You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kalliopi Alpantaki | -- | 2303 | 2024-01-31 09:11:19 | | | |
| 2 | Sirius Huang | Meta information modification | 2303 | 2024-02-01 01:40:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spernovasilis, N.; Karantanas, A.; Markaki, I.; Konsoula, A.; Ntontis, Z.; Koutserimpas, C.; Alpantaki, K. Brucella Spondylitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/54566 (accessed on 24 December 2025).
Spernovasilis N, Karantanas A, Markaki I, Konsoula A, Ntontis Z, Koutserimpas C, et al. Brucella Spondylitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/54566. Accessed December 24, 2025.
Spernovasilis, Nikolaos, Apostolos Karantanas, Ioulia Markaki, Afroditi Konsoula, Zisis Ntontis, Christos Koutserimpas, Kalliopi Alpantaki. "Brucella Spondylitis" Encyclopedia, https://encyclopedia.pub/entry/54566 (accessed December 24, 2025).
Spernovasilis, N., Karantanas, A., Markaki, I., Konsoula, A., Ntontis, Z., Koutserimpas, C., & Alpantaki, K. (2024, January 31). Brucella Spondylitis. In Encyclopedia. https://encyclopedia.pub/entry/54566
Spernovasilis, Nikolaos, et al. "Brucella Spondylitis." Encyclopedia. Web. 31 January, 2024.
Copy Citation
The most prevalent zoonotic disease is brucellosis, which poses a significant threat for worldwide public health. Particularly in endemic areas, spinal involvement is a major source of morbidity and mortality and can complicate the course of the disease.
Brucella
brucellosis
spine
spondylitis
spondylodiscitis
1. Introduction
Brucellosis is a zoonotic infection caused by the bacterial genus Brucella. Humans represent occasional hosts, but brucellosis remains a major public health problem globally and is the most common zoonotic infection. Spinal involvement may complicate the course of the disease and is a significant cause of morbidity and mortality, especially in endemic areas [1].
2. Epidemiology
Brucellosis is caused by a group of small (diameter: 0.5–0.7; length: 0.6–1.5 μm), non-motile, non-spore-forming, slow-growing, facultative intracellular, Gram-negative coccobacilli [2]. It is an ancient disease known by various names, including Mediterranean fever, Malta fever, and undulant fever. The genus Brucella was named after David Bruce in 1887. He isolated and identified the causative bacterium from the spleen of a British soldier who had died of a febrile illness that was common among military personnel stationed in Malta [3]. Twelve species are known to date [4], and each has its preferred animal host, although it can also infect other hosts [5]. The major Brucella species known to cause disease in humans are B. melitensis (sheep and goats), B. abortus (cattle, including the vaccine strain RB51), B. suis (pigs), and B. canis (dogs) [5]. The vast majority of human cases worldwide are associated with B. melitensis [6].
The disease can be transmitted to humans through the consumption of unpasteurized animal products (especially raw milk, soft cheese, butter, and ice cream), direct skin or mucous membrane contact with infected animal tissue, or inhalation of infected aerosol particles [6]. The risk of transmission is generally greater for people working with the bacteria in laboratories, slaughterhouses, veterinarians, hunters, shepherds, and meat-packing plant workers. In rare cases, human-to-human transmission has been documented through sexual contact, breastfeeding, congenital transmission, bone marrow transplantation, blood transfusion, and aerosol from an infected patient [7].
Although accurate epidemiologic data are not available for many endemic areas, it is estimated that more than 500,000 new human cases are reported worldwide each year [8]. The disease is most common in people who have travelled to or live in areas where the disease is endemic in animals along the Mediterranean basin (Portugal, Spain, Southern France, Italy, Greece, Turkey, and North Africa), Mexico, South and Central America, Eastern Europe, Asia, Africa, and the Middle East [9][10]. Even though it is a nationally notifiable disease in most countries and must be reported to the local health authorities, this is not always the case, and official numbers represent only a fraction of the actual incidence of the disease [10].
