Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Tziastoudi | -- | 1675 | 2024-01-30 19:13:23 | | | |
| 2 | Sirius Huang | Meta information modification | 1675 | 2024-01-31 01:58:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tziastoudi, M.; Pissas, G.; Golfinopoulos, S.; Filippidis, G.; Dousdampanis, P.; Eleftheriadis, T.; Stefanidis, I. Iron Deficiency in Heart Failure and CKD. Encyclopedia. Available online: https://encyclopedia.pub/entry/54547 (accessed on 07 February 2026).
Tziastoudi M, Pissas G, Golfinopoulos S, Filippidis G, Dousdampanis P, Eleftheriadis T, et al. Iron Deficiency in Heart Failure and CKD. Encyclopedia. Available at: https://encyclopedia.pub/entry/54547. Accessed February 07, 2026.
Tziastoudi, Maria, Georgios Pissas, Spyridon Golfinopoulos, Georgios Filippidis, Periklis Dousdampanis, Theodoros Eleftheriadis, Ioannis Stefanidis. "Iron Deficiency in Heart Failure and CKD" Encyclopedia, https://encyclopedia.pub/entry/54547 (accessed February 07, 2026).
Tziastoudi, M., Pissas, G., Golfinopoulos, S., Filippidis, G., Dousdampanis, P., Eleftheriadis, T., & Stefanidis, I. (2024, January 30). Iron Deficiency in Heart Failure and CKD. In Encyclopedia. https://encyclopedia.pub/entry/54547
Tziastoudi, Maria, et al. "Iron Deficiency in Heart Failure and CKD." Encyclopedia. Web. 30 January, 2024.
Copy Citation
Heart failure (HF) and chronic kidney disease (CKD) are associated with high mortality. In both disorders, impaired iron homeostasis, mostly in the form of a functional iron deficiency, is a frequent co-morbidity. In HF, functional iron deficiency and management by i.v. iron supplementation have been proven to affect both prognosis and functional capacity. In the same context, iron supplementation is routine for the adequate management of renal anemia in CKD.
heart failure
chronic kidney disease
anemia
iron deficiency
SGLT2i
1. Iron Metabolism
Iron exists mainly in two forms within the human body: the trivalent iron ion (Fe2+) and the ferrous ion (Fe2+). The body absorbs and utilizes iron primarily in the Fe2+ form, while it transports it in the Fe2+ form [1]. The uptake of Fe2+ occurs through the divalent metal transporter 1 (DMT1), which is located on the luminal side of small intestinal epithelial cells [2]. Following absorption in the small intestine, a portion of Fe2+ is utilized to synthesize ferritin in intestinal mucosal epithelial cells, while the remainder enters the bloodstream [3].
Ferritin facilitates the transfer of Fe2+ to blood circulation through ferroportin (FPN), the sole known cellular iron-exporting protein, situated on the basolateral membrane side. The regulation of serum iron levels is tightly controlled by the interaction between hepcidin and FPN [4]. In instances of in vivo iron deficiency, decreased hepcidin expression allows iron to be released into the plasma via FPN [5].
Upon entering the bloodstream, Fe2+ undergoes conversion into Fe2+ facilitated by hephaestin (HP) or ceruloplasmin. The resulting Fe2+ then binds with transferrin, the primary iron transporter, and is transported to various tissues [6]. Transferrin attaches to transferrin receptor 1 (TFR1) and transferrin receptor 2 (TFR2) on the cell surface of iron-deficient cells, entering the cells in a controlled manner [7].
The cornerstone of diagnosing iron deficiency primarily relies on the assessment of serum ferritin levels, which serve as a valuable indicator of cellular iron stores within the body. In healthy individuals, iron deficiency is diagnosed at a serum ferritin level < 30 μg/L. However, in the context of HF and CKD, the diagnostic criteria for iron deficiency are more stringent, with a ferritin level of less than 100 μg/L or a transferrin saturation (TSAT) of 20% or less when serum ferritin is below 300 μg/L [8]. These laboratory findings are indirect markers, reflecting in a clinically relevant manner, cytosolic bioreactive iron (Fe2+). Other biomarkers, except for ferritin and iron levels as well as transferrin saturation, include unsaturated iron-binding capacity, soluble transferrin receptor, plasma hepcidin, and erythropoietin [9]. Certainly, iron deficiency, whether absolute or relative (functional), occurs when the cytosolic iron level is insufficient to support the requirements of heme biosynthesis for proper erythropoiesis. The diagnostic criteria in HF and CKD reflect the increased complexity and multifactorial nature of iron deficiency in these patient populations, underlining the importance of precise assessment and tailored management to address their unique iron-related challenges.
