Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hongyu Ding | -- | 2759 | 2024-01-31 07:57:09 | | | |
| 2 | Rita Xu | Meta information modification | 2759 | 2024-02-02 07:22:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ding, H.; Zhang, Q.; Yao, K. High Entropy Amorphous Alloys. Encyclopedia. Available online: https://encyclopedia.pub/entry/54559 (accessed on 07 February 2026).
Ding H, Zhang Q, Yao K. High Entropy Amorphous Alloys. Encyclopedia. Available at: https://encyclopedia.pub/entry/54559. Accessed February 07, 2026.
Ding, Hongyu, Qi Zhang, Kefu Yao. "High Entropy Amorphous Alloys" Encyclopedia, https://encyclopedia.pub/entry/54559 (accessed February 07, 2026).
Ding, H., Zhang, Q., & Yao, K. (2024, January 31). High Entropy Amorphous Alloys. In Encyclopedia. https://encyclopedia.pub/entry/54559
Ding, Hongyu, et al. "High Entropy Amorphous Alloys." Encyclopedia. Web. 31 January, 2024.
Copy Citation
High entropy amorphous alloys (HEAAs) are materials that have received much attention in recent years. They exhibit many unique properties; however, research on their composition design method has not been deep enough.
high entropy alloy
amorphous alloy
bulk metallic glass
glass-forming ability
1. Introduction
Material innovation has become one of the most important driving forces for promoting human civilization progress, as well as promoting the development of technology and industrial upgrading. Amorphous alloys and high entropy alloys are two types of high-performance materials that have developed rapidly in the past several decades. Since its first report in 1960 [1], amorphous alloys have undergone significant development and have now expanded to dozens of material systems. Inoue et al. developed a copper mold casting method that greatly reduced fabrication costs [2][3]. Peker and Johnson designed a very famous amorphous alloy, Zr41.2Ti13.8Cu12.5Ni10Be22.5, which was also called Vit1 since it possesses very good glass-forming ability (GFA) [4]. Vit1 played an important role in promoting the industrialization of amorphous alloys. Inoue presented a long review of amorphous alloys, and it got more than 5600 citations [5]. After entering the 21st century, more and more amorphous alloys were developed, such as Cu–Zr–Ti–Sn [6], Ni–Nb–Sn [7], Pt–Co–Ni–Cu–P [8], Zr–Al–Co [9], Zr–Cu–Al [10], Cu–Zr–Ag [11], etc.
One of the main challenges in developing amorphous alloys is how to improve its GFA. Li et al. found that similar atom substitution may be an effective way [12][13]. Santos et al. proposed a topological instability (λ) criterion to evaluate GFA in an Ni–Nb–Zr system [14]. Zhang et al. developed the Ti32.8Zr30.2Ni5.3Cu9Be22.7 quinary bulk amorphous alloy; it possesses good GFA and its critical diameter exceeds 50 mm [15]. Nishiyama et al. prepared the world’s biggest glassy alloy, namely the Pd42.5Cu30Ni7.5P20 cylindrical glassy alloy sample. Its diameter was 80 mm and it was obtained by fluxing and water quenching method [16]. Apart from experiments, several parameters were proposed for evaluating the GFA of an alloy, such as Trg [17], ΔTx [18], γ [19], etc. The atomic simulation method was also applied to enhance the GFA of Ni–Nb–Ti amorphous alloys by Li et al. [20]. Amorphous alloys possess outstanding performance, such as high strength, high hardness, good corrosion resistance, and wear resistance, etc. As a result, amorphous alloys are used as low-loss power transformers, precise forming parts, micro-electro-mechanical system components, etc.
The concept of a high entropy alloy (HEA) has received widespread attention from the material research community since its first report in 2004 [21]. High entropy alloys usually contain five or more elements while the concentration of each element was in the range between 5% and 35%. In other words, the configurational entropy of high entropy alloy should be greater than 1.5 R, in which R represents the ideal gas constant (8.314 J/(mol·K)). For alloys containing five elements with equal atomic concentration, the configurational entropy reaches 1.61 R. In this sense, they are called high entropy alloys. In thermodynamics, entropy is a parameter characterizing the degree of disorder in a system. The degree of disorder increases as the number of constituent elements increases. Unlike traditional alloys that are based on one or two principle elements with other elements as minor additions, high entropy alloys belong to multicomponent, non-principle element alloy systems. Due to its breakthrough in traditional alloy design concepts, a new door for material research has opened up, making thousands of material combinations possible. As indicated by calculation, an array choice including 13 mutually miscible metallic elements enables 7099 high entropy alloy systems with 5 to 13 elements in equal molar ratios [21]. It provides a wide range of space and possibilities for developing new alloys.
Cantor et al. developed a series of multicomponent alloys. It was found that the total number of phases is always well below the maximum equilibrium number allowed by the Gibbs phase rule. Among them, a Fe20Cr20Mn20Ni20Co20 alloy possesses an FCC structure [22]. This alloy was called “Cantor alloy”, and it was intensively studied by other researchers. For example, Gludovatz et al. found that the Cantor alloy possesses exceptional damage tolerance with tensile strengths above 1 GPa and fracture toughness values exceeding 200 MPa·m1/2; it is fracture resistant for cryogenic application [23]. Shahmir et al. provided an overview on microstructural engineering of the Cantor alloy in the past twenty years [24]. Zhang et al. proposed that phase formation of HEA can be separated by mixing enthalpy ΔHmix and atomic-size difference δ, it provides important guidance in designing HEAs with desired phases and microstructure [25]. Yamabe-Mitarai et al. studied the stability of Ti-containing high-entropy alloys, it was found that strengths of the BCC HEAs were greater than those of the HCP HEAs at 873 K, they were also greater than that of the commercial Ti alloy TIMETAL 834, indicating that BCC HEAs may be applied at elevated temperatures [26]. Uporov et al. found that ScGdTbDyHo HEA possesses good magnetocaloric properties and it can be influenced by the synthesis route [27]. Most HEAs were prepared by casting method; recently it was found that additive manufacturing characterized by net-shape processing is suitable for elevating the properties of HEAs [28][29][30]. Overall, great progress has been made in phase forming rules, composition design, processing, and application of HEA under various circumstances; they may be potential materials applied in many fields, such as heat-resistant and wear-resistant coatings, magnetic materials, and extreme high/low-temperature materials, etc.
Many factors could affect the phase formation of HEAs. In most cases, HEAs form solid solutions (especially BCC, FCC, and HCP) or intermetallics. However, under certain conditions, an amorphous structure could also be formed; this is high entropy amorphous alloy (HEAA). HEAAs possess both the long-range disordered atomic structure stacking characteristics of amorphous alloys and compositional complex characteristics in high entropy alloys. They are a new type of multiple component-disordered alloy. In other words, HEAA comprises five or more elements with an atomic ratio of each element between 5% and 35%, while it possesses an amorphous structure at room temperature. From a scientific research perspective, HEAAs provide a model material connecting amorphous alloys and high entropy alloys; it is helpful for intensive research on the amorphous forming rule of amorphous alloys and the phase evolution mechanism of high entropy alloys. From an industrial application perspective, due to complicated composition and structural characteristics, HEAAs exhibit a series of unique physical, chemical, and mechanical properties; they may be applied on certain specific occasions.