Osteoarticular involvement is one of the most common complications of brucellosis and varies in the literature from 10% to 85% of patients [11][12][13][14][15]. The wide range between reports in the literature may be due to the characteristics of the study populations, the radio-diagnostic methods used, and the different diagnostic criteria [13]. It may present as sacroiliitis, spondylitis, osteomyelitis, peripheral arthritis, bursitis, and tenosynovitis [14]. The type of skeletal involvement depends in part on the age of the patient [1]. The most common osteoarticular finding in children is monoarticular arthritis (usually of the knees and hips) [16], whereas in adults, the sacroiliac (up to 80%) and spinal (up to 54%) joints are most commonly involved [17]. According to one study, patients with osteoarticular brucellosis have a longer duration of illness before diagnosis [11].
Brucella spondylitis is among the most serious manifestations of the disease and is associated with complications such as epidural, paravertebral, and psoas abscesses, and possible resultant nerve compression [17]. The incidence of spondylitis among the cases of brucellosis varies in the literature between 2 and 60% [18]. In a review study regarding spinal brucellosis, the predominant radiologic finding was spondylitis or spondylodiscitis, which was documented in 92% of cases, followed by a pre- or paravertebral abscess at a rate of 18% [18]. According to several studies, spondylitis is more common in men and in patients aged between 50 and 60 years [11][19][20]. It mainly affects the lumbar spine, followed by the thoracic, sacral, and cervical areas [21]. The most frequently involved site of infection is the L5–S1 level [15][21]. One study showed that although the lumbar spine is most commonly affected, the involvement of the thoracic spine was more frequent in severely complicated cases [19]. Notably, multilevel vertebral involvement has been reported to occur in 2–36% of cases of Brucella spondylitis [11][18][19][20][21][22][23].
3. Pathogenesis
Brucellosis may present as a multisystemic disease. Infectious organisms have been described to reach the spine by hematogenous or non-hematogenous routes, such as direct external bacterial inoculation or contiguous spread from an adjacent infectious site [24]. As for Brucella species, they mainly spread to the spine hematogenously through the nutrient arterioles of the vertebral bodies [25] or, rarely, by retrograde flow through the venous plexus of Batson, which was first described in an attempt to explain the preference of metastatic disease for the posterior aspect of the vertebral body [26][27]. As the vascularization of the vertebral bodies has been meticulously studied, the natural history of Brucella spondylitis can be explained sufficiently. Early Brucella spondylitis involves the anterior portion of the vertebral rim as the arterial vascularization of the vertebral bodies is anatomically denser on that surface [28]. Later, the infection progresses to the remainder of the vertebral body using the medullary spaces, eventually reaching the disc annulus and the nucleus pulposus [1][25]. It is worth noting that in adult life intra-osseous arteries are end arteries and therefore, in the event of septic emboli entrapment, extensive destruction of the vertebral body cannot be prevented by the presence of an anastomotic network [29]. The most commonly affected sites are the lumbar spine, followed by the thoracic and cervical spine, while multilevel involvement has also been described [21][30].
Before diving deeper into the pathophysiological mechanisms that orchestrate the deleterious effects of a Brucella infection on joints and bones, this section will first analyze the key aspects of normal bone physiology. Bone is primarily comprised of cells and an extracellular matrix, the osteoid, which becomes mineralized after the deposition of calcium and phosphate in the form of hydroxyapatite, a process essential for the structural integrity of the bone. There are three types of bone cells: osteoblasts, osteoclasts, and osteocytes [31]. Osteoblasts are bone-forming cells responsible for bone mineralization and the production of the receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin, which induce and suppress osteoclastogenesis, respectively [32]. Osteocytes are terminally differentiated osteoblasts that become entrapped in the mineralized matrix [31]. Finally, osteoclasts are bone-resorbing cells with the unique ability to digest the calcified bone matrix. Until recently, it was established that the formation of osteoclasts can be accomplished either by the fusion of osteoclast progenitor cells that originate from the monocyte/macrophage lineage of the bone marrow or through the differentiation of osteal macrophages, which are the bone marrow resident macrophages [33][34]. Nonetheless, the latest research has demonstrated that peripheral blood mononuclear cells can also fuse and become mature multinucleated osteoblasts and that these may significantly contribute to the bone damage seen during inflammatory conditions such as rheumatoid arthritis [35][36].
