Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Letizia Berloni | -- | 2467 | 2024-01-30 14:52:47 | | | |
| 2 | Rita Xu | -7 word(s) | 2460 | 2024-01-31 03:56:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tonet, E.; Amantea, V.; Lapolla, D.; Assabbi, P.; Boccadoro, A.; Berloni, M.L.; Micillo, M.; Marchini, F.; Chiarello, S.; Cossu, A.; et al. Coronary Computed Tomography Angiography. Encyclopedia. Available online: https://encyclopedia.pub/entry/54533 (accessed on 05 March 2026).
Tonet E, Amantea V, Lapolla D, Assabbi P, Boccadoro A, Berloni ML, et al. Coronary Computed Tomography Angiography. Encyclopedia. Available at: https://encyclopedia.pub/entry/54533. Accessed March 05, 2026.
Tonet, Elisabetta, Veronica Amantea, Davide Lapolla, Paolo Assabbi, Alberto Boccadoro, Maria Letizia Berloni, Marco Micillo, Federico Marchini, Serena Chiarello, Alberto Cossu, et al. "Coronary Computed Tomography Angiography" Encyclopedia, https://encyclopedia.pub/entry/54533 (accessed March 05, 2026).
Tonet, E., Amantea, V., Lapolla, D., Assabbi, P., Boccadoro, A., Berloni, M.L., Micillo, M., Marchini, F., Chiarello, S., Cossu, A., & Campo, G. (2024, January 30). Coronary Computed Tomography Angiography. In Encyclopedia. https://encyclopedia.pub/entry/54533
Tonet, Elisabetta, et al. "Coronary Computed Tomography Angiography." Encyclopedia. Web. 30 January, 2024.
Copy Citation
The use of coronary computed tomography angiography (CCTA) in the setting of stable coronary artery disease is highly recommended for low-risk patients. High-risk patients, such as symptomatic subjects with prior revascularization, are suggested to be investigated with noninvasive functional tests or invasive coronary angiography.
cardiovascular computed tomography
coronary artery disease
stenting
1. Introduction
It is well-established that cardiac computed tomography angiography (CCTA) has recently played a key role in the area of ischemic heart disease (IHD). For individuals with a low to moderate pre-test risk of coronary artery disease (CAD), the most recent guidelines for chronic coronary syndrome advocate for using CCTA as the primary anatomical test [1]. Regarding acute chest pain, this approach was designed to both confirm and exclude patients in the emergency department when electrocardiograms and laboratory biomarkers did not provide conclusive results [2]. In patients with previous coronary revascularization, both with percutaneous and surgical techniques, the anatomical assessment of coronary arteries has been a prerogative of invasive coronary angiography because of several limitations of CCTA. As a matter of fact, the metallic elements of stented coronary segments lead to a blooming effect, beam-hardening artifacts, and a partial volume effect that makes the assessment of in-stent disease progression challenging. All these factors reduce visualization of the true in-stent lumen, and it has been estimated that 12% of all coronary stents cannot be imaged with adequate diagnostic quality [2]. However, the development of new technology has allowed us to overcome these limitations. The newly established multi-slice CT scan employs multi-row detector array systems permitting a rapid imaging modality allowing views of cardiac structures during one breath hold. Novel CT scanner developments have introduced photon-counting detector technology, which is a sophisticated system improving spatial resolution thanks to a smaller detector scheme when compared with the conventional one [2]. Additionally, the introduction of stress CT perfusion and CT-derived fractional flow reserve (FFR-CT) allows for the functional assessment of coronary stenosis detected with CCTA [3]. The introduction of all this new technology has inspired the use of CCTA for the evaluation of patients already treated with a coronary artery bypass graft (CABG), complex percutaneous coronary intervention (PCI), and a bioresorbable scaffold (BRS) (Graphical abstract). The availability of a noninvasive anatomic test for patients with previous coronary revascularization and its possible association with functional assessment in a single exam could play a key role in the follow-up management of these subjects, especially considering the rate of false-positive and negative results of functional examinations.
2. New Technologies in CCTA
In recent years, new technological developments have been introduced in the field of CT scanners. Advanced multi-detector CT scanners from newer generations exhibit enhanced spatial and temporal resolution, along with comprehensive heart coverage using wide-detector or dual-source CT. The former offers a coverage area of 16 cm and can capture heart images in a single heartbeat. Dual-source CT can image the heart in about 300 ms. It uses two X-ray tubes and two detectors arranged at 90° angles; this technology allows the reconstruction of images at one quarter of the gantry rotation time, improving temporal resolution. It also shows a good diagnostic image quality in patients with fast heart rates, limiting the use of beta-blockers. Additionally, thinner detectors and faster gantry rotation have also allowed for good image quality in patients with coronary stents and CABG [3].
