Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikola Ferara | -- | 1598 | 2024-01-30 00:42:17 | | | |
| 2 | Camila Xu | Meta information modification | 1598 | 2024-01-30 01:13:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferara, N.; Špoljar, S.; Lugović-Mihić, L.; Grginić, A.G.; Vranješ, V.R.; Bešlić, I.; Perović, J.; Džombeta, T.R. Purpureocillium lilacinum. Encyclopedia. Available online: https://encyclopedia.pub/entry/54497 (accessed on 07 February 2026).
Ferara N, Špoljar S, Lugović-Mihić L, Grginić AG, Vranješ VR, Bešlić I, et al. Purpureocillium lilacinum. Encyclopedia. Available at: https://encyclopedia.pub/entry/54497. Accessed February 07, 2026.
Ferara, Nikola, Sanja Špoljar, Liborija Lugović-Mihić, Ana Gverić Grginić, Violeta Rezo Vranješ, Iva Bešlić, Judita Perović, Tihana Regović Džombeta. "Purpureocillium lilacinum" Encyclopedia, https://encyclopedia.pub/entry/54497 (accessed February 07, 2026).
Ferara, N., Špoljar, S., Lugović-Mihić, L., Grginić, A.G., Vranješ, V.R., Bešlić, I., Perović, J., & Džombeta, T.R. (2024, January 30). Purpureocillium lilacinum. In Encyclopedia. https://encyclopedia.pub/entry/54497
Ferara, Nikola, et al. "Purpureocillium lilacinum." Encyclopedia. Web. 30 January, 2024.
Copy Citation
Purpureocillium lilacinum is a ubiquitous hyaline fungus that is widely distributed in the environment. This fungus has a well-established place in agriculture as a biological nematicide, due to its ability to parasitize nematodes and their eggs while producing secondary metabolites that can promote plant growth. Despite being previously considered an extremely rare pathogen in humans, it has the ability to cause infections of the skin and other sites in both immunosuppressed and healthy individuals.
cutaneous fungal infections
cutaneous hyalohyphomycosis
invasive fungal infections
1. Epidemiology
P. lilacinum is an emerging pathogen among immunocompromised individuals [1]. The most frequent predisposing factors for invasive infections are: hematological and oncological diseases (30.7% of invasive infections), solid organ transplantation (SOT), steroid treatments, and diabetes mellitus [2]. In this group, the most frequent type of infection is a local cutaneous one, followed by invasive sinusitis, pneumonia, and CVC (central venous catheter)-associated fungemia [3]. However, P. lilacinum also causes infections among immunocompetent individuals, mostly ocular infections (keratitis and endophthalmitis) related to ophthalmic surgery, non-surgical trauma, and skin infections [1]. It is also an opportunistic pathogen in infections associated with medical devices, such as a cardiac prosthesis or dialysis catheters [4]. A recent review identified 101 cases with invasive P. lilacinum worldwide in a period between 1974 and 2020, with the highest number of cases in the United States. Patients were mostly male (61.4%) and the median age was 53 years [2].
2. Pathogenesis
Due to the ubiquitous distribution of P. lilacinum in the environment, there are multiple possible modes of infection [5]. The most frequent infection sites are the skin, subcutaneous tissue, and eyes, although it can spread through the bloodstream, causing infections in various organs, such as the lungs, sinuses, and CNS [6]. Infection commonly occurs via the inhalation of the fungus and its consequent dissemination to the skin and other sites or directly, through inoculation at a site of skin or mucosal breakdown [7]. There have been reports of hospital-acquired P. lilacinum infections due to contaminated medical supplies, including lotions and catheters, or even tattoo-related infections, due to the contamination of the tattoo needle or ink [1]. An interesting feature of this fungus is that it can infect human phagocytic cells (macrophages and dendritic cells), thus escaping local immune defenses and migrating via the lymph flow [2]. Also, it exhibits the phenomenon of adventitious sporulation, which is the term used for the presence of fungal reproductive structures, phialides and conidia, within the infected tissue. This phenomenon is associated with a rapid rate of dissemination and a high prevalence of positive blood cultures, due to the sustained release of fungal spores into the bloodstream, along with angioinvasion, which has been observed in some other fungi, such as Fusarium spp. [8]. According to a recent review, P. lilacinum caused disseminated diseases in 22% of cases [2]. Both adventitious sporulation and the escape from the immune system could be responsible for the high rates of recurrent infections and the lack of a spontaneous resolution, as is often seen in P. lilacinum infections [4]. Although the immune response towards the fungus differs between immunocompetent and immunosuppressed individuals, P. lilacinum is capable of causing damage in both groups [9][10]. An experimental murine study showed that, despite not developing any clinical signs of infection, infected immunocompetent mice did have evident tissue damage, assessed using a histopathological analysis, which revealed conidia-like structures in the lung tissue of these mice [10]. Also, in contrast to previous studies, de Sequeira et al. found that P. lilacinum has the ability to infect and cause disease in immunocompetent and immunosuppressed mice with low levels of inoculum [10]. This could explain why P. lilacinum causes infections related to prostheses and medical devices.