So far, positive progress has been achieved and dozens of HEAAs have been developed. The first batch of HEAAs was reported in 2002 by Ma et al., namely Ti20Zr20Hf20Cu20M20 (M = Fe, Co, Ni) alloys. Among them, the Ti20Zr20Hf20Cu20Ni20 alloy can form bulk metallic glass (BMG) with a critical diameter of 1.5 mm. They were called multicomponent glassy alloys or non-base glassy alloys at that time [31]. Later in 2011, the research work continued. Zhao et al. prepared a Zn20Ca20Sr20Yb20(Li0.55Mg0.45)20 BMG and it possesses homogeneous flow behavior at room temperature [32]. Takeuchi et al. developed the Pd20Pt20Cu20Ni20P20 alloy, its critical diameter reaches 10 mm and the concept of high entropy bulk metallic glass (HE-BMG) was proposed [33]. Li et al. developed CaMgZnSrYb HE-BMG with good biodegradable properties [34]. Later, Yao’s group developed a series of Ti–Zr–(Hf)–Cu–Ni–Be HE-BMGs with good GFA [35][36][37][38]; the composition design method will be discussed in detail later. Kim et al. developed Er–Gd–Y–Al–Co HE-BMGs and found that the relation between the fragility and elastic properties of these alloys is quite different from traditional BMGs [39]. Xu et al. developed Fe25Co25Ni25 (P, C, B, Si)25 HE-BMGs with good magnetic properties [40]. Huo et al. developed a denary HE-BMG with a large magnetocaloric effect [41]. Bizhanova et al. developed quinary Zr31Ti27Be26Cu10M6 (M = Ag, Al, Ni, V, Cr, and Fe) and senary Zr28Ti24Be23Cu9Ni10N6 (N = V, Cr, Fe, Ag, and Al) alloys with critical diameters of 6–15 mm [42]. Inoue et al. found that Fe43Cr16Mo16C15B10 HE-BMG and Zr–Al–(TM1, TM2) pseudo-HE-BMG can confer useful heat resistance at elevated temperatures [43]. Wada et al. developed septenary Zr–Hf–Ti–Al–Co–Ni–Cu high-entropy bulk metallic glasses with centimeter-scale glass-forming ability [44]. Panahi et al. studied the glass forming range of (FeCoCrNi)–(B,Si) HEAAs, the crystallization process, and the influence of Si element on the microstructure was elucidated [45]. Szyba et al. studied structural and electrochemical properties of resorbable Ca32Mg12Zn38Yb18–xBx (x = 1, 2, 3) metallic glasses in Ringer’s solution; it was found that the HEAA had significantly higher corrosion resistance than CaMgZn alloys [46]. Law et al. compared the magnetocaloric properties of amorphous and crystalline HEAs; it was found that the magneto-entropy change of HEAAs was generally larger than its crystalline counterpart, while the transition temperature was relatively lower [47]. Calin et al. found that Ti–Zr–Nb–Hf–Si HEAAs exhibit excellent corrosion properties in simulated body fluids. Moreover, its weak paramagnetic nature and superior radiopacity offer compatibility with medical diagnostic imaging systems [48]. Jalali et al. studied the thermal and deformation behavior of Zr33Hf8Ti6Cu32Ni10Co5Al6 HE-BMG; the correlation between fragility, structural relaxation enthalpy, diffusion, free volume and deformation behavior was discussed compared with the Cu–Zr–Al prototype BMG [49][50]. Jia et al. created a nanosponge-like architecture from PdPtCuNiP HEAA; it possesses outstanding hydrogen evolution reaction activity [51]. Makarov et al. studied temperature dependencies of enthalpy change in the initial (as-quenched) and relaxed (aged) HE-BMGs; the calculated results agreed with interstitialcy theory [52]. Alvi et al. reported that a thin film of HfMoNbTaTiVWZr HEAA showed thermal stability up to 750 °C, and it can resist Ar-ion irradiation [53].
2. Composition Design of HEAAs
2.1. Designing HEAA Based on Quinary Bulk Metallic Glasses
It is well known that the key point for preparing amorphous alloys is avoiding crystallization of high-temperature alloy melt during the cooling process. When an amorphous alloy could be obtained at a low cooling rate, or the critical size for obtaining an amorphous metallic sample is large, the alloy is recognized as possessing good or large glass-forming ability (GFA). Among traditional amorphous alloys, Zr41.2Ti13.8Cu12.5Ni10Be22.5 (Vit1) [4] and Ti32.8Zr30.2Ni5.3Cu9Be22.7 [15] quinary bulk amorphous alloys possess good GFA and a critical diameter over 50 mm. It implies that the five elements, Ti, Zr, Cu, Ni, and Be, are structural and chemically compatible to form bulk metallic glasses. So it is reasonable to suppose that the Ti20Zr20Cu20Ni20Be20 high entropy alloy may possess good glass-forming ability and a big glassy sample might be made.
Figure 1 shows the composition design approach of the Ti20Zr20Cu20Ni20Be20 HEAA. An equal-atomic Ti20Zr20Cu20Ni20Be20 high entropy alloy was designed from quinary BMGs with good GFA. The Ø3 mm Ti20Zr20Cu20Ni20Be20 rod sample was prepared by the copper mold casting technique. Its XRD spectra was shown in Figure 2a. No sharp diffraction peak corresponding to the crystalline phase was observed in the Ø3 mm Ti20Zr20Cu20Ni20Be20 sample, indicating that this alloy possesses a fully amorphous structure. However, the critical diameter of the Ti20Zr20Cu20Ni20Be20 BMG sample is only 3 mm, much smaller than that of Zr41.2Ti13.8Cu12.5Ni10Be22.5 (Vit1) and Ti32.8Zr30.2Ni5.3Cu9Be22.7 BMGs. The glass transition temperature (Tg), initial crystallization temperature (Tx), melting temperature (Tm), and liquidus temperature (Tl) are marked with arrows in Figure 3. Tg, Tx, Tm, and Tl are measured to be 683 K, 729 K, 1076 K, and 1161 K, respectively. This high-entropy BMG possesses a high compressive fracture strength of 2315 MPa for Ø3 mm × 6 mm sample, higher than that of Vit1 alloy, which is attributed to high entropy effect as well as high Ni content (Figure 4) [35]. In a uniaxial compressive experiment, it breaks in a brittle manner without plasticity. The present result provides a successful example of HEAA composition design by selecting five elements from quinary BMG with good GFA, despite the fact that the GFA of the designed high-entropy BMG is not large enough. Then, further study for improving the GFA of the high-entropy amorphous alloys is important and necessary.
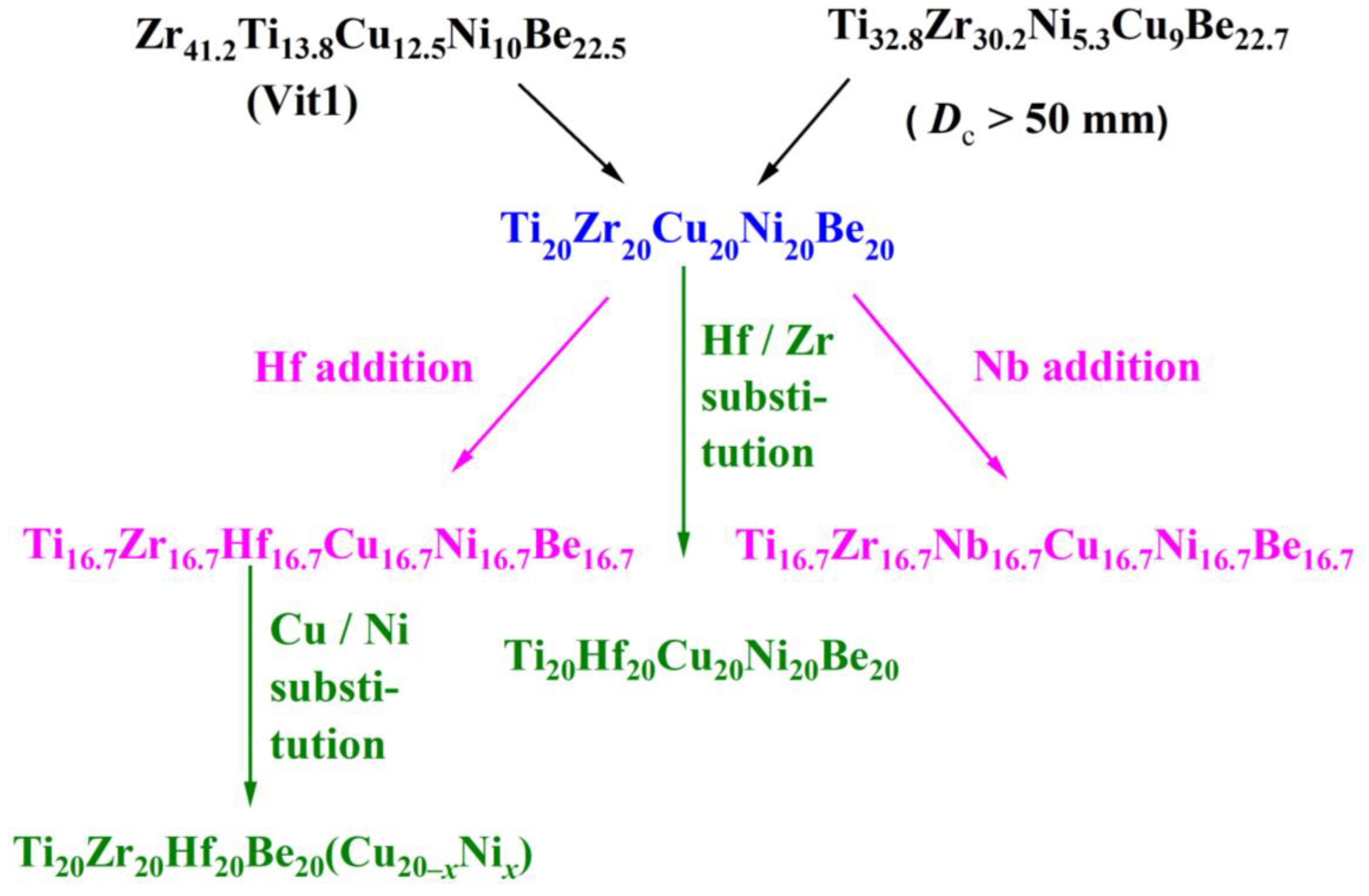
Figure 1. Composition design approach for Ti–(Zr, Hf, Nb)–Cu–Ni–Be HEAAs.