Photon-counting computed tomography (PCCT) is a recently introduced CT technology. It is based on a new generation of X-ray detectors; they are composed of semiconductor materials that directly convert each X-ray photon into electron-hole pairs. This mechanism enables the counting of photons and their classification into energy levels, avoiding noise at the electronic level. In this way, a substantial spatial resolution improvement can be achieved. Additionally, this technology is related to a decrease in the radiation dose and the amount of contrast media [3][4].
It must be noted that only 49% of significant CCTA stenoses are associated with abnormal invasive fractional flow reserve, such that current recommendations emphasize that patients with a stenosis of 50% or more are recommended to undergo further investigation with a functional test to guide revascularization. The FFR-CT technique has been developed with the aim of obtaining a noninvasive functional assessment of coronary stenosis. It works on the basis that by considering anatomical coronary features and applying computational flow dynamic algorithms, the coronary reply to adenosine administration can be estimated. A patient-specific three-dimensional (3D) anatomic coronary artery model is obtained, and a physiologic model is then derived based on patient-specific inflow and outflow hemodynamic conditions, with the resting myocardial blood flow proportional to the myocardial mass and the mathematical estimation of microvascular resistance. The decreased hyperemic microvascular resistance to adenosine is also predicted, with no need for adenosine administration. In this way, this analysis can forecast the performance of coronary circulation during conditions of maximum hyperemia [3]. FFR-CT does not require additional scan data, and it is associated with fast processing times. The addition of FFR-CT to coronary CTA improves its specificity by evaluating lesion-specific ischemia, enhances its role as a gatekeeper for ICA by decreasing nonobstructive disease at ICA, and offers guidance for revascularization decisions and planning.
Another technique for functional assessment of coronary stenosis is stress CT perfusion. It takes into account that under resting conditions, the coronary circulation maintains a consistent pressure gradient thanks to an autoregulation mechanism; in the presence of a hyperemic stimulus, this autoregulation is disrupted, resulting in reduced myocardial perfusion when coronary stenosis is present. A rest/stress protocol is recommended, using adenosine as a stressor.