3. Cutaneous Infections
The clinical presentations of cutaneous and subcutaneous P. lilacinum infections are variable, are non-specific, and can be misleading. They can vary from small erythematous papules and plaques with a central umbilication to hemorrhagic vesicles, soft or indurated cutaneous or subcutaneous nodules, or even cellulitis and ulcerations (Figure 1 and Figure 2). In one experimental murine study, the subcutaneous inoculation of P. lilacinum caused comparable damage to animal tissue, including dermatitis, panniculitis, and skin ulcerations with a diffuse inflammatory infiltrate in both immunosuppressed and immunocompetent mice. However, the lesions in immunosuppressed mice were more severe, including extensive areas of ulcers covered with crust, dermatitis, and suppurative panniculitis, with more fungal structures observed on histological slides [9]. Ulcerations are the result of angioinvasion, in both humans and mice. Even though some of the lesions can be dramatic and extensive, they are usually completely asymptomatic [5][11]. Skin infections are mostly located on the lower limbs, reinforcing the theory of skin being the inoculation site. Most skin infections are not accompanied by general symptoms, such as a fever or malaise [5]. The latest reports point out that P. lilacinum infections of the lower limbs in immunocompromised patient can be easily mistaken for typical bacterial cellulitis, caused by Streptococcus and Staphylococcus, and P. lilacinum is usually suspected only after the patient is unresponsive to antibiotic therapy [7]. A distinctive feature of P. lilacinum infections is the complete lack of a spontaneous resolution and a tendency towards recurrent infections [4], which is why they need to be properly diagnosed, taken seriously, and treated accordingly.
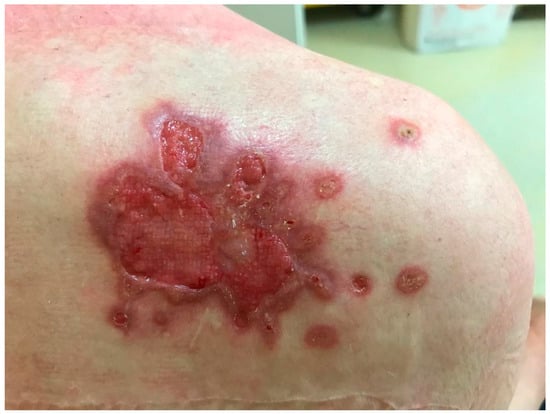
Figure 1. Ulcerative skin lesion on the skin of right scapula as a manifestation of P. lilacinum skin infection (from archives of the authors).