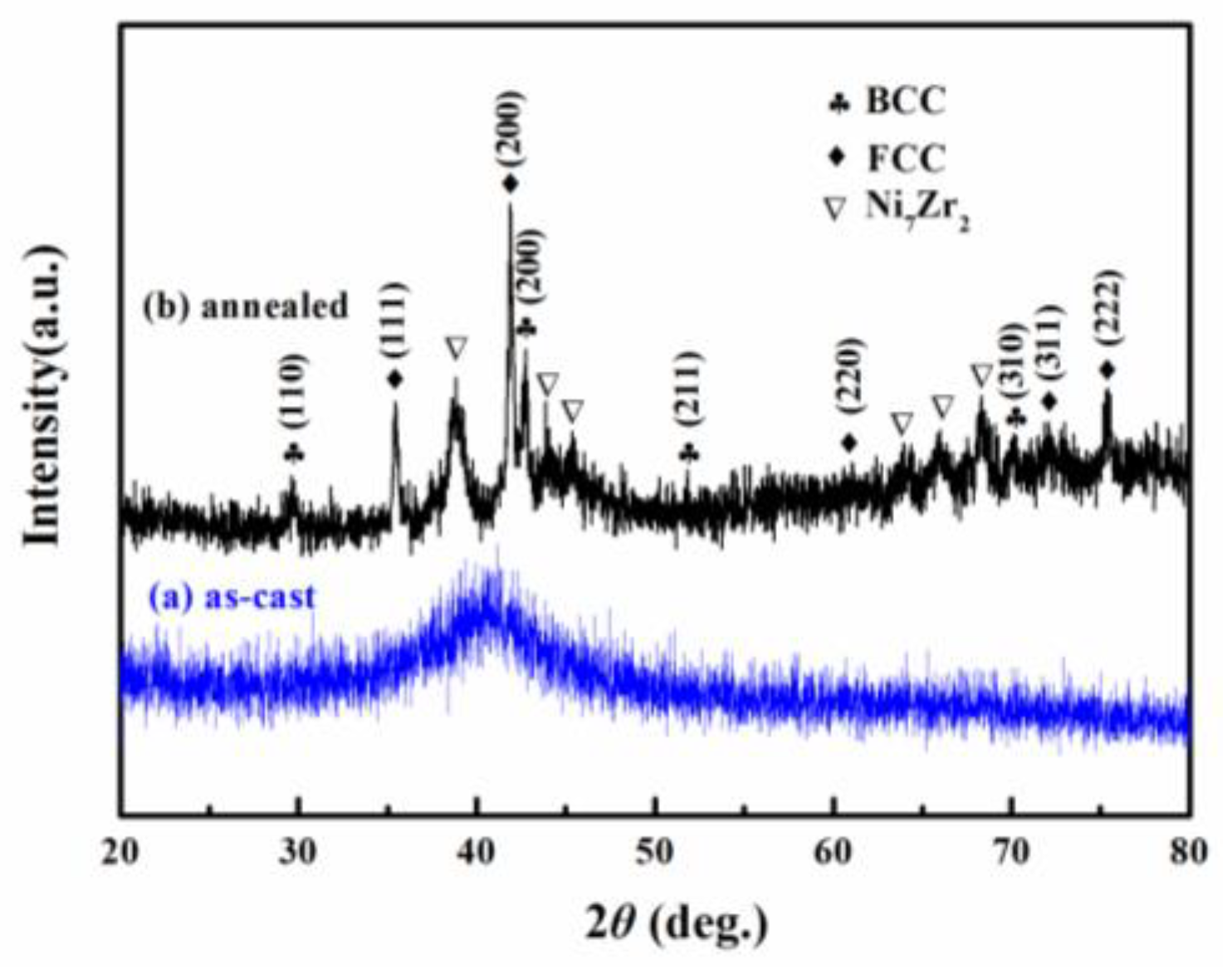
Figure 2. XRD patterns of Ø3 mm Ti20Zr20Cu20Ni20Be20 rod sample (a) as-cast, (b) annealed.
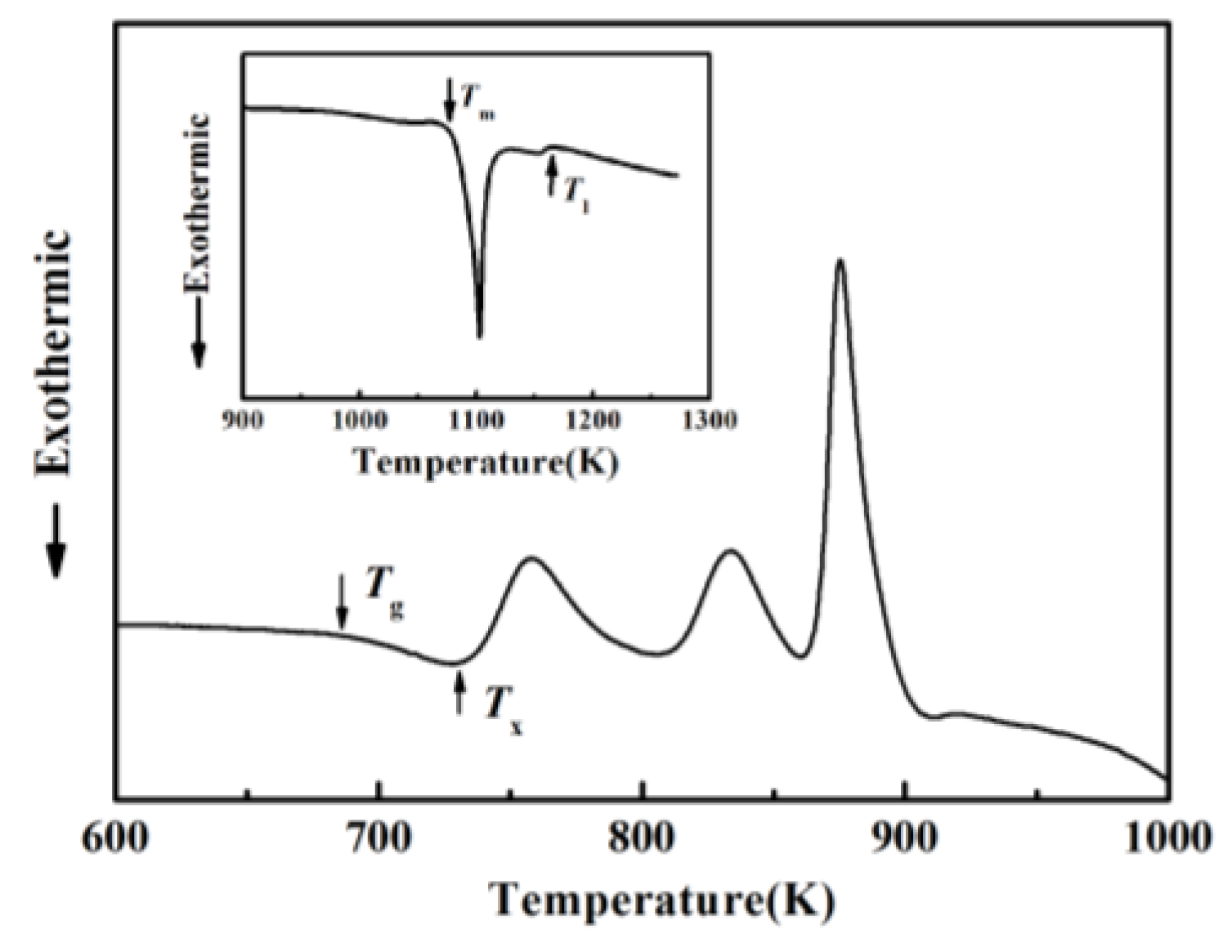
Figure 3. DSC curve of Ø3 mm Ti20Zr20Cu20Ni20Be20 sample. Inset shows melting behavior.
