Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | William Bruno | -- | 2244 | 2024-01-26 11:04:56 | | | |
| 2 | Jason Zhu | Meta information modification | 2244 | 2024-01-29 03:29:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Andreotti, V.; Vanni, I.; Pastorino, L.; Ghiorzo, P.; Bruno, W. Germline POT1 Variants. Encyclopedia. Available online: https://encyclopedia.pub/entry/54409 (accessed on 08 February 2026).
Andreotti V, Vanni I, Pastorino L, Ghiorzo P, Bruno W. Germline POT1 Variants. Encyclopedia. Available at: https://encyclopedia.pub/entry/54409. Accessed February 08, 2026.
Andreotti, Virginia, Irene Vanni, Lorenza Pastorino, Paola Ghiorzo, William Bruno. "Germline POT1 Variants" Encyclopedia, https://encyclopedia.pub/entry/54409 (accessed February 08, 2026).
Andreotti, V., Vanni, I., Pastorino, L., Ghiorzo, P., & Bruno, W. (2024, January 26). Germline POT1 Variants. In Encyclopedia. https://encyclopedia.pub/entry/54409
Andreotti, Virginia, et al. "Germline POT1 Variants." Encyclopedia. Web. 26 January, 2024.
Copy Citation
Protection of Telomere 1 (POT1) was deemed as a novel high-penetrance susceptibility gene to cutaneous melanoma nearly 10 years ago. Thereafter, various cancers have been proposed as associated with germline POT1 variants in the context of the so-called POT1 Predisposition Tumor Syndrome (POT1–TPD). While the key role, and related risks, of the alterations in POT1 in melanoma are established, the correlation between germline POT1 variants and the susceptibility to other cancers partially lacks evidence, due also to the rarity of POT1–TPD.
POT1
pathogenic variants
germline
melanoma
cancer predisposition
1. Introduction
Protection of Telomere 1 (POT1), encoded by a gene on chromosome 7q31.33, is a key subunit of the shelterin telomere binding complex, which protects cells from inappropriate DNA damage response and plays a critical role in telomere regulation. In particular, OB1 and OB2 domains at the N terminus of this essential subunit bind the terminal portions of single-stranded telomeres (ssDNA, TTAGGG repeats) through the interaction with TPP1/ACD, another component of the shelterin complex. The shortening of telomeres occurs at each cell cycle, and POT1 and the shelterin complex protect the ends of chromosomes from instability and regulate their length [1][2]. As an integral part of a complex capable of controlling cellular senescence mechanisms, POT1 plays a key role in telomere homeostasis, while it also acts as a genomic stabilizer [3]. Studies showed that pathogenic variants in the POT1 gene are associated with the alteration of the telomere length and a subsequent increased risk for cancer. Therefore, it is a gene of interest in studying cancer progression and predisposition syndromes [4][5][6] (see Figure 1).
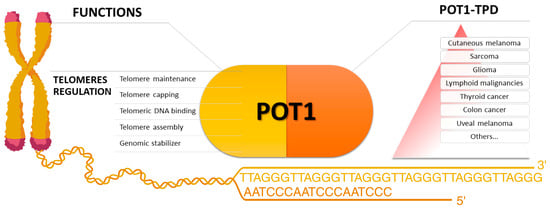
Figure 1. On the left, the main functions in which the protein Protection of Telomere 1 is involved. On the right, a summary of the main cancers proposed as associated with POT1 Tumor Predisposition Syndrome. Cutaneous melanoma is at the top of the pyramid for the consistency of the data supporting its association with the syndrome (created with BioRender.com).
In 2014, POT1 was deemed as a novel high-penetrance susceptibility gene to cutaneous melanoma by two independent studies [5][7]. Functional and animal model studies thoroughly investigated the susceptibility variants found in cutaneous melanoma families, but after that, the number of cancers proposed as associated with the clinical spectrum of the POT1 predisposition tumor syndrome (POT1–TPD) increased [8].