Stress CT perfusion images can be obtained using both static and dynamic protocols. The static protocol involves capturing a complete dataset of images throughout the entire cardiac volume during the passage of the contrast medium.
The dynamic protocol, on the other hand, involves capturing multiple datasets that correspond to the contrast kinetics within the cardiac chambers, allowing for the derivation of time-attenuation curves.
This approach enables the assessment of myocardial blood flow in each individual myocardial segment [3].
3. CCTA after Complex PCI
PCI of the left main (LM) improves survival, and in most cases, it is not inferior to surgical revascularization [4]. Intra-stent restenosis (ISR) is a complication of paramount importance, especially in the setting of LM revascularization, because of its relationship to adverse events. With current stents, ISR is due to neo-atherogenesis, which leads to a higher risk of destabilization and stent thrombosis. In the setting of LM, ISR at 15-month follow-up has been revealed to be present in up to 16% of total subjects, requiring invasive revascularization in 7% of cases. Coronary angiography represents the best technique for ISR assessment [4]. Planned Angiography Control (PAC) has been proposed to diagnose and treat ISR promptly, but its benefit remains to be established. An increased rate of percutaneous coronary interventions (PCI) without a reduction in cardiovascular events has been mainly reported. Some technical issues about the use of CCTA in coronary stent imaging have been described, such as the blooming effect, partial volume effect, motion artifacts, and inadequate intravascular contrast enhancement. The blooming effect is the most important issue, corresponding to a phenomenon in which stent struts appear thicker, causing an underestimation of stent lumen. However, with new technology development, CCTA provides a precise, noninvasive reconstruction of the coronary tree and may offer an alternative to invasive coronary angiography [5]. Figure 1 shows three multiplanar reconstructions of LM and left anterior descending with a drug-eluting stent previously implanted: struts of the stent appear clearly detectable, the stent seems to be well-positioned, and regarding stent lumen, it can be noted that there is good opacification and no evidence of ISR. Medium and distal tracts of the left anterior descending seem to be free from plaque proliferation and/or stenosis. Figure 2, instead, shows an ISR of a stent implanted in the proximal segment of the left circumflex coronary artery. Therefore, Figure 1 and Figure 2 demonstrate the feasibility of LM and proximal