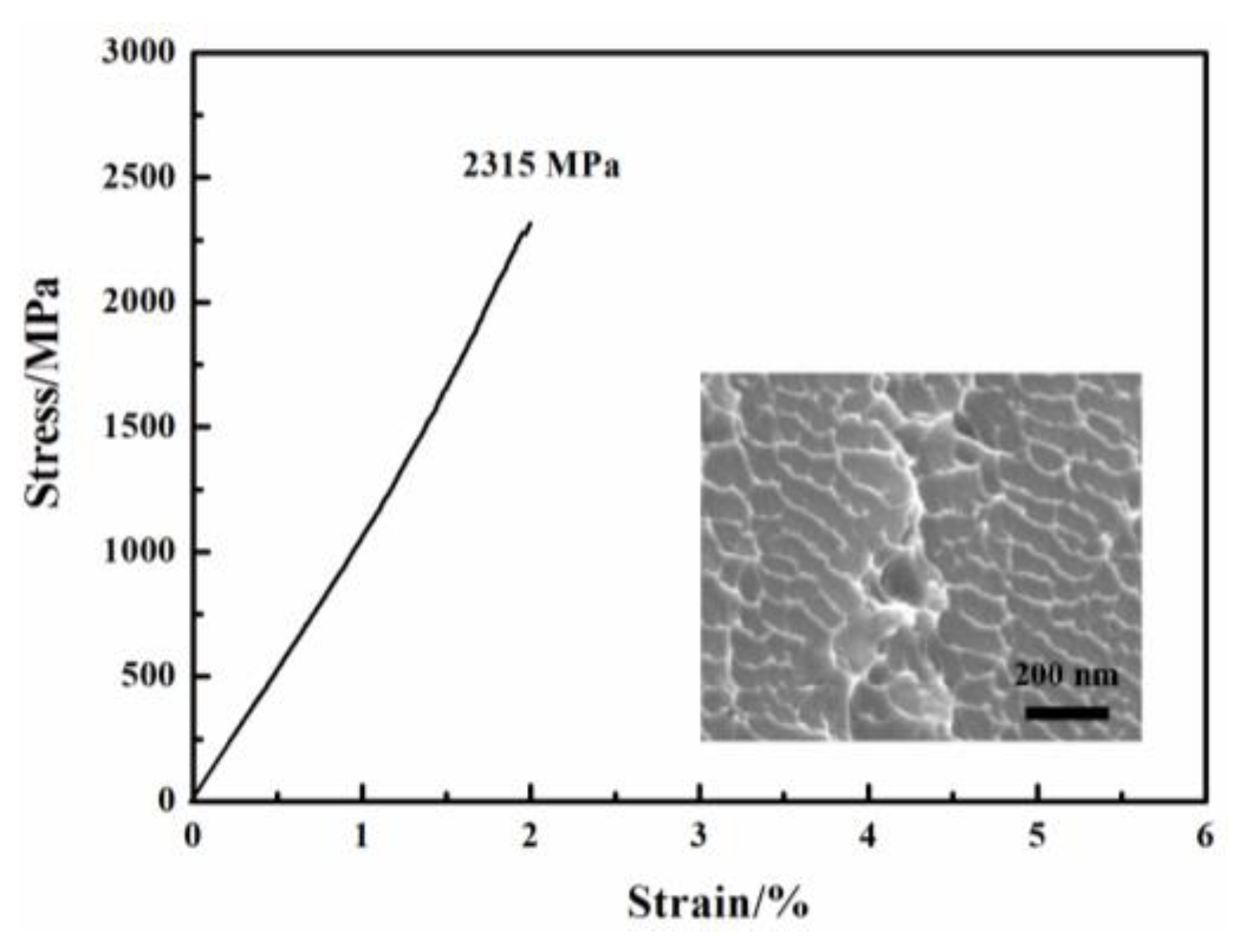
Figure 4. Stress–strain curve of Ø3 mm × 6 mm Ti20Zr20Cu20Ni20Be20 sample. Inset shows fracture morphology after compression.
2.2. Designing HEAAs by Similar Element Substitution/Addition
Similar element substitution/addition was proved to be an effective composition design method in traditional bulk metallic glasses [12][13], so it is reasonable to suppose that it may still work in HEAA. Hf and Zr are members of the same group in the periodic table of elements; they also possess similar atomic radii and chemical properties. Then Hf was used to replace the Zr element in Ti20Zr20Cu20Ni20Be20 HE-BMG. Therefore, a Ti20Hf20Cu20Ni20Be20 alloy was designed. It possesses an amorphous structure and its critical diameter is 2 mm, as shown in Figure 5 [54]. Moreover, Nb and Zr are also very close in the periodic table of elements, so Hf and Nb were added to the Ti20Zr20Cu20Ni20Be20 HE-BMG as a sixth alloying element, respectively. Accordingly, Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 with a critical diameter of 1.5 mm (Figure 5) [54] and Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 with a critical diameter of 15 mm (Figure 6a,b) [36] were designed and developed. Surprisingly, the Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 senary HE-BMG possesses a critical size 10 times that of Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 HE-BMG, and it refreshes our cognition about GFA in an equal-atomic high entropy alloy system. Before this alloy, the largest HE-BMG with an equal-atomic concentration is the Pd20Pt20Cu20Ni20P20 alloy, and its critical diameter is 10 mm by fluxing method [33]. The composition design approach for the Ti20Hf20Cu20Ni20Be20 alloy, Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 alloy and Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 alloy is also demonstrated in Figure 1.