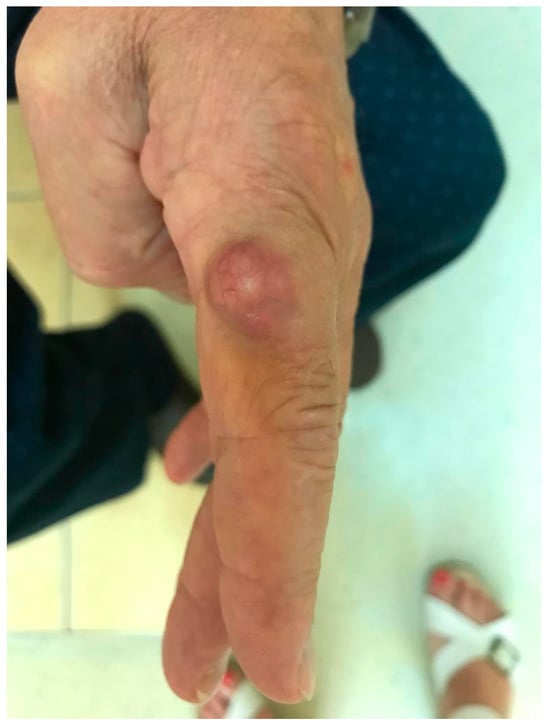
Figure 2. Cutaneous nodule on the left index finger as a manifestation of P. lilacinum infection (from archives of the authors).
4. Diagnosis
The gold standard for making a diagnosis of a P. lilacinum infection is cultivation from lesions suspected to be the sites of infection [11]. P. lilacinum can grow on a conventional fungal culture medium in a rather rapid manner, and mature colonies can be obtained within three days [4]. Its growth is characterized by violaceous colonies with a woolly surface, while microscopic examination reveals branching, hyaline hyphae and phialides tapering at their distal end as chain-like conidiophores [11]. However, its growth in a culture can sometimes be the result of a contaminated laboratory environment. Moreover, due to its ubiquity, P. lilacinum is usually considered a contaminant in cultures until it is confirmed through a histopathological analysis. That is why it is mandatory to obtain a skin biopsy, since a histological examination with routine stains can detect hyphae and reproductive structures, such as phialides and conidia [4]. Additionally, the Grocott methenamine silver (GMS) stain is commonly used, since it imparts a black color to the fungal profiles and a pale green color to the background [12]. Due to its ability to sporulate in tissues, P. lilacinum can be confused with Blastomyces dermatitidis, but it is differentiated by the presence of hyphal elements within a tissue biopsy, elevated (1-3)-β-d-glucan, and growth on cultures [13]. In doubtful cases, it is advisable to confirm the diagnosis with MALDI-TOF mass spectrometry, DNA sequencing, or polymerase chain reaction (PCR) amplification of the 18 S RNA [4]. Identifying the exact fungus is of utmost importance due to the major differences in the sensitivity to antifungal agents within the same species, with an example being Purpureocilium lilacinum vs. P. variotti [5]. Additionally, when a disseminated disease is suspected, imaging techniques should be performed to assess the severity of the disease. The most commonly used imaging techniques are chest and paranasal CT (computed tomography) scans, followed by CNS imaging and the use of both a CT scan and MR [2]. Figure 3 summarizes the clinical presentation, the patient characteristics, and the diagnostic steps in a P. lilacinum infection.
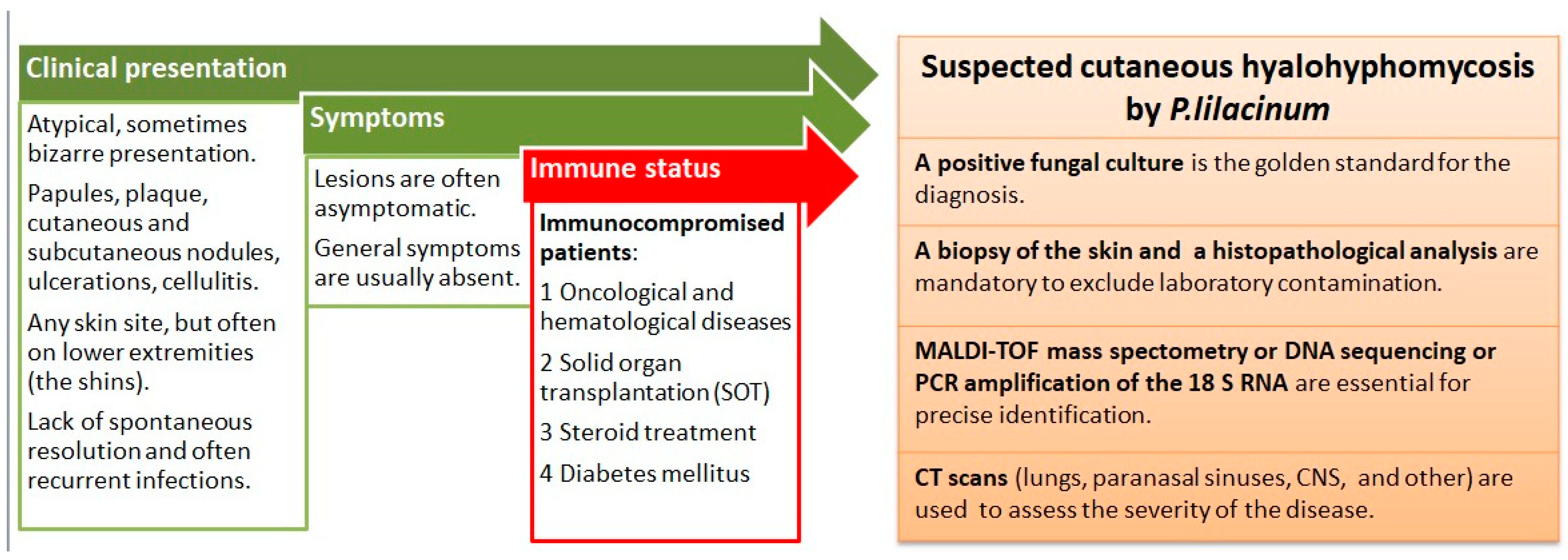
Figure 3. Summary of clinical presentation, patient characteristics, and diagnostic steps in P. lilacinum infection.
5. Treatment