Since more than 50 variants in POT1 were identified either as germline predisposition alleles, or somatic mutations in different cancers, evidence was collected to consider POT1 as a relevant gene in the tumorigenesis process [9]. POT1 has a positive Z-score for both missense and loss-of-function variants, indicating an intolerance to variation [10], hence the low frequency of POT1 variants. Furthermore, their apparently incomplete penetrance, or evidence from mouse models that they may contribute to tumorigenesis, but that other mechanisms, e.g., p53 deficiency, may be required [11], need to be considered when evaluating the strength of proposed clinical associations.
As aforementioned, germline POT1 variants were reported as associated with cancers other than melanoma, such as glioma, lymphoid malignancies, colon and thyroid cancer, uveal melanoma, and sarcomas [9]. Hence, a Li–Fraumeni-like surveillance protocol for POT1 germline variant carriers was also proposed [12], although a standardization on this issue is missing.
2. Glioma
In 2014, a study reported the finding of three variants in three out of 301 glioma families: p.G95C, p.E450X, and p.D617Efs*9 (reported as p.D617Efs*8), the first two segregating in two affected relatives of these small families and with a likely incomplete penetrance [13].
The binding between POT1 and telomeres or TPP1/ACD was predicted to be disrupted. As a functional study, Telomere Length (TL) analysis was performed. As noted in a relative commentary, and its reply, it is not excluded that the finding of the variants could be by chance, and the clinical association lacked further functional validation and reproducibility of the data [14][15].
Interestingly, a study on a native mouse glioma model demonstrated that only p.G95C, a missense variant in the POT1 OB1 DNA-binding domain, can play a proliferative role in glioma initiation. The authors also suggest that, due to the complexity of gliomagenesis and peculiar effects potentially dependent on POT1 variants, e.g., sexual dimorphism, further mechanisms should be investigated [16].
On the sidelines, another study suggested a correlation between glioma risk and POT1, albeit as a risk factor locus, along with others involved in telomere regulation [17].
3. Colon Cancer
One study reported that whole-exome (WES) and whole-genome sequencing (WGS) performed on a total amount of 6558 cases of ColoRectal Cancer (CRC) allowed to find in three different cases three POT1 variants: the aforementioned p.D617Efs*9, p.Arg363Ter, and p.Asn75LysfsTer16 [18].
A subset-based meta-analyses of 204,993 SNPs, among 61,851 cancer cases, reported 13 SNPs as associated with cancer risk. Only the rs116895242 near POT1 was evaluated as inversely associated with colorectal, ovarian, and lung cancers [19].
An association among seven SNPs in POT1 and susceptibility to CRC was reported. Still, only one SNP (rs7794637) was previously associated with a cancer predisposition, i.e., to breast cancer, and no further evaluations were performed [20].
4. Thyroid Cancer
In 2017, the p.K90E variant was identified via WES in a family of eight members affected by cutaneous melanoma. Seven of the eight patients with melanoma carried the variant [21].
The variant segregates with individuals who developed cutaneous melanoma, with a positive family history of endocrine diseases, not only thyroid-related, and is currently classified as likely pathogenic. Two of the relatives were diagnosed with thyroid cancer, one along with cutaneous melanoma, and one along with renal cell cancer, but not cutaneous melanoma, yet their carrier status was not assessed.
In 2020, a WGS study on five families with a history of Non-Medullary Thyroid Cancer (NMTC), multinodular goiter, and thyroid benign nodules found the variant p.V29L in one family [22].
Four family members, who presented papillary thyroid carcinoma, carried the variant, while one out of three family members, with only benign nodules, carried it.
In silico studies predicted a weak binding of the OB1 domain to telomeric ssDNA, also demonstrated by a Chip assay. The binding to TPP1/ACD was not tested.
No significant differences were observed regarding protein expression in HEK293T transfected cells or TL between V29L carriers and not carriers (only in transfected cells).
To date this is the only study reporting the p.V29L variant.
Targeted testing of seven NMTC families found no variant nor in POT1 or other genes allegedly associated with NMTC susceptibility [23].