Bone is often regarded as a metabolically inert structure with an innate resistance to infection. Nevertheless, osteoarticular brucellosis is the most frequent complication of a Βrucella infection in humans [11][12]. The underlying mechanisms involved in this process have only recently been elucidated (Figure 1). The available data are mainly derived from research regarding B. abortus but can be safely used for the understanding of the pathogenesis of Brucella spondylitis in general. By now, it is evident that Brucella’s success as a pathogen relies on its ability to maintain an intracellular lifestyle, primarily by invading and replicating within macrophages. However, macrophages are not the only intracellular niche that Brucella can penetrate [37]. Firstly, it has been established that B. abortus can infect and replicate within osteoblasts in vitro [38][39]. Once inside osteoblasts, Βrucella interferes with the physiological functions of these cells via, principally, three mechanisms: the induction of osteoblast apoptosis and the hampering of their differentiation; the inhibition of mineralization and organic matrix deposition; and the upregulation of RANKL [39]. These changes are the result of the direct effect of Brucella on osteoblasts, but also the result of Brucella-infected macrophages, the ones that already reside in the bone and the ones that are attracted to the site of infection. The induction of apoptosis is largely dependent upon the phosphorylation of p38 and extracellular signal-regulated kinase 1 and 2 (ERK1/2), which is activated in Brucella-infected osteoblasts. P38 and ERK1/2 are mitogen-activated protein kinases (MAPK) that regulate a plethora of functions in terms of cell growth, development, and survival [40]. Another critical function of these pathways is the production of monocyte chemotactic protein 1 (MCP-1) by osteoblasts, which is responsible for the attraction of monocytes and macrophages to the site of infection. In turn, these cells secrete tumor necrosis factor alpha (TNF-a) that results in osteoblast apoptosis, decreased bone mineralization, and upregulation of RANKL [39].
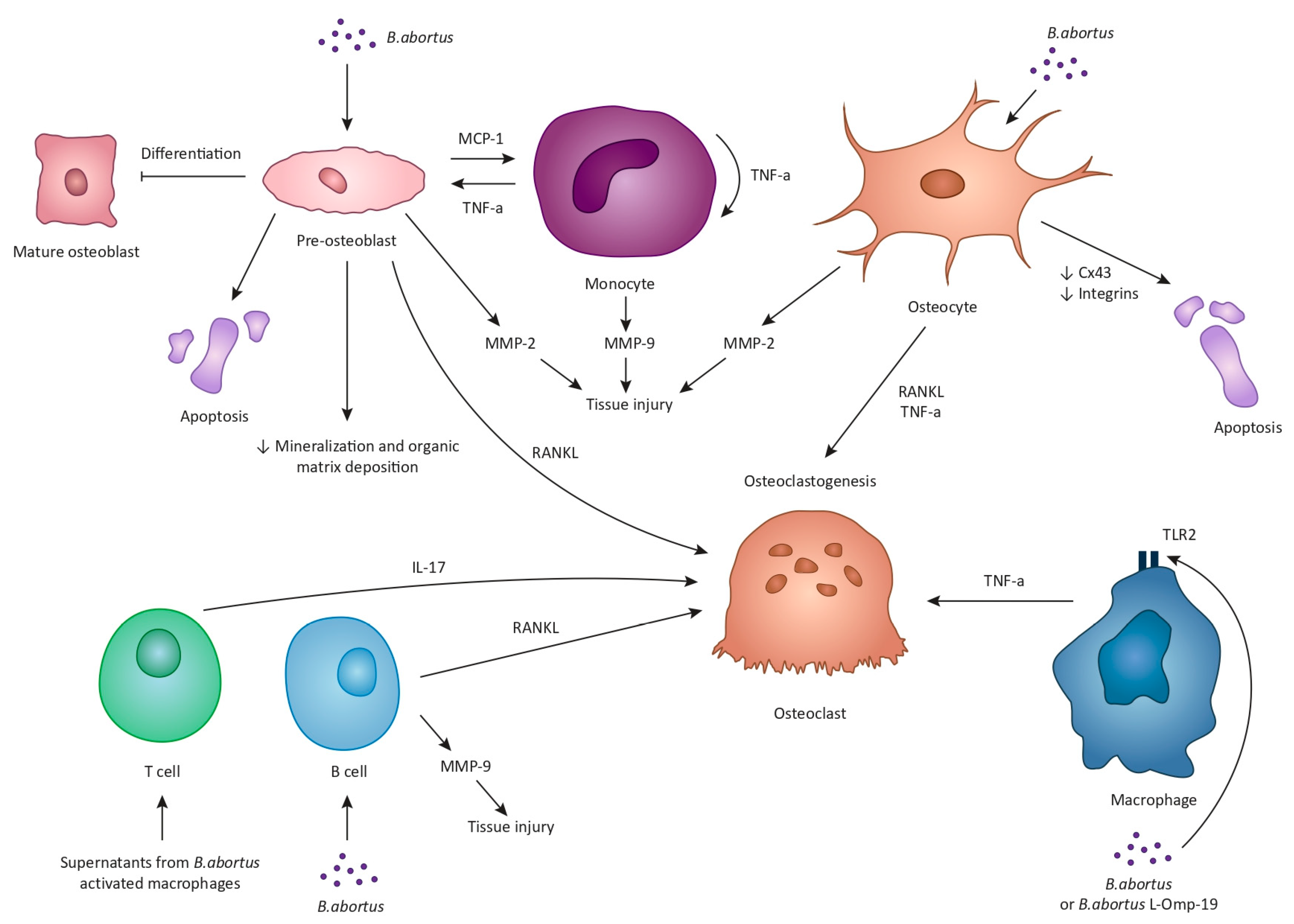