There is no standard treatment for a cutaneous P. lilacinum infection, and treatment is often difficult. Clinical management consists of an antifungal treatment, surgery, or a combination of both [14]. This fungus is intrinsically resistant to many antifungal agents, including itraconazole, terbinafine, griseofulvin, and amphotericin B [6][14]. Because of this, the treatment of P. lilacinum infections should be tailored according to the in vitro susceptibility results. Second-generation triazoles, such as voriconazole, posaconazole, isavuconazole, and ravuconazole, are promising treatment options [15]. Most of the recent reports show that posaconazole and variconazole have the lowest minimum inhibitory concentration (MIC) [2]. In most of the successful treatments of cutaneous P. lilacinum infections, voriconazole was the agent of choice [1]. It is also the preferable agent if the CNS is involved [15]. Accetta et al. reported the first successful treatment of an invasive P. lilacinum infection with isavuconazole [6]. Isavuconazole previously showed good results in treating invasive fungal infections in patients that were intolerant of variconazole and posaconazole [6]. It is interesting to note that combination therapy (e.g., amphotericin B + azole) did not result in a statistically significantly lower mortality rate in comparison to monotherapy (18.5% vs. 20%). Also, the use of amphotericin B is associated with a significantly higher mortality rate, which is in accordance with its intrinsic resistance [2]. The accumulated body of evidence shows that surgery plays an important part in the management of P. lilacinum infections (strength of recommendation: B, quality of evidence: III), and subcutaneous skin infections cure faster with surgery [14]. In a recent case report of a chronic subcutaneous infection of P. lilacinum in a female patient who received a hepato-renal allograft transplant, the authors emphasized the importance of a complete surgical intervention and foreign body search; complementary to antifungal agents, these interventions proved to be beneficial in preventing a recurrence or relapse of the cutaneous infection [5]. This was backed up by another case report in which a recurrent deep necrotic ulcer of the shin caused by P. lilacinum was successfully managed only after surgical debridement followed by split-thickness skin grafting, which resulted in the absence of recurrences at a two-year follow-up [11]. The necessity for surgical interventions in the treatment of P. lilacinum infections may be related to its ability to sporulate in tissues [15].
References
- Trinh, S.A.; Angarone, M.P. Purpureocillium lilacinum tattoo-related skin infection in a kidney transplant recipient. Transpl. Infect. Dis. 2017, 19, e12689.