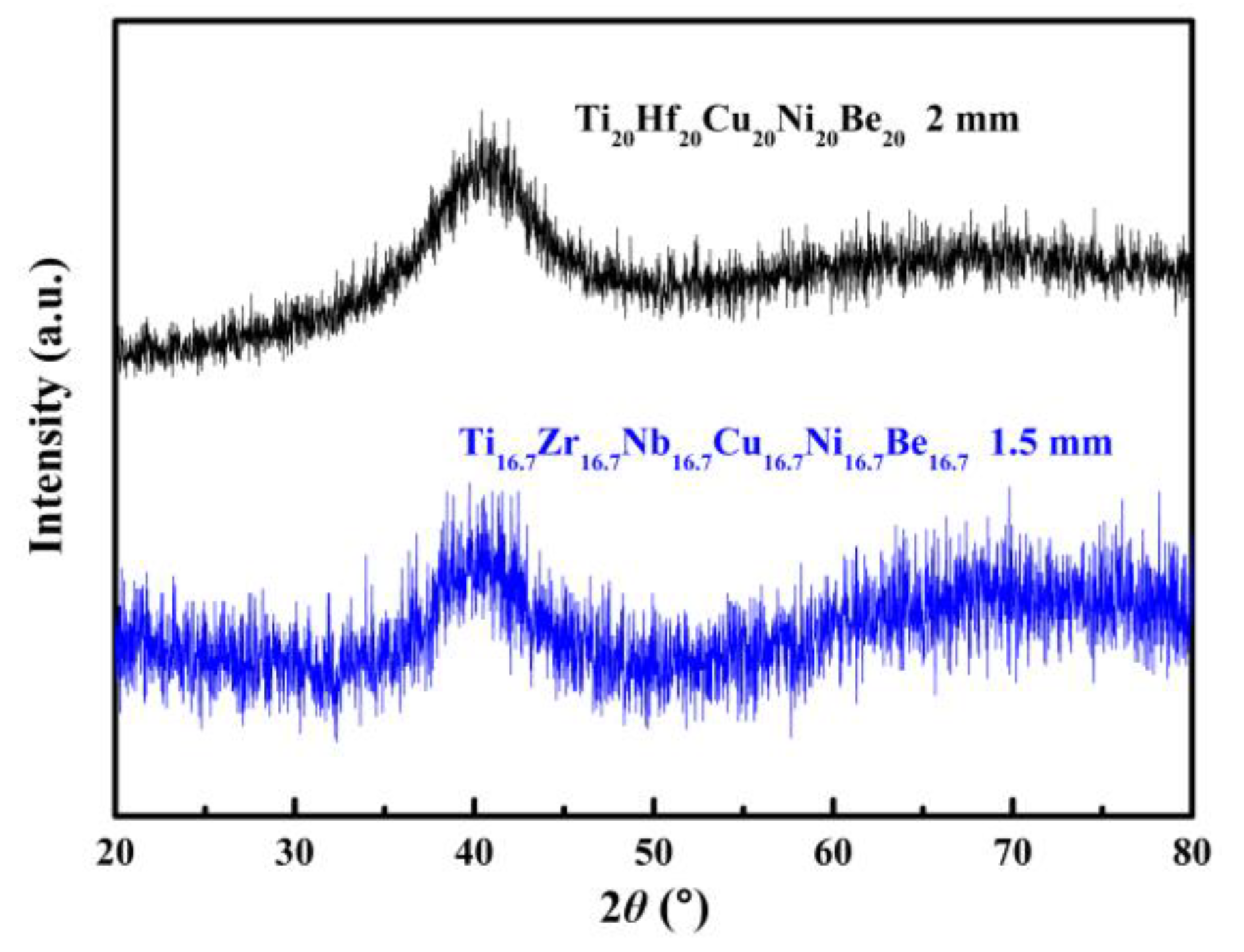
Figure 5. XRD patterns of Ø2 mm Ti20Hf20Cu20Ni20Be20 and Ø1.5 mm Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 sample.
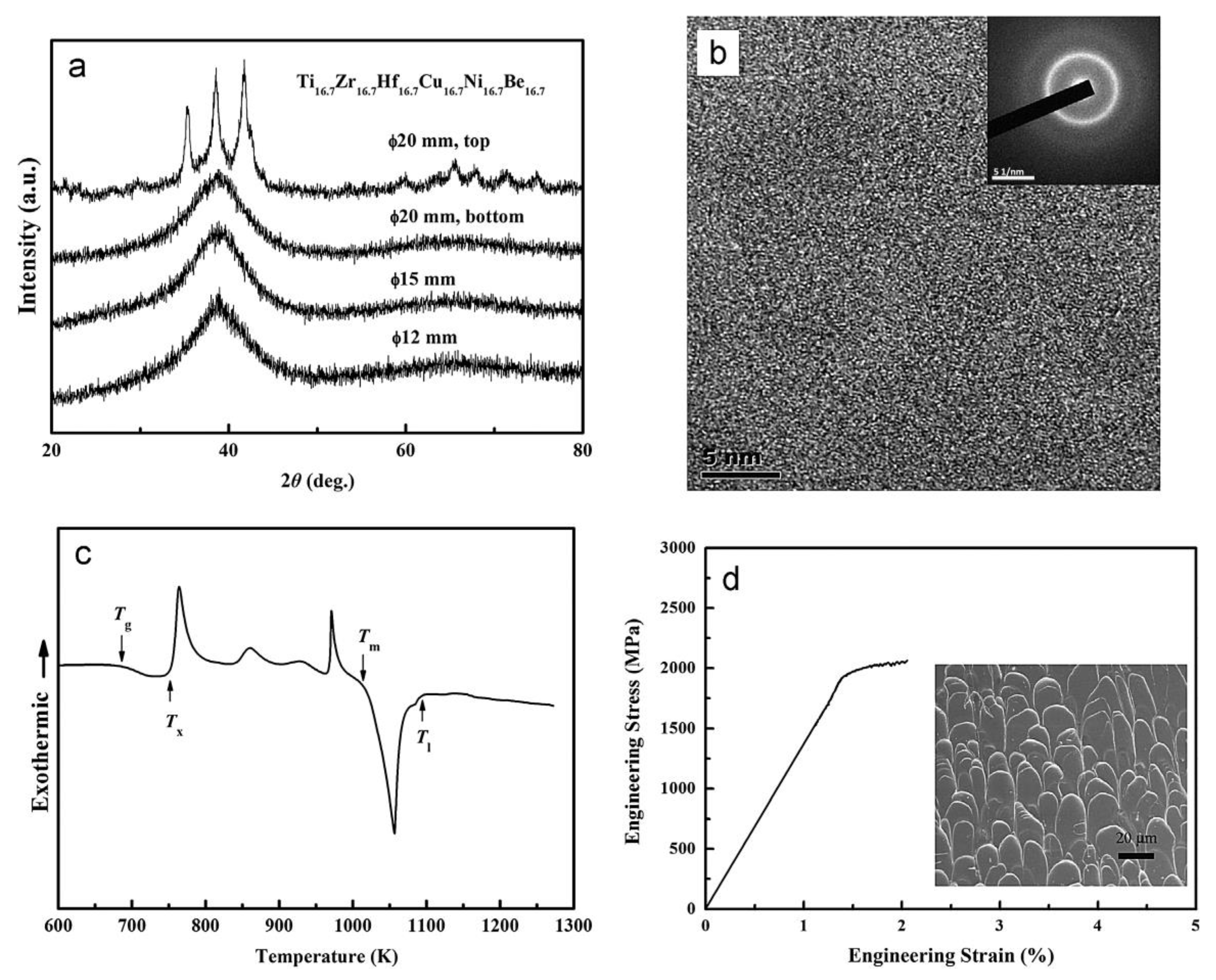
Figure 6. (a) XRD spectra of the Ø12 mm, Ø15 mm and Ø20 mm rod samples of Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 alloy. (b) The HRTEM image of the Ø15 mm glassy rod (inset: SAED pattern). (c) The DSC curve and (d) stress–strain curve of a Ø3 mm × 6 mm glassy sample (inset: SEM image).
For Ti20Hf20Cu20Ni20Be20 alloy, Tg, Tx, Tm, and Tl are measured to be 717 K, 760 K, 1095 K, and 1220 K, respectively (Figure 7). Its compressive fracture strength is 2425 MPa for Ø2 mm × 4 mm sample, and it also breaks without plasticity (Figure 8) [54]. For Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 alloy, Tg, Tx, Tm, and Tl are measured to be 684 K, 739 K, 1066 K, and 1218 K, respectively (Figure 7). Its yield strength, fracture strength, and plasticity are 2330 MPa, 2450 MPa, and 0.5% for the Ø1.5 mm × 3 mm sample, respectively (Figure 8) [54]. For the Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 alloy, Tg, Tx, Tm, and Tl are measured to be 681 K, 751 K, 1019 K, and 1100 K, respectively (Figure 6c). Its yield strength, fracture strength, and plasticity are 1943 MPa, 2064 MPa, and 0.6% for the Ø3 mm × 6 mm sample, respectively (Figure 6d) [36]. These alloys possess high thermal stability and high strength; the relationship between the high entropy effect and properties will be discussed later.