Figure 1. The underlying mechanisms of Brucella-induced osteoarticular disease are multiple, complex, and largely rely on experimental data from B. abortus studies. B. abortus can infect and replicate within osteoblasts and interfere with the physiological functions of these cells via three mechanisms: the induction of osteoblast apoptosis and the hampering of their differentiation; the inhibition of mineralization and organic matrix deposition; and the upregulation of receptor activator of nuclear factor kappa-B ligand (RANKL). Brucella-infected osteoblasts also secrete monocyte chemotactic protein 1 (MCP-1) that attracts monocytes and macrophages to the site of infection. In turn, these cells secrete tumor necrosis factor alpha (TNF-a) that, similarly, results in osteoblast apoptosis, decreased bone mineralization, and upregulation of RANKL. Brucella-infected osteoblasts and monocytes can also secrete matrix metalloproteinases, MMP-2 and MMP-9, respectively. Specifically, MMP-9 production is the result of the autocrine function of TNF-a produced by monocytes. Additionally, Brucella can multiply within osteocytes and lead to the production of MMP-2, RANKL, TNF-a, and proinflammatory cytokines. Moreover, Brucella and supernatants from Brucella-infected macrophages inhibit the expression of connexin 43 along with the expression of integrins, ultimately leading to osteocyte apoptotic cell death. Upon Brucella infection or in response to B. abortus, lipidated outer membrane protein 19 macrophages release inflammatory mediators such as TNF-a, eventually enhancing osteoclastogenesis. Moreover, supernatants from B. abortus-activated macrophages stimulate T cells to produce interleukin-17 which promotes osteoclast differentiation through the induction of proinflammatory cytokines. Finally, B. abortus-infected B cells produce MMP-9 and RANKL.
Matrix metalloproteinases (MMPs) also contribute to the osteoarticular damage in the context of brucellosis. Specifically, two types of MMPs, MMP-2 and MMP-9, which aid in the degradation of type I collagen present in bones and type II collagen present in cartilage, have been demonstrated to be involved in Brucella-induced tissue injury [41][42]. In particular, in vitro studies have shown that B. abortus-infected osteoblasts produce MMP-2 in a process that is largely mediated by the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by the same cells [42]. In addition, as mentioned above, Brucella-infected osteoblasts produce MCP-1 that attracts monocytes, which then secrete MMP-9 [42]. MMP-9 production is the result of the autocrine function of TNF-a produced by monocytes in response to GM-CSF [42].