In the same year, a study proposed an association between the SNP rs58722976, corresponding to a POT1 intronic variant, and found in three out of 110 childhood cancer patients, and the risk for subsequent thyroid malignant neoplasm. The SNP was also found in 14 out of 4596 patients who did not develop thyroid cancer. An increased TL was also associated with this variant. However, its low frequency did not allow further studies consolidating the clinical association, e.g., focused on possible ancestry differences and biases regarding the predisposition to thyroid diseases [24].
The variant p.I49Mfs*7 was found in a 65-year-old patient with papillary thyroid carcinoma, cutaneous melanoma, and splenic marginal zone lymphoma. The variant was assessed as germline by next-generation sequencing on skin fibroblasts and once reported in a patient with glioma as a putative predisposition variant [25]. Further personal and family history information was unavailable, and no functional studies were performed [26].
5. Uveal Melanoma
A few genes, e.g., BAP1 and MDB4, are the only established genes correlated to hereditary and syndromic forms of Uveal Melanoma (UM) [27][28].
A study reported two deleterious variants in two out of 20 UM patients [29]. Both patients also developed cutaneous melanoma. However, the patients were from Australia, the country with the highest melanoma incidence, and melanoma was diagnosed after 65 years of age. Segregation and TL measurement, as functional analysis, were evaluated for both cases. While the first variant, p.Q94Rfs*13 (reported as p.Q94Rfs*12), is predicted as likely pathogenic, the second one, written as c.1851_1852del, is the same discussed above in association with glioma risk, or reported in a CRC cohort (p.D617Efs*9).
6. Sarcoma
A peculiar scenario of variant-specific phenotype is the one described for the risk of sarcomas, e.g., cardiac angiosarcoma, in correlation with the founder variant p.R117C, identified in four TP53-negative Li–Fraumeni-Like (LFL) Spanish families [30].
In a subsequent study, the authors identified four additional variants predicted to be damaging in two out of 10 LFL families probands with angiosarcoma (p.Gln301*, p.T497K, being reported as T497L) and in two out of 30 sporadic cardiac angiosarcoma cases (p.R116C, c.547-1G>A). In contrast, one sporadic case carried the p.R117C variant. Of note, no POT1 variant was found in 24 LFL families without angiosarcoma cases [31].
Above all, data from in vitro studies confirmed in silico prediction about p.R117C, in terms of reduced telomeres, TPP1/ACD binding of POT1, and abnormal TL.
In 2022, a further convincing study observed the same effect on TL for this variant and the development of angiosarcomas in the knock-in mice model [32].
A European WES and targeted sequencing study on 1244 patients with osteosarcoma led to the identification of likely pathogenic (c.124G>T, p.Gln376Arg, c.205C>T, D224N) and pathogenic variants (c.1851_1852delTA) in POT1, among a few unexpected genes (CDKN2A, MEN1, VHL, APC, MSH2, ATRX), other than in TP53. However, functional analysis for the specific variants was not performed, and the authors also declared some limitations, such as the lack of family history assessment and methodological biases [33].
On a side note, a WES study identified the p.P35L variant in one out of 13 probands of LFL families. The variant is not reported in any database, and its pathogenicity is predicted by in silico structural analysis [34].
7. Lymphoid Malignancies
In 2013, a GWAS, integrated with metanalyses, mapped new susceptibility loci for Chronic Lymphocytic Leukemia (CLL) to 7q31.33 and proposed an association between the POT1 3′UTR SNP rs17246404 and a small CLL increased risk [35]. In 2016, a WES study from the same authors identified four deleterious POT1 variants, each segregating in four out of 66 CLL families [36]. p.Tyr36Cys, previously reported at somatic level in CLL cases [37][38], was predicted by a structural prediction tool to impact POT1 and telomeric DNA affinity, whereas c.1164-1G>A, p.Gln358SerFTer13, and p.Gln376Arg were predicted to impair POT1-TPP1/ACD interaction. For the latter variant, an analysis of 1083 cases vs. 5854 controls showed a higher risk of CLL (3.61-fold). Nonetheless, while being in an evolutionarily conserved residue, it was predicted to not affect the protein stability and was not in linkage disequilibrium with SNP rs17246404. No significant difference in TL was observed for all the variants.