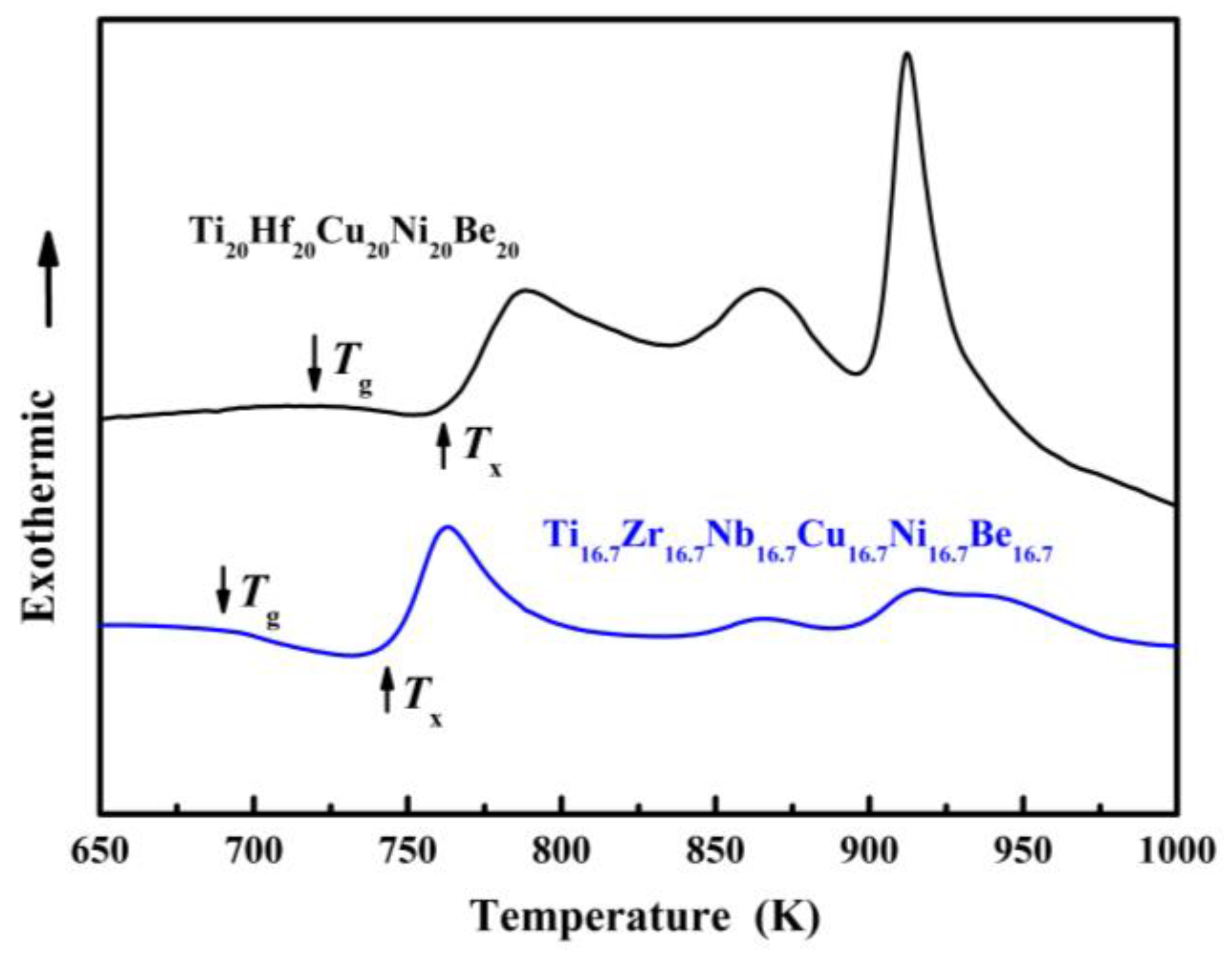
Figure 7. DSC curves of Ø2 mm Ti20Hf20Cu20Ni20Be20 and Ø1.5 mm Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 sample.
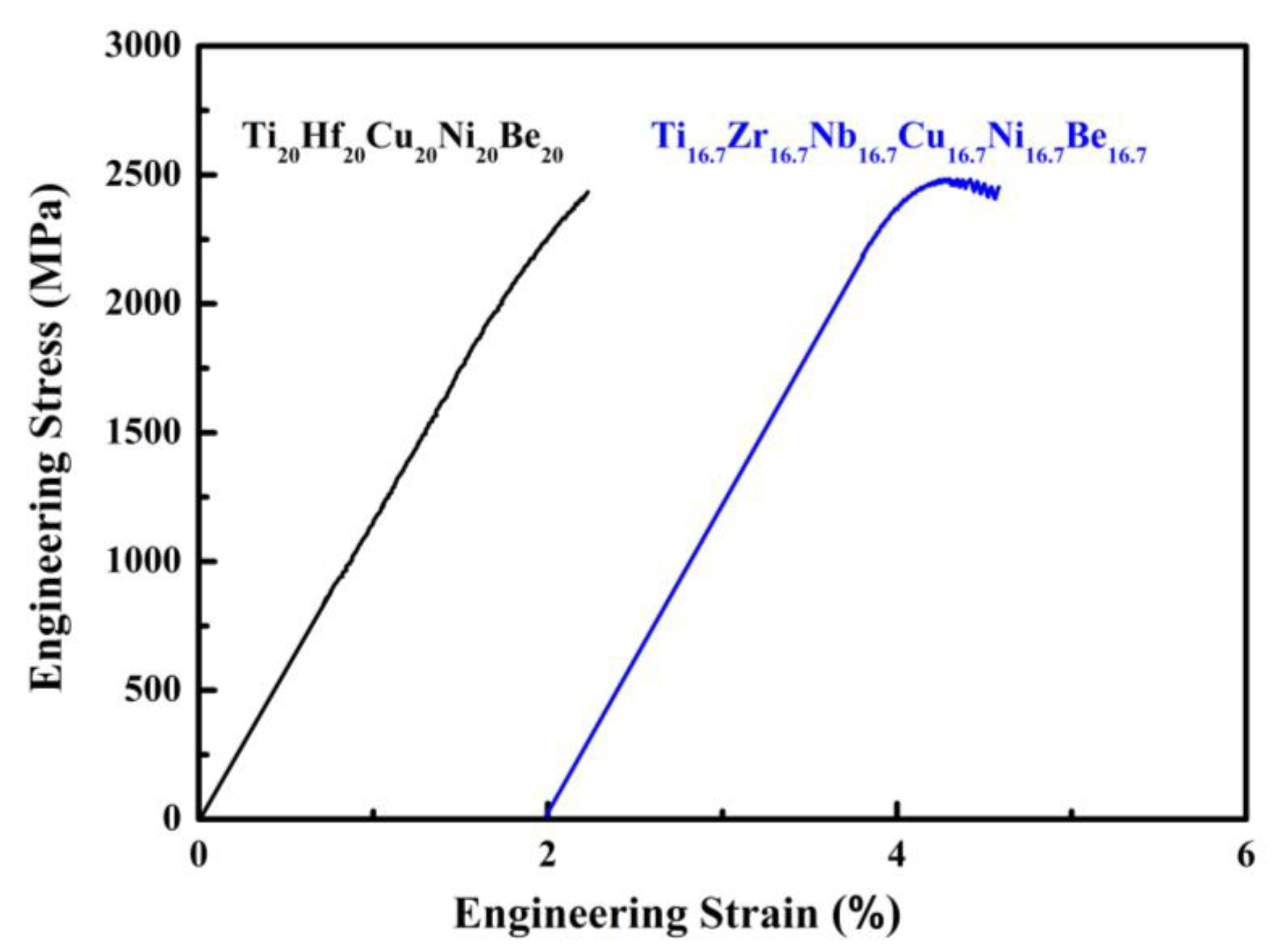
Figure 8. Stress–strain curves of Ø2 mm × 4 mm Ti20Hf20Cu20Ni20Be20 and Ø1.5 mm × 3 mm Ti16.7Zr16.7Nb16.7Cu16.7Ni16.7Be16.7 sample.
The fracture surface morphology of Ti20Zr20Cu20Ni20Be20 was shown as an inset in Figure 4. The nanowave structure is observed on the fracture surface. This is consistent with its brittle failure feature. In contrast, a typical vein pattern has been observed for the Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 alloy (inset in Figure 6d); it also coincides with its plastic deformation behavior. The fracture surface morphology is in agreement with compression experiment results [35][36].
Stimulated by the Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 alloy with good GFA (critical diameter reaches 15 mm), the Ti–Zr–Hf–Cu–Ni–Be alloys with varied Cu/Ni ratio have been studied since Cu and Ni are also very close in the periodic table of elements, and the atomic radius difference is very small. Experimental results show that a series of Ti20Zr20Hf20Be20(Cu(Cu20–xNix) (x = 0, 2.5, 5, 7.5, 10, 12.5, 15, 17.5, 20) HE-BMGs with critical diameters of 12–30 mm were designed and developed (Figure 9) [37][38].