2. Iron Deficiency Anemia in Heart Failure and Chronic Kidney Disease
Iron deficiency represents a state characterized by a diminished delivery of iron to cells, resulting in a cascade of biological repercussions. This deficiency ultimately leads to impaired production of essential components, such as heme and iron–sulfur compounds, which play pivotal roles in the biosynthesis of hemoglobin and oxidative phosphorylation, a critical cellular energy-producing process. This impairment leads thus to reduced erythropoiesis by erythroid precursors in the bone marrow and to reduced cellular production of ATP in mitochondria (e.g., the mitochondria of cardiomyocytes). Therefore, iron deficiency exerts a wide-ranging influence on cellular and systemic functions, highlighting its crucial role in maintaining overall health and well-being.
Iron deficiency is a complex condition that can manifest itself in various forms, including both absolute and relative (functional) iron deficiency. Absolute iron deficiency represents a situation where there is either a complete absence or a severe reduction in the total body iron stores. Iron stores are typically found in macrophages and hepatocytes, and their scarcity can have profound effects on various physiological processes. On the other hand, functional iron deficiency refers to a condition in which the body possesses an adequate amount of iron stores, but it struggles to mobilize these stores effectively for critical functions like erythropoiesis (red blood cell production) or other cellular processes. In functional iron deficiency, the issue is not the lack of iron stores but rather an impairment in the ability to utilize the available iron stores efficiently.
The presence of absolute iron deficiency in HF and individuals with CKD often stems from a combination of factors. These factors encompass reduced iron intake, such as anorexia, which can limit the body’s access to this essential mineral. Additionally, impaired iron absorption, potentially caused by gastrointestinal edema, can further hinder the body’s ability to maintain adequate iron stores. Moreover, the use of anticoagulation and anti-platelet aggregation drugs in these patient populations can increase the risk of gastrointestinal blood loss, contributing to the depletion of iron stores [8]. In the context of CKD, another notable contributor to absolute iron deficiency is iatrogenic blood loss. This can occur through frequent blood tests that are necessary for monitoring the condition, as well as losses that may happen in the vascular access and extracorporeal circuits commonly used in the management of CKD patients [10]. These factors compound the challenge of maintaining sufficient iron stores in individuals with CKD, necessitating careful management and monitoring of iron levels to ensure optimal health outcomes.
Relative iron deficiency in HF often arises from a state of chronic inflammation, which is characterized by elevated levels of inflammatory cytokines, resembling the pattern seen in anemia from chronic disease (Figure 1). This inflammatory milieu includes an increase in cytokines, notably interleukin-6 (IL-6), which exerts a cascade of effects on iron metabolism. IL-6 and other inflammatory mediators can upregulate the production of hepcidin in the liver. Hepcidin, in turn, plays a central role in regulating iron balance by inactivating ferroportin, the primary cellular iron exporter. This results in an inhibition of iron mobilization from its cellular stores, such as hepatocytes and macrophages. Importantly, this hepcidin-mediated inactivation of ferroportin in enterocytes within the gastrointestinal tract further hampers iron absorption, contributing significantly to the development of iron deficiency [11]. There is a significant reduction in iron and transferrin saturation in the blood and an upregulation of transferrin receptor (TfR) on the cell membranes of heme-synthesizing cells. Apart from the effects on hepcidin, the inflammatory mediators promote ferritin synthesis, resulting in the entrapment of iron in its intracellular stores, independent of iron concentration adequacy [8].