Brucella can also infect and multiply within osteocytes in vitro [43]. Infected osteocytes then secrete MMP-2, RANKL, TNF-a, and proinflammatory cytokines [43]. This response ultimately leads bone marrow-derived monocytes (BMM) to undergo osteoclastogenesis. At this point it should be mentioned that one of the ways by which coordinated communication among osteocytes and between osteocytes and osteoblasts is achieved is via gap junctions, and the most abundant protein in these gap junctions is connexin 43 (Cx43) [44]. Interestingly, the B. abortus infection has been found to reduce the expression of Cx43 [43]. Moreover, the interaction between osteocytes and supernatants from Brucella-infected macrophages inhibits the expression of Cx43 along with the expression of integrins [43], which also participate in osteocyte adhesion and signaling [45]. The outcome of these changes is osteocyte apoptotic cell death [43]. Based on these findings, it can be safely deducted that Brucella harms osteocyte activity and viability, directly and indirectly, thus contributing to the tissue damage observed in an osteoarticular infection.
The role of macrophages and monocytes in the pathophysiology of tissue damage noted in Brucella infections is not limited to their interaction with osteoblasts and osteocytes. Upon infection with Brucella or in response to Brucella lipoproteins, such as the lipidated outer membrane protein 19 (L-Omp19), macrophages release inflammatory mediators such as TNF-a, interleukin-6 (IL-6), and IL-1β in a toll-like receptor 2-dependent manner (TLR2) [46]. In turn, TNF-a production results in the differentiation of BMM into osteoclasts [46]. Another intriguing observation is that supernatants from B. abortus-infected monocytes or L-Omp19-stimulated monocytes are able to induce, again, through TNF-a production, the differentiation of human monocytes to osteoclasts [46]. It should be pointed out that osteoclastogenesis associated with B. abortus does not require bacterial viability but is equally elicited by structural bacterial components. It is established that these components are the Brucella lipoproteins but not the Brucella lipopolysaccharide [46][47].
Brucella affects the bone tissue not only through macrophages and monocytes but also through T cells and B cells by exploiting them to induce bone loss. Specifically, stimulation of activated T cells with supernatants from B. abortus-activated macrophages results in the production of RANKL and IL-17 which promote osteoclastogenesis in vitro [48]. In addition, it appears that IL-17 is the main driving force for osteoclast differentiation through the induction of proinflammatory cytokines, primarily TNF-a, by osteoclast precursors [48]. This phenomenon has also been replicated in vivo when injection of mice tibiae with T cells that were treated with supernatants from Brucella-infected macrophages induced extensive osteoclastogenesis [48]. Similarly, B. abortus-infected B cells produce MMP-9, proinflammatory cytokines, and RANKL, the latter being the main mediator of B cell-induced osteoclastogenesis in vitro [49].
Finally, the role of several cytokines, their receptors, and single-nucleotide polymorphisms for cytokine-encoded genes in the inflammatory damaged observed during Brucella spondylitis is still unclear and demands further research [50][51][52].
In summary, the osteoarticular damage observed in a Brucella infection is the aftereffect of the direct changes that the bacterium causes on bone cells and also the result of the intricate interactions between Brucella, bone cells, and the immune system.
References
- Tali, E.T.; Koc, A.M.; Oner, A.Y. Spinal brucellosis. Neuroimaging Clin. N. Am. 2015, 25, 233–245.
- Percin, D. Microbiology of Brucella. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 13–17.
- Bruce, D. Note on Discovery of a Micrococcus in Malta Fever. Practicioner 1887, 39, 161–170.
- El-Sayed, A.; Awad, W. Brucellosis: Evolution and expected comeback. Int. J. Vet. Sci. Med. 2018, 6, S31–S35.
- Rajendhran, J. Genomic insights into Brucella. Infect. Genet. Evol. 2021, 87, 104635.
- Pappas, G.; Akritidis, N.; Bosilkovski, M.; Tsianos, E. Brucellosis. N. Engl. J. Med. 2005, 352, 2325–2336.
- Tuon, F.F.; Gondolfo, R.B.; Cerchiari, N. Human-to-human transmission of Brucella—A systematic review. Trop. Med. Int. Health 2017, 22, 539–546.
- CDC. CDC Yellow Book 2024: Health Information for International Travel; Oxford University Press: Oxford, UK, 2023.
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99.
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398.