segment stent assessment by CCTA. Its use in the PAC setting has been investigated and may provide relevant advantages as it is a noninvasive examination. There are three different methods to determine the degree of ISR with CCTA: qualitative, semi-quantitative, and quantitative. The first technique provides that significant ISR (reduction of luminal diameter >50%) is visually detected: ISR is identified as a hypodense layer between the struts and the lumen. The second method is characterized by a four-point scale where 1 corresponds to the patency of the stent and 4 results in stent occlusion. Finally, the third technique provides that the percentage of stenosis is calculated as the ratio between diameters in the short axis of the narrowest stent lumen and of the proximal and distal reference segments [5]. Roura G et al. evaluated the agreement between CCTA and intravascular ultrasound (IVUS) to assess in-stent lumen diameters and lumen area of LM stents: the study highlighted a good agreement between the two techniques so that CCTA can be considered to analyze LM ISR [6]. A study by Van Mieghem CAG et al. assessed the performance of CCTA in the analysis of LM stenting: they enrolled 74 patients scheduled for follow-up coronary angiography after LM stenting, and they performed CCTA before coronary angiography. The study demonstrated that the accuracy of CCTA for detecting LM ISR was 98%; in particular, diagnostic accuracy was 98% both for patients with stented LM and with distal LM bifurcation lesions and only one side branch treated [7]. In subjects with complex bifurcation stenting (i.e., LM and both major side branches), the reliability of CCTA was 83%. The low number of false-positive scans leading to unnecessary diagnostic coronary angiograms should be acceptable, taking into account the potentially serious consequences of LM ISR [7]. Furthermore, the study underlined that the evaluation of stent diameter and area by CCTA had a good correlation with IVUS assessment. One of the most important issues was the high radiation dose required for the analysis of stents by CCTA. However, as previously reported, new scanner developments (i.e., dual-source CT scanners) reduced patient dose.
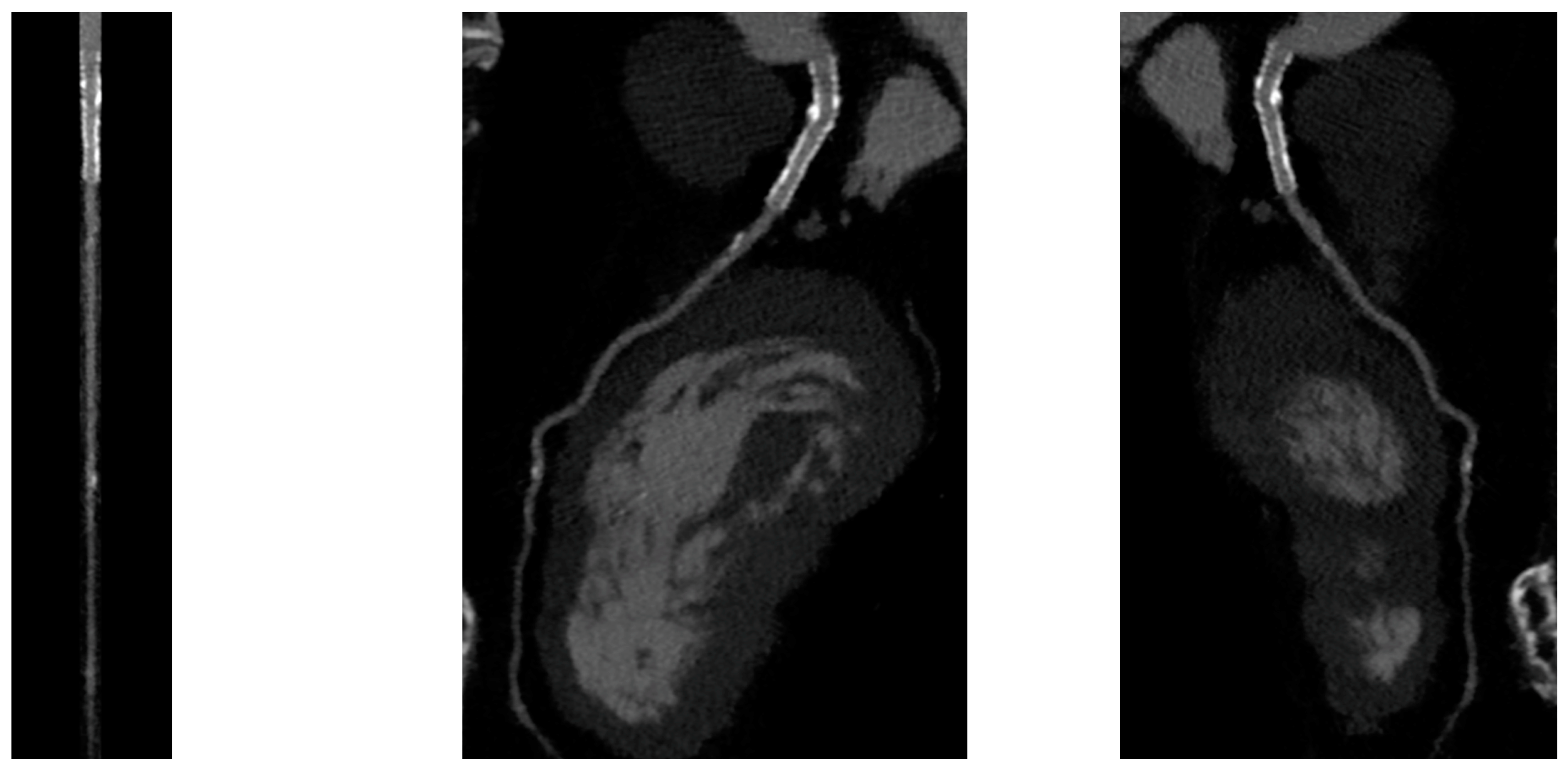
Figure 1. CCTA assessment of left main drug-eluting stent (Lumen image on the left, multiplanar reconstruction in the center and on the right). With a new CT scan, beam-hardening artifacts are reduced, and the stent lumen can be assessed with good performance.
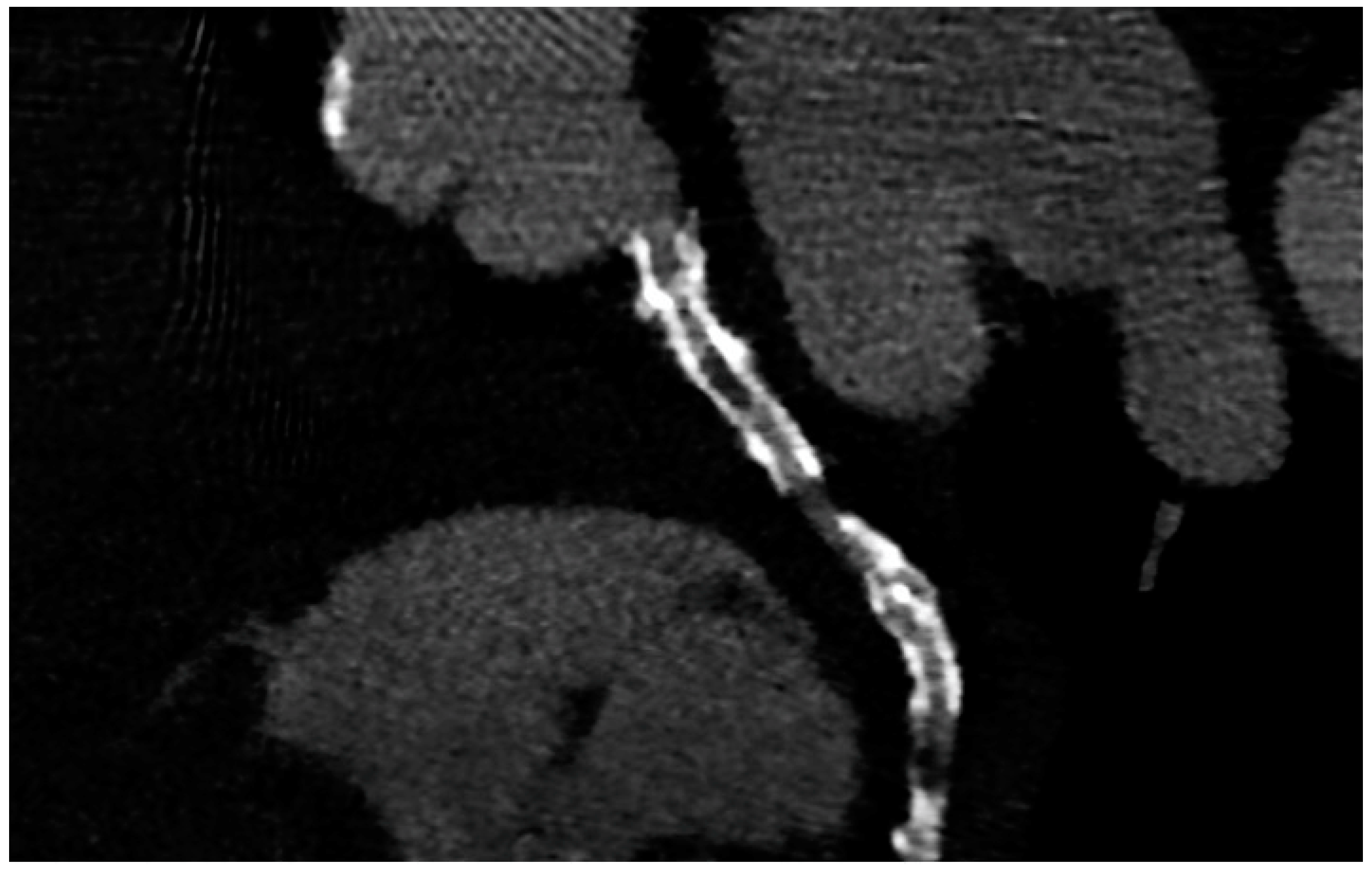
Figure 2. ISR in a stent previously implanted in the proximal segment of the left circumflex artery.