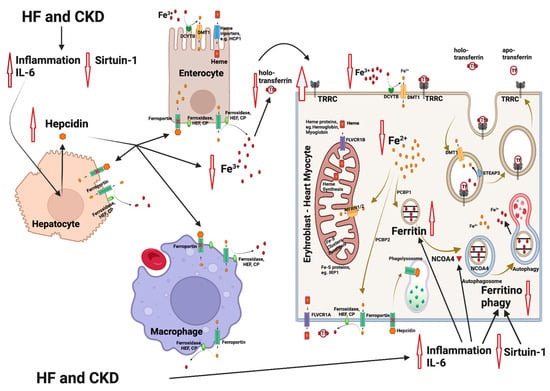
Figure 1. Mechanisms involved in iron deficiency and iron deficiency anemia in patients with heart failure (HF) and chronic kidney disease (CKD). HF and CKD lead to activation of inflammation through elevation of interleukin-6 (IL-6) levels and to an inhibition of sirtuin 1 (SIRT1) expression. As a result, hepcidin is upregulated, leading to an inhibition of the Fe2+ efflux from enterocytes and macrophages (resident in spleen and liver). The Fe2+ flow to the extracellular space and to the blood is blocked, followed by a reduction in the intracellular Fe2+ in heme and/or Fe-S-cluster protein-producing cells (e.g., erythroblasts and cardiomyocytes). Parallel, IL-6 also inhibits the nuclear receptor coactivator 4 (NCOA4), which normally mediates ferritinophagy, leading to the release and export of iron to the cytosol for use in various processes (e.g., mitochondrial heme synthesis). NCOA4 inhibition leads to enhanced entrapment of iron in ferritin and to a further reduction in the available cytoplasmic Fe2+. The resulting state is functional iron deficiency [12][13]. Abbreviations: CP: ceruloplasmin, DMT1: divalent metal transporter 1, DCYTB: duodenal cytochrome b, FLVCR1A/B: feline leukemia virus subgroup C receptor 1A/B, HEF: hephestin, IRP1: iron-regulating protein 1, MFRN1/2: mitoferrin 1/2, NCOA4: nuclear receptor coactivator 4, PCBP1/2: poly r(C)-binding protein 1/2, SGLT2i: SGLT2 inhibitors, SIRT1: sirtuin 1, STEAP: six-transmembrane epithelial antigen of prostate, Tf: transferrin, TFRC: transferrin receptor. Created with BioRender.com (accessed on 10 November 2023).
In the context of CKD, the state of chronic inflammation is a recurrent theme, closely linked to enhanced hepcidin levels and contributing to the development of functional iron deficiency. In humans, individuals with chronic infections or severe inflammatory diseases exhibit elevated levels of urinary hepcidin. In hepatic cell cultures, the expression of hepcidin can be stimulated by cytokines, especially interleukin-6 (IL-6) [14]. An additional reason for increased hepcidin in CKD is reduced renal clearance [15][16]. More specifically, in chronic kidney disease, elevated plasma hepcidin levels, driven by inflammation and compromised renal clearance, impede duodenal iron absorption and result in the sequestration of iron in macrophages [17][18]. Erythropoietin deficiency is the main cause of anemia in CKD, and, according to guidelines, an erythropoiesis-stimulating agent (ESA) treatment is standard [19]. ESA administration in CKD may be an additional cause of a functional iron deficiency, which results from the enhanced iron need for ESA-induced erythropoiesis [10].
Treatment of anemia with erythropoietin in HF has no impact on the overall prognosis, and darbepoietin has even been associated with an increased rate of thromboembolism [8]. In CKD, target hemoglobin levels are 10–11.5 g/dL, while a hemoglobin level > 13 mg/dL is associated with increased cardiovascular risk and is contraindicated [20]. Intravenous iron supplementation in iron-deficient patients with HF had a positive impact on morbidity (hospitalization) and on cardiovascular and overall mortality [21][22]. In a CKD population study, the prevalence of anemia was 20.6% [23]. In this large observational trial, iron deficiency anemia, either absolute (TSAT ≤ 20%, ferritin < 100 μg/L) or relative (functional TSAT ≤ 20%, ferritin 100–500 μg/L), was associated with an increased cardiovascular hospitalization rate. Specifically, in the group with functional iron deficiency anemia (TSAT ≤ 20%, ferritin 100–500 μg/L) and in the group with ferritin levels > 500 μg/L, a higher risk of mortality was observed [23]. However, randomized clinical trials concerning iron supplementation in CKD are missing [24].
A noninferiority trial involving 2141 patients focused on the maintenance of intravenous iron was conducted exclusively in chronic hemodialysis cases. Patients were randomly assigned to receive high-dose iv iron sucrose proactively (400 mg monthly, independent of ferritin level < 700 μg/L or TSAT < 40%) or low-dose iron sucrose reactively (given if ferritin < 200 μg/L or TSAT < 20%). At the follow-up of 2.1 years, there was no difference between the groups in the composite end-point (nonfatal myocardial infarction, nonfatal stroke, hospitalization for HF, or death) [24].
References
- Mackenzie, B.; Garrick, M.D. Iron Imports. II. Iron Uptake at the Apical Membrane in the Intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G981–G986.
- Yanatori, I.; Kishi, F. DMT1 and Iron Transport. Free Radic. Biol. Med. 2019, 133, 55–63.
- Zhang, X.-D.; Liu, Z.-Y.; Wang, M.-S.; Guo, Y.-X.; Wang, X.-K.; Luo, K.; Huang, S.; Li, R.-F. Mechanisms and Regulations of Ferroptosis. Front. Immunol. 2023, 14, 1269451.