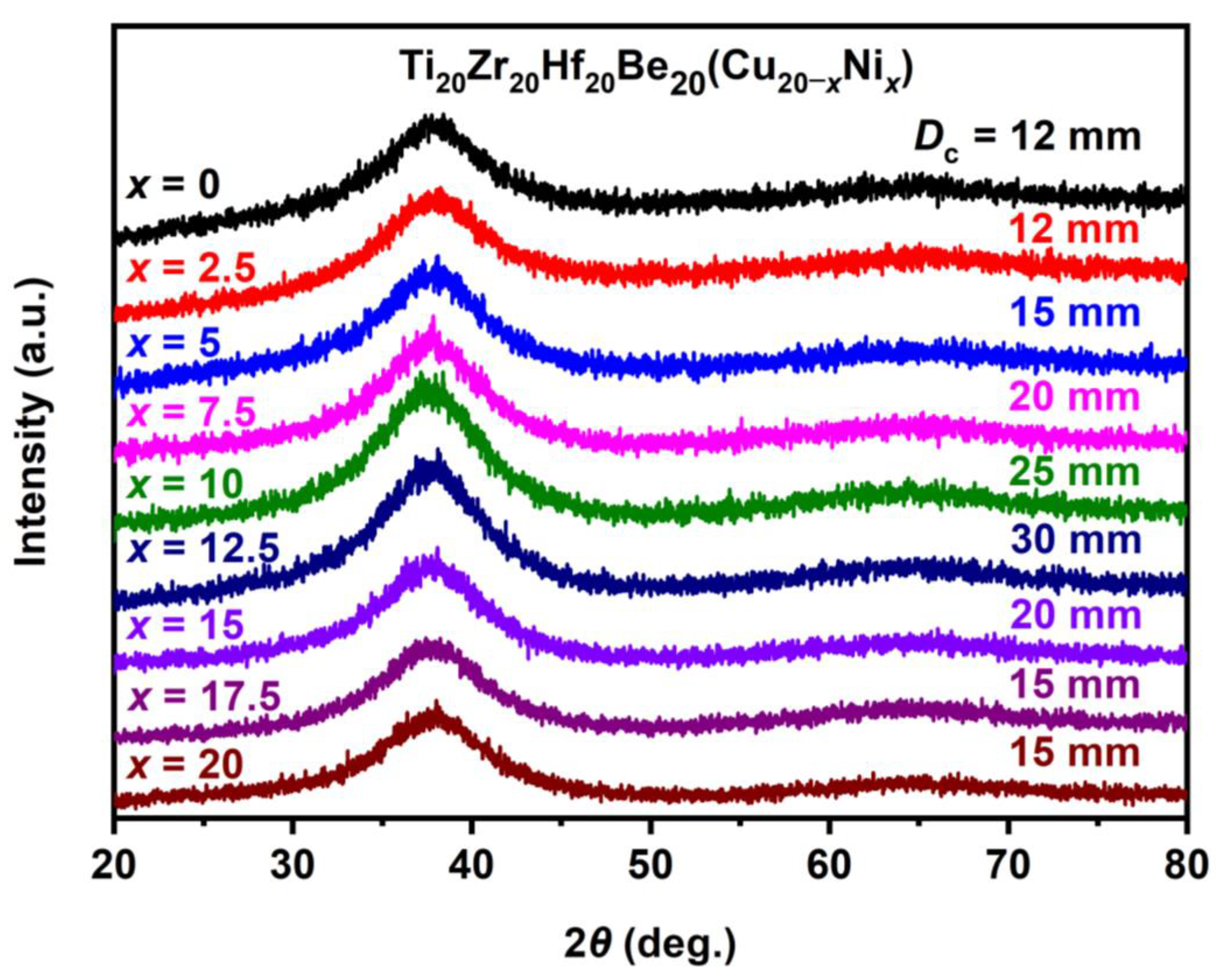
Figure 9. XRD patterns of Ti20Zr20Hf20Be20(Cu20–xNix) (x = 0, 2.5, 5, 7.5, 10, 12.5, 15, 17.5, 20) HE-BMGs.
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline structure in solidified gold-silicon alloys. Nature 1960, 187, 869–870.
- Inoue, A.; Zhang, T.; Masumoto, T. Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 177–183.
- Zhang, T.; Inoue, A.; Masumoto, T. Amorphous Zr-Al-TM (TM=Co, Ni, Cu) alloys with significant supercooled liquid region of over 100 K. Mater. Trans. JIM 1991, 32, 1005–1010.
- Peker, A.; Johnson, W.L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10.0Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344.
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306.
- Zhang, Q.S.; Zhang, H.F.; Deng, Y.F.; Ding, B.Z.; Hu, Z.Q. Bulk metallic glass formation of Cu-Zr-Ti-Sn alloys. Scr. Mater. 2003, 49, 273–278.
- Yim, H.C.; Xu, D.H.; Johnson, W.L. Ni-based bulk metallic glass formation in the Ni-Nb-Sn and Ni-Nb–Sn-X (X=B,Fe,Cu) alloy systems. Appl. Phys. Lett. 2003, 82, 1030–1032.
- Schroers, J.; Johnson, W.L. Highly processable bulk metallic glass-forming alloys in the Pt-Co-Ni-Cu-P system. Appl. Phys. Lett. 2004, 84, 3666–3668.
- Zhang, X.F.; Wang, Y.M.; Qiang, J.B.; Wang, Q.; Wang, D.H.; Li, D.J.; Shek, C.H.; Dong, C. Optimum Zr-Al-Co bulk metallic glass composition Zr53Al23.5Co23.5. Intermetallics 2004, 12, 1275–1278.
- Wang, D.; Tan, H.; Li, Y. Multiple maxima of GFA in three adjacent eutectics in Zr-Cu-Al alloy system-A metallographic way to pinpoint the best glass forming alloys. Acta Mater. 2005, 53, 2969–2979.
- Duan, G.; Blauwe, K.D.; Lind, M.L.; Schramm, J.P.; Johnson, W.L. Compositional dependence of thermal, elastic, and mechanical properties in Cu-Zr-Ag bulk metallic glasses. Scr. Mater. 2008, 58, 159–162.
- Li, R.; Pang, S.J.; Ma, C.L.; Zhang, T. Influence of similar atom substitution on glass formation in (La–Ce)–Al–Co bulk metallic glasses. Acta Mater. 2007, 55, 3719–3726.
- Li, G.H.; Wang, W.M.; Bian, X.F.; Wang, L.; Zhang, J.T.; Li, R.; Huang, T. Influences of similar elements on glass forming ability and magnetic properties in Al-Ni-La amorphous alloy. J. Mater. Sci. Technol. 2010, 26, 146–150.
- Santos, F.S.; Kiminami, C.S.; Bolfarini, C.; de Oliveira, M.F.; Botta, W.J. Evaluation of glass forming ability in the Ni-Nb-Zr alloy system by the topological instability (λ) criterion. J. Alloys Compd. 2010, 495, 313–315.
- Tang, M.Q.; Zhang, H.F.; Zhu, Z.W.; Fu, H.M.; Wang, A.M.; Li, H.; Hu, Z.Q. TiZr-base bulk metallic glass with over 50 mm in diameter. J. Mater. Sci. Technol. 2010, 26, 481–486.
- Nishiyama, N.; Takenaka, K.; Miura, H.; Saidoh, N.; Zeng, Y.Q.; Inoue, A. The world’s biggest glassy alloy ever made. Intermetallics 2012, 30, 19–24.
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488.
- Inoue, A.; Zhang, T.; Masumoto, T. Glass-forming ability of alloys. J. Non-Cryst. Solids 1993, 156–158, 473–480.
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512.
- Li, Y.; Li, J.H.; Liu, J.B.; Liu, B.X. Atomic approach to the optimized compositions of Ni-Nb-Ti glassy alloys with large glass-forming ability. RSC Adv. 2015, 5, 3054–3062.
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principle elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218.
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158.
- Shahmir, H.; Mehranpour, M.S.; Shams, S.A.A.; Langdon, T.G. Twenty years of the CoCrFeNiMn high-entropy alloy: Achieving exceptional mechanical properties through microstructure engineering. J. Mater. Res. Technol. 2023, 23, 3362–3423.