4. CCTA after Scaffold Implantation
Bioresorbable scaffolds (BRS) were designed to combine the short-term advantages of permanent stents with the long-term benefit of complete reabsorption, facilitating the restoration of vasomotor and endothelial function. This technology helps prevent prolonged inflammation, maintains the integrity of distal bypass grafting sites, and allows unimpeded future vessel imaging. Despite the promising theoretical benefits of BRS, the initial generation of BRS devices exhibited higher rates of stent thrombosis in comparison to other stents [8]. Newer generation devices appear to present a viable alternative to drug-eluting stents in the management of acute coronary syndromes (ACS) for several reasons: their different composition when compared to first-generation BRS, the optimized deployment technique, and the lesion selection. Notably, ACS lesions show specific characteristics based on the pathophysiology of the disease [8]. Thus, various factors create favorable conditions for BRS implantation in ACS patients, including the vulnerable nature of the plaque, minimal calcification, the presence of a thrombus, and the relative youth of patients. BRS is radiolucent except for two metallic radio-opaque markers located at both extremities. This design feature aids in visualization during imaging procedures, ensuring accurate placement and monitoring of the scaffold. Thus, CCTA can delineate the contours of the scaffolded segment: markers easily enable the location of where BRS was implanted, and they can be distinguished from calcification because of the difference in attenuation [9]. Figure 3 shows a CCTA analysis of scaffolded coronary segments: as highlighted by orange brackets, there is evidence of two little markers of the scaffold that appear completely reabsorbed: indeed, no struts are detectable, and the vessel lumen can be analyzed in depth also in the scaffolded part with no evidence of plaque proliferation. The diagnostic accuracy of coronary CT angiography in poli-LLA (poly-L-lactide) Everolimus scaffold was studied in the ABSORB II study (A Bioresorbable Everolimus-Eluting Scaffold Versus a Metallic Everolimus-Eluting Stent II) [10]. The study provided the randomization of enrolled patients to receive treatment with BRS or drug-eluting stent. At the 3-year follow-up, patients treated with BRS underwent coronary angiography with intravascular ultrasound (IVUS) evaluation and CCTA. The study demonstrated that the CCTA diagnostic accuracy for detecting in-scaffold obstruction and luminal dimensions was similar