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787.
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493.
- Dev, S.; Babitt, J.L. Overview of Iron Metabolism in Health and Disease. Hemodial. Int. 2017, 21 (Suppl. S1), S6–S20.
- Andrews, N.C.; Schmidt, P.J. Iron Homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85.
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98.
- Docherty, K.F.; Welsh, P.; Verma, S.; De Boer, R.A.; O’Meara, E.; Bengtsson, O.; Køber, L.; Kosiborod, M.N.; Hammarstedt, A.; Langkilde, A.M.; et al. Iron Deficiency in Heart Failure and Effect of Dapagliflozin: Findings From DAPA-HF. Circulation 2022, 146, 980–994.
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468.
- Singer, C.E.; Vasile, C.M.; Popescu, M.; Popescu, A.I.S.; Marginean, I.C.; Iacob, G.A.; Popescu, M.D.; Marginean, C.M. Role of Iron Deficiency in Heart Failure-Clinical and Treatment Approach: An Overview. Diagnostics 2023, 13, 304.
- Packer, M. How Can Sodium-Glucose Cotransporter 2 Inhibitors Stimulate Erythrocytosis in Patients Who Are Iron-Deficient? Implications for Understanding Iron Homeostasis in Heart Failure. Eur. J. Heart Fail. 2022, 24, 2287–2296.
- Camaschella, C.; Pagani, A. Advances in Understanding Iron Metabolism and Its Crosstalk with Erythropoiesis. Br. J. Haematol. 2018, 182, 481–494.
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a Putative Mediator of Anemia of Inflammation, Is a Type II Acute-Phase Protein. Blood 2003, 101, 2461–2463.
- Zaritsky, J.; Young, B.; Wang, H.-J.; Westerman, M.; Olbina, G.; Nemeth, E.; Ganz, T.; Rivera, S.; Nissenson, A.R.; Salusky, I.B. Hepcidin--a Potential Novel Biomarker for Iron Status in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1051–1056.
- Eleftheriadis, T.; Liakopoulos, V.; Antoniadi, G.; Kartsios, C.; Stefanidis, I. The Role of Hepcidin in Iron Homeostasis and Anemia in Hemodialysis Patients. Semin. Dial. 2009, 22, 70–77.
- Ashby, D.R.; Gale, D.P.; Busbridge, M.; Murphy, K.G.; Duncan, N.D.; Cairns, T.D.; Taube, D.H.; Bloom, S.R.; Tam, F.W.K.; Chapman, R.S.; et al. Plasma Hepcidin Levels Are Elevated but Responsive to Erythropoietin Therapy in Renal Disease. Kidney Int. 2009, 75, 976–981.
- Ganz, T.; Nemeth, E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 87–93.
- Ku, E.; Del Vecchio, L.; Eckardt, K.-U.; Haase, V.H.; Johansen, K.L.; Nangaku, M.; Tangri, N.; Waikar, S.S.; Więcek, A.; Cheung, M.; et al. Novel Anemia Therapies in Chronic Kidney Disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023, 104, 655–680.
- Pfeffer, M.A.; Burdmann, E.A.; Chen, C.-Y.; Cooper, M.E.; De Zeeuw, D.; Eckardt, K.-U.; Feyzi, J.M.; Ivanovich, P.; Kewalramani, R.; Levey, A.S.; et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2009, 361, 2019–2032.
- Anker, S.D.; Kirwan, B.-A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of Ferric Carboxymaltose on Hospitalisations and Mortality Rates in Iron-Deficient Heart Failure Patients: An Individual Patient Data Meta-Analysis. Eur. J. Heart Fail. 2018, 20, 125–133.
- Yamani, N.; Ahmed, A.; Gosain, P.; Fatima, K.; Shaikh, A.T.; Qamar, H.; Shahid, I.; Arshad, M.S.; Almas, T.; Figueredo, V. Effect of Iron Supplementation in Patients with Heart Failure and Iron Deficiency: A Systematic Review and Meta-Analysis. IJC Heart Vasc. 2021, 36, 100871.
- Awan, A.A.; Walther, C.P.; Richardson, P.A.; Shah, M.; Winkelmayer, W.C.; Navaneethan, S.D. Prevalence, Correlates and Outcomes of Absolute and Functional Iron Deficiency Anemia in Nondialysis-Dependent Chronic Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, 129–136.
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.V.; Murray, H.; Tomson, C.R.V.; Wheeler, D.C.; et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N. Engl. J. Med. 2019, 380, 447–458.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
503
Revisions:
2 times
(View History)
Update Date:
31 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




