T.G.; Rempel, S.P.; Agnol, L.D.; Bianchi, O. The main blow spun polymer systems: Processing conditions and applications. J. Polym. Res. 2020, 27, 205.
- Dadol, G.C.; Kilic, A.; Tijing, L.D.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B.; Stojanovska, E.; Polat, Y. Solution blow spinning (SBS) and SBS-spun nanofibers: Materials, methods, and applications. Mater. Today Commun. 2020, 25, 101656.
- Abdal-Hay, A.; Oh, Y.S.; Yousef, A.; Pant, H.R.; Vanegas, P.; Lim, J.K. In vitro deposition of Ca-P nanoparticles on air jet spinning Nylon 6 nanofibers scaffold for bone tissue engineering. Appl. Surf. Sci. 2014, 307, 69–76.
- Zou, W.; Chen, R.Y.; Zhang, G.Z.; Zhang, H.C.; Qu, J.P. Recent advances in centrifugal spinning preparation of nanofibers. Adv. Mater. Res. 2014, 1015, 170–176.
- Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers 2023, 15, 1253.
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010, 10, 2257–2261.
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157.
- Mokhtari, F.; Salehi, M.; Zamani, F.; Hajiani, F.; Zeighami, F.; Latifi, M. Advances in electrospinning: The production and application of nanofibers and nanofibrous structures. Text. Prog. 2016, 48, 119–219.
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro-and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160.
- Ahn, S.Y.; Mun, C.H.; Lee, S.H. Microfluidic spinning of fibrous alginate carrier having highly enhanced drug loading capability and delayed release profile. RSC Adv. 2015, 5, 15172–15181.
- Ray, S.S.; Chen, S.S.; Li, C.W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514.
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572.
- Kong, L.; Ziegler, G.R. Quantitative relationship between electrospinning parameters and starch fiber diameter. Carbohydr. Polym. 2013, 92, 1416–1422.
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
851
Revisions:
2 times
(View History)
Update Date:
01 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