to invasive coronary angiography (ICA) and IVUS. Analyzing scaffold segments, the sensitivity, specificity, and negative predictive values were 71%, 82%, and 97%, respectively, using IVUS as a reference. One limitation was its use of a 3-year follow-up period, which did not address the crucial question of assessing the occurrence of restenosis within the initial 12 months. It is during this period that most restenosis events occur, coinciding with the presence of BRS with thicker struts in place [11]. Salinas P et al. performed the first case series of Magnesium bioresorbable scaffold investigated with CCTA at 1 year of follow-up [12]. The CCTA in-scaffold percentage diameter stenosis and area stenosis were 22% and 39%, respectively, underlying plaque growth. Additionally, performing plaque characterization, the segments treated with RMS showed that the most common component of the plaque was the fibrous one (69% of the cases), suggesting that RMS allows for the stabilization of culprit lesions [12]. Furthermore, anatomical findings can be combined with noninvasive fractional flow reserve derived from CCTA (FFR-CT) to distinguish the presence or absence of flow-limiting disease [13]. A study by Tonet E et al. investigated the performance of CCTA and FFR-CT in 26 patients treated with Magnesium bioresorbable scaffold: all patients underwent CCTA 18 months after BRS implantation. The left anterior descending artery was the most commonly affected vessel. CCTA revealed patent scaffolded segments, with complete strut reabsorption observed in 93% of cases. FFR-CT demonstrated to be feasible in scaffolded segments with a median value of 0.88 [0.81–0.91]. Figure 4 shows a case from the above-reported study: BRS (orange bracket) appears to be characterized by plaque proliferation with a prevalent calcific component. FFR-CT analysis highlighted a significant stenosis related to the plaque. In conclusion, these results suggest that CCTA plus FFR-CT is a valuable noninvasive tool for the assessment of coronary arteries in subjects treated with BRS. Scaffolded segments can be easily distinguished, allowing for quantitative measurements and the calculation of noninvasive FFR. The analysis also indicates a tendency to observe plaque stabilization in the scaffolded segments with fibrosis and calcium [14]. However, further evidence is needed in this setting of BRS patients.
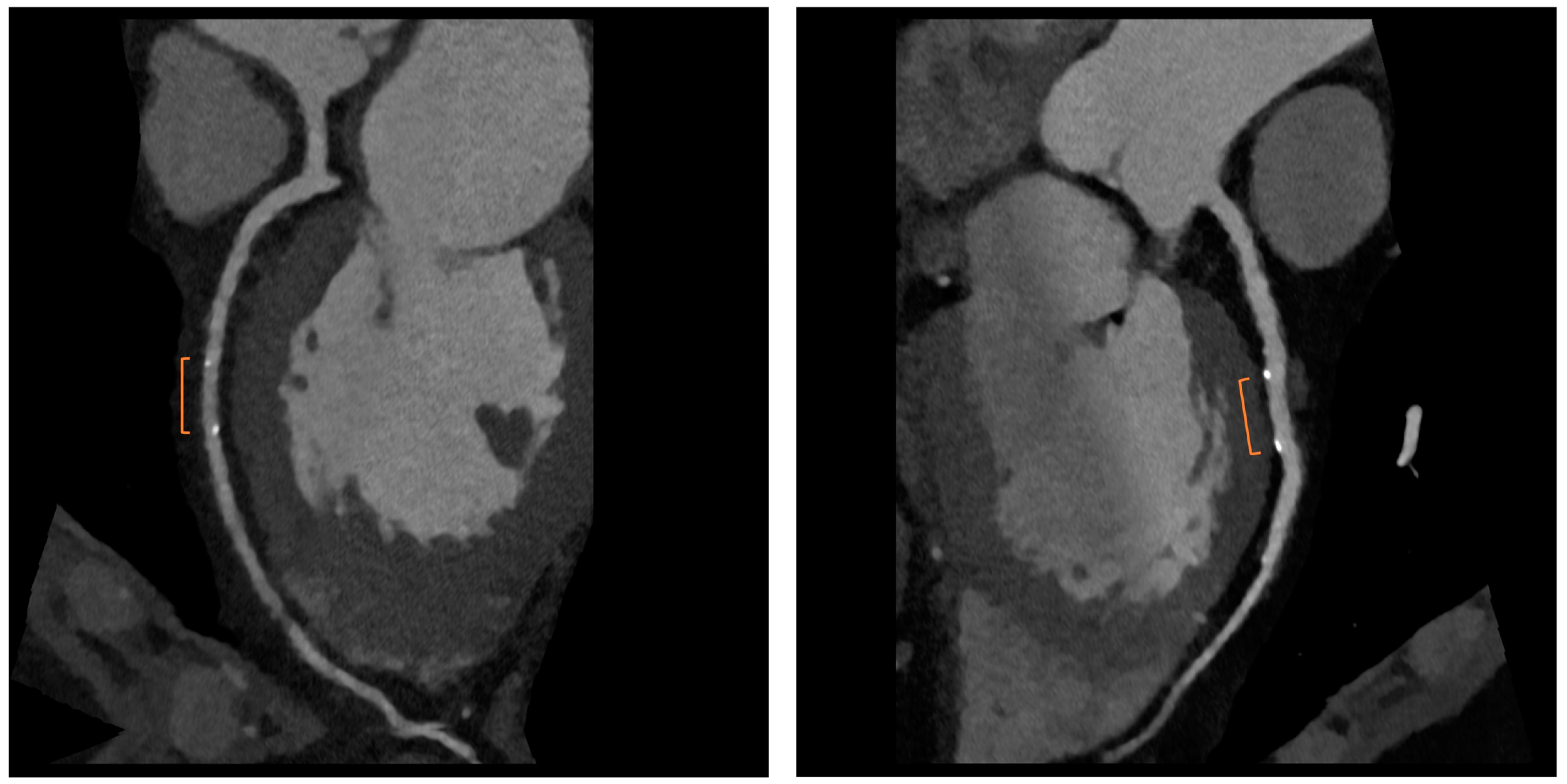
Figure 3. CCTA assessment of BRS. Orange brackets highlight the two markers of BRSs whose struts result in complete reabsorption. The vessel is also well analyzed in the scaffolded segment.