- Sprute, R.; Salmanton-García, J.; Sal, E.; Malaj, X.; Ráčil, Z.; Ruiz de Alegría Puig, C.; Falces-Romero, I.; Barać, A.; Desoubeaux, G.; Kindo, A.J.; et al. Invasive infections with Purpureocillium lilacinum: Clinical characteristics and outcome of 101 cases from FungiScope® and the literature. J. Antimicrob. Chemother. 2021, 76, 1593–1603.
- Jacobs, S.E.; Wengenack, N.L.; Walsh, T.J. Non-Aspergillus Hyaline Molds: Emerging Causes of Sino-Pulmonary Fungal Infections and Other Invasive Mycoses. Semin. Respir. Crit. Care Med. 2020, 41, 115–130.
- Saghrouni, F.; Saidi, W.; Ben Said, Z.; Gheith, S.; Ben Said, M.; Ranque, S.; Denguezli, M. Cutaneous hyalohyphomycosis caused by Purpureocillium lilacinum in an immunocompetent patient: Case report and review. Med. Mycol. 2013, 51, 664–668.
- Albert, R.; Lemaignen, A.; Desoubeaux, G.; Bailly, E.; Bernard, L.; Lacasse, M. Chronic subcutaneous infection of Purpureocillium lilacinum in an immunocompromised patient: Case report and review of the literature. Med. Mycol. Case Rep. 2022, 38, 5–8.
- Accetta, J.; Powell, E.; Boh, E.; Bull, L.; Kadi, A.; Luk, A. Isavuconazonium for the treatment of Purpureocillium lilacinum infection in a patient with pyoderma gangrenosum. Med. Mycol. Case Rep. 2020, 29, 18–21.
- Paul, J.; Czech, M.M.; Balijepally, R.; Brown, J.W. Diagnostic and therapeutic challenges of treating opportunistic fungal cellulitis: A case series. BMC Infect. Dis. 2022, 22, 435.
- Lockwood, M.B.; Crescencio, J.C. Adventitious sporulation in Fusarium: The yeast that were not. IDCases 2015, 3, 5–7.
- Corrêa-Moreira, D.; de Lima Neto, R.G.; da Costa, G.L.; de Moraes Borba, C.; Oliveira, M.M.E. Purpureocillium lilacinum an emergent pathogen: Antifungal susceptibility of environmental and clinical strains. Lett. Appl. Microbiol. 2022, 75, 45–50.
- de Sequeira, D.C.M.; Menezes, R.C.; Oliveira, M.M.E.; Antas, P.R.Z.; De Luca, P.M.; Oliveira-Ferreira, J.D.; Borba, C.D.M. Experimental hyalohyphomycosis by Purpureocillium lilacinum: Outcome of the infection in C57BL/6 murine models. Front. Microbiol. 2017, 8, 1617.
- Chen, W.Y.; Lin, S.R.; Hung, S.J. Successful Treatment of Recurrent Cutaneous Purpureocillium lilacinum (Paecilomyces lilacinus) Infection with Posaconazole and Surgical Debridement. Acta Derm.-Venereol. 2019, 99, 1313–1314.
- Sowmya, S.V.; Augustine, D.; Hemanth, B.; Prathab, A.G.; Alamoudi, A.; Bahammam, H.A.; Bahammam, S.A.; Bahammam, M.A.; Haragannavar, V.C.; Prabhu, S.; et al. Alternate Special Stains for the Detection of Mycotic Organisms in Oral Cyto-Smears—A Histomorphometric Study. Microorganisms 2022, 10, 1226.
- Lundstrom, Z.T.; Abu Saleh, O.M. The Blastomycosis Bluff by Purpureocillium lilacinum. Mayo Clin. Proc. 2019, 94, 735–736.
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20 (Suppl. S3), 27–46.
- Corbeddu, M.; Ferreli, C.; Cappai, R.; Ferraguti, P.; Atzori, L.; Pilloni, L.; Rongioletti, F. Fatal hyalohyphomycosis with cutaneous involvement caused by Purpureocillium lilacinum in an immunocompromised patient with bullous pemphigoid. Acta Biomed. 2021, 92, e2021139.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
965
Revisions:
2 times
(View History)
Update Date:
30 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