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538.
- Yamabe-Mitarai, Y.; Yanao, K.; Toda, Y.; Ohnuma, I.; Matsunaga, T. Phase stability of Ti-containing high-entropy alloys with a bcc or hcp structure. J. Alloys Compd. 2022, 911, 164849.
- Uporov, S.A.; Estemirova, S.K.; Sterkhov, E.V.; Balyakin, I.A.; Rempel, A.A. Magnetocaloric effect in ScGdTbDyHo high-entropy alloy: Impact of synthesis route. Intermetallics 2022, 151, 107678.
- Kuwabara, K.; Kimura, T.; Chen, M.C.; Shiratori, H.; Shinagawa, K.; Daigo, Y. Microstructural transformation and corrosion property improvement of CoCrFeNiTi-based multi-principal element alloys fabricated via laser powder bed fusion. Adv. Eng. Mater. 2022, 24, 2101272.
- Smith, T.M.; Kantzos, C.A.; Zarkevich, N.A.; Harder, B.J.; Heczko, M.; Gradl, P.R.; Thompson, A.C.; Mills, M.J.; Gabb, T.P.; Lawson, J.W. A 3D printable alloy designed for extreme environments. Nature 2023, 617, 513–518.
- Ron, T.; Shirizly, A.; Aghion, E. Additive manufacturing technologies of high entropy alloys (HEA): Review and prospects. Materials 2023, 16, 2454.
- Ma, L.Q.; Wang, L.M.; Zhang, T.; Inoue, A. Bulk glass formation of Ti–Zr–Hf–Cu–M (M=Fe,Co,Ni) alloys. Mater. Trans. 2002, 43, 277–280.
- Zhao, K.; Xia, X.X.; Bai, H.Y.; Zhao, D.Q.; Wang, W.H. Room temperature homogeneous flow in a bulk metallic glass with low glass transition temperature. Appl. Phys. Lett. 2011, 98, 141913.
- Takeuchi, A.; Chen, N.; Wada, T.; Yokoyama, Y.; Kato, H.; Inoue, A.; Yeh, J.W. Pd20Pt20Cu20Ni20P20 high-entropy alloy as a bulk metallic glass in the centimeter. Intermetallics 2011, 19, 1546–1554.
- Li, H.F.; Xie, X.H.; Zhao, K.; Wang, Y.B.; Zheng, Y.F.; Wang, W.H.; Qin, L. In vitro and in vivo studies on biodegradable CaMgZnSrYb high-entropy bulk metallic glass. Acta Biomater. 2013, 9, 8561–8573.
- Ding, H.Y.; Yao, K.F. High entropy Ti20Zr20Cu20Ni20Be20 bulk metallic glass. J. Non-Cryst. Solids 2013, 364, 9–12.
- Ding, H.Y.; Shao, Y.; Gong, P.; Li, J.F.; Yao, K.F. A senary TiZrHfCuNiBe high entropy bulk metallic glass with large glass-forming ability. Mater. Lett. 2014, 125, 151–153.
- Zhao, S.F.; Yang, G.N.; Ding, H.Y.; Yao, K.F. A quinary Ti-Zr-Hf-Be-Cu high entropy bulk metallic glass with a critical size of 12 mm. Intermetallics 2015, 61, 47–50.
- Zhao, S.F.; Shao, Y.; Liu, X.; Chen, N.; Ding, H.Y.; Yao, K.F. Pseudo-quinary Ti20Zr20Hf20Be20(Cu20–xNix) high entropy bulk metallic glasses with large glass forming ability. Mater. Des. 2015, 87, 625–631.
- Kim, J.; Oh, H.S.; Kim, J.; Ryu, C.W.; Lee, G.W.; Chang, H.J.; Park, E.S. Utilization of high entropy alloy characteristics in Er-Gd-Y-Al-Co high entropy bulk metallic glass. Acta Mater. 2018, 155, 350–361.
- Xu, Y.Q.; Li, Y.H.; Zhu, Z.W.; Zhang, W. Formation and properties of Fe25Co25Ni25(P, C, B, Si)25 high-entropy bulk metallic glasses. J. Non-Cryst. Solids 2018, 487, 60–64.
- Huo, J.T.; Wang, J.Q.; Wang, W.H. Denary high entropy metallic glass with large magnetocaloric effect. J. Alloys Compd. 2019, 776, 202–206.
- Bizhanova, G.; Li, F.W.; Ma, Y.F.; Gong, P.; Wang, X.Y. Development and crystallization kinetics of novel near-equiatomic high-entropy bulk metallic glasses. J. Alloys Compd. 2019, 779, 474–486.
- Inoue, A.; Kong, F.L.; Zhu, S.L.; Greer, A.L. Multicomponent bulk metallic glasses with elevated-temperature resistance. MRS Bull. 2019, 44, 867–872.
- Wada, T.; Jiang, J.; Yubuta, K.; Kato, H.; Takeuchi, A. Septenary Zr–Hf–Ti–Al–Co–Ni–Cu high-entropy bulk metallic glasses with centimeter-scale glass-forming ability. Materialia 2019, 7, 100372.
- Panahi, S.L.; Garcia-Ramon, M.; Pineda, E.; Bruna, P. New (FeCoCrNi)-(B,Si) high-entropy bulk metallic glasses, study of the crystallization processes by X-ray diffraction and Mossbauer spectroscopy. J. Non-Cryst. Solids 2020, 547, 120301.
- Szyba, D.; Bajorek, A.; Babilas, R. Structural and electrochemical study of resorbable Ca32Mg12Zn38Yb18–xBx (x=1, 2, 3) metallic glasses in Ringer’s solution. J. Alloys Compd. 2020, 815, 152313.
- Law, J.Y.; Franco, V. Pushing the limits of magnetocaloric high-entropy alloys. APL Mater. 2021, 9, 080702.
- Calin, M.; Vishnu, J.; Thirathipviwat, P.; Popa, M.M.; Krautz, M.; Manivasagam, G.; Gebert, A. Tailoring biocompatible Ti-Zr-Nb-Hf-Si metallic glasses based on high-entropy alloys design approach. Mater. Sci. Eng. C 2021, 121, 111733.
- Jalali, A.; Malekan, M.; Park, E.S.; Rashidi, R.; Bahmani, A.; Yoo, G.H. Thermal behavior of newly developed Zr33Hf8Ti6Cu32Ni10Co5Al6 high-entropy bulk metallic glass. J. Alloys Compd. 2021, 892, 162220.
- Jalali, A.; Malekan, M.; Park, E.S.; Rashidi, R.; Bahmani, A.; Yoo, G.H. Deformation behavior of Zr33Hf8Ti6Cu32Ni10Co5Al6 high-entropy bulk metallic glass and Cu47Zr47Al6 low-entropy bulk metallic glass at room and high temperatures. Mater. Sci. Eng. A 2022, 832, 142499.
- Jia, Z.; Nomoto, K.; Wang, Q.; Kong, C.; Sun, L.G.; Zhang, L.C.; Liang, S.X.; Lu, J.; Kruzic, J.J. A self-supported high-entropy metallic glass with a nanosponge architecture for efficient hydrogen evolution under alkaline and acidic conditions. Adv. Funct. Mater. 2021, 31, 2101586.
- Makarov, A.S.; Goncharova, E.V.; Qiao, J.C.; Kobelev, N.P.; Khonik, V.A. Relaxation-induced changes in high-entropy bulk metallic glasses. J. Exp. Theor. Phys. 2021, 133, 175–182.
- Alvi, S.; Milczarek, M.; Jarzabek, D.M.; Hedman, D.; Kohan, M.G.; Levintant-Zayants, N.; Vorniero, A.; Akhtar, F. Enhanced mechanical, thermal and electrical properties of high-entropy HfMoNbTaTiVWZr thin film metallic glass and its nitrides. Adv. Eng. Mater. 2022, 24, 2101626.
- Ding, H.Y.; Luan, H.W.; Bu, H.T.; Xu, H.J.; Yao, K.F. Designing high entropy bulk metallic glass (HE-BMG) by similar element substitution/addition. Materials 2022, 15, 1669.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