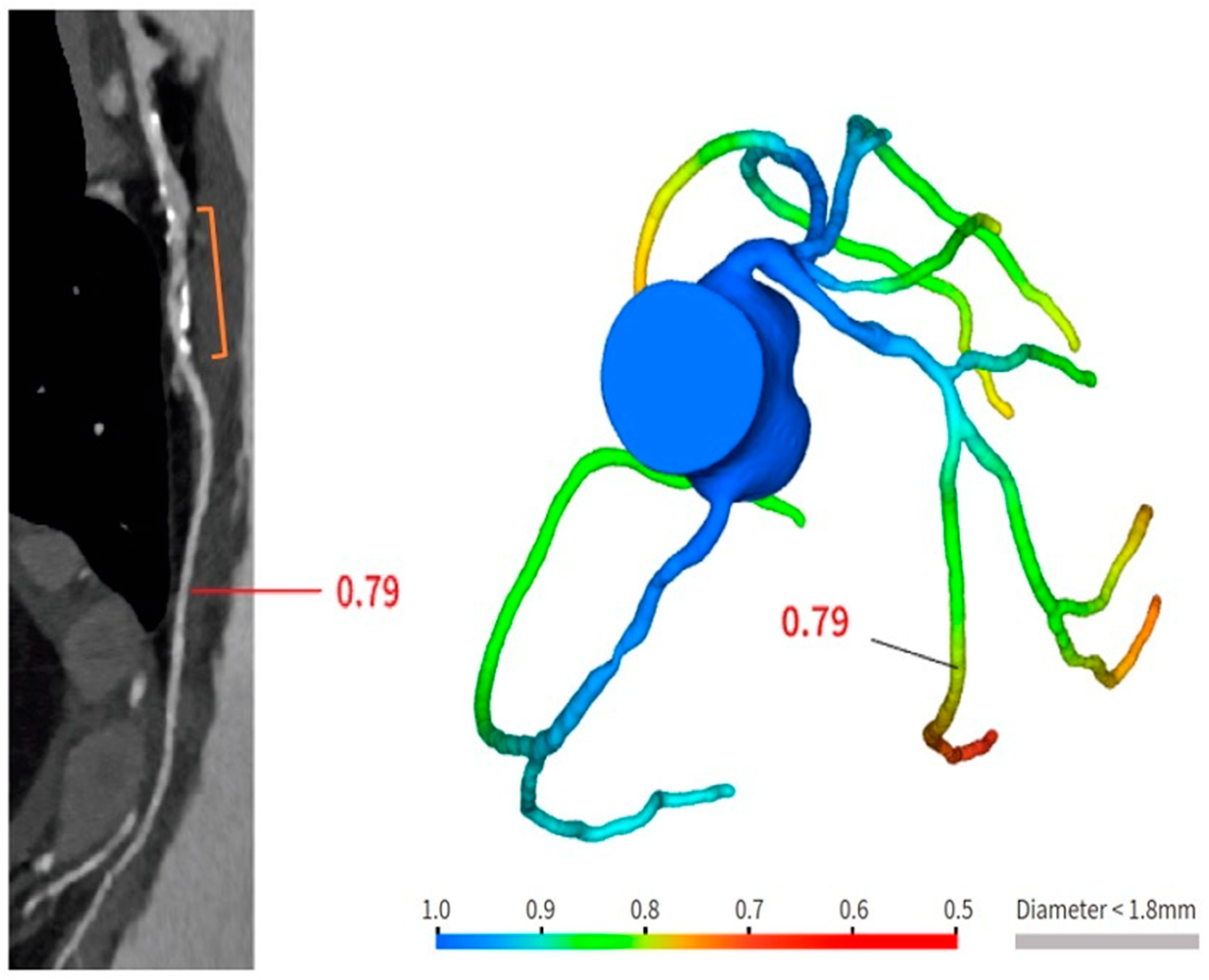
Figure 4. Anatomical and functional assessment of ISR in a scaffold (orange bracket). ISR appears to be characterized by a major calcific part, and the stenosis results were significant under the FFR-CT assessment. FFR-CT was performed with DeepVessel FFR (DVFFR) software (Keya Medical, Seattle, WA, USA).