- Bosilkovski, M.; Krteva, L.; Caparoska, S.; Dimzova, M. Osteoarticular involvement in brucellosis: Study of 196 cases in the Republic of Macedonia. Croat. Med. J. 2004, 45, 727–733.
- Geyik, M.F.; Gur, A.; Nas, K.; Cevik, R.; Sarac, J.; Dikici, B.; Ayaz, C. Musculoskeletal involvement of brucellosis in different age groups: A study of 195 cases. Swiss Med. Wkly. 2002, 132, 98–105.
- Buzgan, T.; Karahocagil, M.K.; Irmak, H.; Baran, A.I.; Karsen, H.; Evirgen, O.; Akdeniz, H. Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int. J. Infect. Dis. 2010, 14, e469–e478.
- Arkun, R.; Mete, B.D. Musculoskeletal brucellosis. Semin. Musculoskelet. Radiol. 2011, 15, 470–479.
- Turan, H.; Serefhanoglu, K.; Karadeli, E.; Togan, T.; Arslan, H. Osteoarticular involvement among 202 brucellosis cases identified in Central Anatolia region of Turkey. Intern. Med. 2011, 50, 421–428.
- Bosilkovski, M.; Kirova-Urosevic, V.; Cekovska, Z.; Labacevski, N.; Cvetanovska, M.; Rangelov, G.; Cana, F.; Bogoeva-Tasevska, S. Osteoarticular involvement in childhood brucellosis: Experience with 133 cases in an endemic region. Pediatr. Infect. Dis. J. 2013, 32, 815–819.
- Esmaeilnejad-Ganji, S.M.; Esmaeilnejad-Ganji, S.M.R. Osteoarticular manifestations of human brucellosis: A review. World J. Orthop. 2019, 10, 54–62.
- Turgut, M.; Turgut, A.T.; Kosar, U. Spinal brucellosis: Turkish experience based on 452 cases published during the last century. Acta Neurochir. 2006, 148, 1033–1044, discussion 1044.
- Ulu-Kilic, A.; Karakas, A.; Erdem, H.; Turker, T.; Inal, A.S.; Ak, O.; Turan, H.; Kazak, E.; Inan, A.; Duygu, F.; et al. Update on treatment options for spinal brucellosis. Clin. Microbiol. Infect. 2014, 20, O75–O82.
- Bozgeyik, Z.; Ozdemir, H.; Demirdag, K.; Ozden, M.; Sonmezgoz, F.; Ozgocmen, S. Clinical and MRI findings of brucellar spondylodiscitis. Eur. J. Radiol. 2008, 67, 153–158.
- Bozgeyik, Z.; Aglamis, S.; Bozdag, P.G.; Denk, A. Magnetic resonance imaging findings of musculoskeletal brucellosis. Clin. Imaging 2014, 38, 719–723.
- Ozaksoy, D.; Yucesoy, K.; Yucesoy, M.; Kovanlikaya, I.; Yuce, A.; Naderi, S. Brucellar spondylitis: MRI findings. Eur. Spine J. 2001, 10, 529–533.
- Harman, M.; Unal, O.; Onbasi, K.T.; Kiymaz, N.; Arslan, H. Brucellar spondylodiscitis: MRI diagnosis. Clin. Imaging 2001, 25, 421–427.
- Mavrogenis, A.F.; Megaloikonomos, P.D.; Igoumenou, V.G.; Panagopoulos, G.N.; Giannitsioti, E.; Papadopoulos, A.; Papagelopoulos, P.J. Spondylodiscitis revisited. EFORT Open Rev. 2017, 2, 447–461.
- Morales, H. Infectious Spondylitis Mimics: Mechanisms of Disease and Imaging Findings. Semin. Ultrasound CT MR 2018, 39, 587–604.
- Batson, O.V. The Function of the Vertebral Veins and Their Role in the Spread of Metastases. Ann. Surg. 1940, 112, 138–149.
- Turunc, T.; Demiroglu, Y.Z.; Uncu, H.; Colakoglu, S.; Arslan, H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J. Infect. 2007, 55, 158–163.
- Ratcliffe, J.F. Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis. A microarteriographic investigation. Acta Radiol. Diagn. 1985, 26, 137–143.