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477.
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367.
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. The Incremental Role of Coronary Computed Tomography in Chronic Coronary Syndromes. J. Clin. Med. 2020, 9, 3925.
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Juni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology/ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619.
- Valgimigli, M.; Chieffo, A.; Lefevre, T.; Colombo, A.; Morice, M.C.; Serruys, P.W. Revisiting the incidence and temporal distribution of cardiac and sudden death in patients undergoing elective intervention for unprotected left main coronary artery stenosis in the drug eluting stent era. Eurointervention 2007, 2, 435–443.
- Roura, G.; Gomez-Lara, J.; Ferreiro, J.L.; Gomez-Hospital, J.A.; Romaguera, R.; Teruel, L.M.; Carreno, E.; Esplugas, E.; Alfonso, F.; Cequier, A. Multislice CT for assessing in-stent dimensions after left main coronary artery stenting: A comparison with three dimensional intravascular ultrasound. Heart 2013, 99, 1106–1112.
- Van Mieghem, C.A.G.; Cademartiri, F.; Mollet, N.R.; Malagutti, P.; Valgimigli, M.; Mejboom, W.B.; Pugliese, F.; McFadden, E.P.; Ligthart, J.; Runza, G.; et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: A comparison with conventional coronary angiography and intravascular ultrasound. Circulation 2006, 114, 645–653.
- Bennett, J.; De Hemptinne, Q.; McCutcheon, K. Magmaris Resorbable Magnesium Scaffold for the Treatment of Coronary Heart Disease: Overview of Its Safety and Efficacy. Expert. Rev. Med. Devices 2019, 16, 757–769.
- Ghafari, C.; Brassart, N.; Delmotte, P.; Brunner, P.; Dghoughi, S.; Carlier, S. Bioresorbable Magnesium-Based Stent: Real-World Clinical Experience and Feasibility of Follow-Up by Coronary Computed Tomography: A New Window to Look at New Scaffolds. Biomedicines 2023, 11, 1150.
- Collet, C.; Chevalier, B.; Cequier, A.; Fajadet, J.; Dominici, M.; Helqvist, S.; Van Boven, A.J.; Dudek, D.; McClean, D.; Almeida, M.; et al. Diagnostic accuracy of coronary CT angiography for the evaluation of bioresorbable vascular scaffolds. J. Am. Coll. Cardiol. Imaging 2018, 11, 722–732.
- Cigarroa, J.E. Coronary CT Angiography and Bioresorbable Vascular Scaffolds: Is There Clarity? JACC Cardiovasc. Imaging 2018, 11, 733–735.
- Salinas, P.; Pozo-Osinalde, E.; Cerrato, E.; Garcia-Blas, S.; Vaudano, G.P.; Parrilla, C.; Sanchis, J.; Varbella, F.; Escaned, J. Cardiac Computed Tomography Angiography Follow-Up of Resorbable Magnesium Scaffolds. Cardiovasc. Revasc Med. 2021, 29, 18–21.
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural Netw. 2020, 123, 82–93.
- Tonet, E.; Cossu, A.; Pompei, G.; Ruggiero, R.; Caglioni, S.; Mele, D.; Boccadoro, A.; Micillo, M.; Cocco, M.; De Raffele, M.; et al. Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold. Life 2022, 12, 1612.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
734
Revisions:
2 times
(View History)
Update Date:
31 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