- Ratcliffe, J.F. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. A microarteriographic study. J. Anat. 1982, 134, 373–382.
- Chelli Bouaziz, M.; Ladeb, M.F.; Chakroun, M.; Chaabane, S. Spinal brucellosis: A review. Skelet. Radiol. 2008, 37, 785–790.
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275.
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011, 343, 289–302.
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Recent Advances in Osteoclast Biological Behavior. Front. Cell Dev. Biol. 2021, 9, 788680.
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front. Immunol. 2021, 12, 664871.
- Sprangers, S.; Schoenmaker, T.; Cao, Y.; Everts, V.; de Vries, T.J. Different Blood-Borne Human Osteoclast Precursors Respond in Distinct Ways to IL-17A. J. Cell Physiol. 2016, 231, 1249–1260.
- Iwamoto, N.; Kawakami, A. The monocyte-to-osteoclast transition in rheumatoid arthritis: Recent findings. Front. Immunol. 2022, 13, 998554.
- Roop, R.M., 2nd; Barton, I.S.; Hopersberger, D.; Martin, D.W. Uncovering the Hidden Credentials of Brucella Virulence. Microbiol. Mol. Biol. Rev. 2021, 85, e00021-19.
- Delpino, M.V.; Fossati, C.A.; Baldi, P.C. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 2009, 77, 984–995.
- Scian, R.; Barrionuevo, P.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus invasion of osteoblasts inhibits bone formation. Infect. Immun. 2012, 80, 2333–2345.
- Roskoski, R., Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143.
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543.
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Granulocyte-macrophage colony-stimulating factor- and tumor necrosis factor alpha-mediated matrix metalloproteinase production by human osteoblasts and monocytes after infection with Brucella abortus. Infect. Immun. 2011, 79, 192–202.
- Pesce Viglietti, A.I.; Arriola Benitez, P.C.; Gentilini, M.V.; Velasquez, L.N.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus Invasion of Osteocytes Modulates Connexin 43 and Integrin Expression and Induces Osteoclastogenesis via Receptor Activator of NF-kappaB Ligand and Tumor Necrosis Factor Alpha Secretion. Infect. Immun. 2016, 84, 11–20.
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192.
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos. Rep. 2019, 17, 195–206.
- Delpino, M.V.; Barrionuevo, P.; Macedo, G.C.; Oliveira, S.C.; Genaro, S.D.; Scian, R.; Miraglia, M.C.; Fossati, C.A.; Baldi, P.C.; Giambartolomei, G.H. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J. Leukoc. Biol. 2012, 91, 285–298.
- Giambartolomei, G.H.; Zwerdling, A.; Cassataro, J.; Bruno, L.; Fossati, C.A.; Philipp, M.T. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 2004, 173, 4635–4642.
- Giambartolomei, G.H.; Scian, R.; Acosta-Rodriguez, E.; Fossati, C.A.; Delpino, M.V. Brucella abortus-infected macrophages modulate T lymphocytes to promote osteoclastogenesis via IL-17. Am. J. Pathol. 2012, 181, 887–896.
- Pesce Viglietti, A.I.; Arriola Benitez, P.C.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus-infected B cells induce osteoclastogenesis. Microbes Infect. 2016, 18, 529–535.
- Hu, X.; Shang, X.; Wang, L.; Fan, J.; Wang, Y.; Lv, J.; Nazierhan, S.; Wang, H.; Wang, J.; Ma, X. The role of CXCR3 and its ligands expression in Brucellar spondylitis. BMC Immunol. 2020, 21, 59.
- Fu, J.; He, H.Y.; Ojha, S.C.; Shi, H.; Sun, C.F.; Deng, C.L.; Sheng, Y.J. Association of IL-6, IL-10 and TGF-beta1 gene polymorphisms with brucellosis: A systematic review with meta-analysis. Microb. Pathog. 2019, 135, 103640.
- Zafari, P.; Zarifian, A.; Alizadeh-Navaei, R.; Taghadosi, M.; Rafiei, A. Association between polymorphisms of cytokine genes and brucellosis: A comprehensive systematic review and meta-analysis. Cytokine 2020, 127, 154949.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
781
Revisions:
2 times
(View History)
Update Date:
01 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