In 2018, a WES study identified two POT1 variants in two out of 41 Hodgkin lymphoma families, D224N and Y36H, the second one seemingly with incomplete penetrance (one carrier was unaffected). For both variants, an increased TL was observed in human cells [39]. A variant similar to Y36H, Y36N, was previously studied, and the binding to the telomeric overhang was not compromised [4], in contrast to another study that identified the same variant as somatic in CLL cases, and demonstrated an impact on telomeric binding [37]. Of note, the susceptibility hypothetically underlying a familial clustering of Hodgkin lymphoma is still far to be uncovered, and other rare variants segregated in the two families. Hence, while a contribution of deleterious POT1 variants may not be ruled out, other genes may be equally worth investigating.
The D224N was aforementioned among the variants found through a WES performed on osteosarcoma cases and reported as segregating in a family with a variety of lymphoid malignancies (Hodgkin lymphoma, Myeloid Chronic Leukemia, Follicular lymphoma) and with a proband who also developed cutaneous melanoma, renal cell carcinoma, colon cancer, and prostate cancer. The father developed a similar cluster of cutaneous melanoma and lymphoid malignancies, although from 62 to 76 years of age. Neoplastic tissues for further studies were not available, and other possible risk factors, e.g., lifestyle, sun exposure, environment, and the median age of diagnosis in the general population, were not reported or highlighted [40].
In 2021, the p.Q199* germline variant was identified in only one child with Acute Myeloid Leukemia for which evidence of pathogenicity was found at cell, protein expression, and mRNA levels [41].
An exome analysis found three POT1 variants in as many multiple myeloma patients out of 128 patients: c.458T>A, c.1594G>C, and c.547-1G>A [42].
The first one was found in a patient also diagnosed with thyroid cancer at 36 years and whose father, affected by multiple myeloma, was not a carrier of the variant. The second one was found in a proband just diagnosed with osteosarcoma at 20 years, multiple myeloma at 66, and acute myeloid leukemia at 70 and previously identified in a cutaneous melanoma family [5]. The third variant, also found in the proband’s mother with a diagnosis of multiple myeloma, is the same described in a family with angiosarcoma [31]. Multiple myeloma was not reported in both of the cutaneous melanoma or sarcoma families. The same study reported the variant c.1A>G (p.Met1Val) in a patient diagnosed with essential thrombocythemia and Monoclonal Gammopathy of Undetermined Significance at 77 and papillary thyroid carcinoma at 78 years of age.
A recent study on a case series of 3323 patients with different diagnoses of myeloproliferative neoplasm identified several somatic, germline, and putative germline POT1 variants. Thirteen variants were established as germline, and for four patients, a history of cancers, allegedly associated with POT1 variants, is reported (cutaneous melanoma, glioma, thyroid cancer).
References
- Aramburu, T.; Plucinsky, S.; Skordalakes, E. POT1-TPP1 telomere length regulation and disease. Comput. Struct. Biotechnol. J. 2020, 18, 1939–1946.
- Aramburu, T.; Kelich, J.; Rice, C.; Skordalakes, E. POT1-TPP1 binding stabilizes POT1, promoting efficient telomere maintenance. Comput. Struct. Biotechnol. J. 2022, 20, 675–684.
- Yu, Y.; Tan, R.; Ren, Q.; Gao, B.; Sheng, Z.; Zhang, J.; Zheng, X.; Jiang, Y.; Lan, L.; Mao, Z. POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions. Aging 2017, 9, 2529–2543.
- Gu, P.; Wang, Y.; Bisht, K.K.; Wu, L.; Kukova, L.; Smith, E.M.; Xiao, Y.; Bailey, S.M.; Lei, M.; Nandakumar, J.; et al. Pot1 OB-fold mutations unleash telomere instability to initiate tumorigenesis. Oncogene 2017, 36, 1939–1951.
- Shi, J.; Yang, X.R.; Ballew, B.; Rotunno, M.; Calista, D.; Fargnoli, M.C.; Ghiorzo, P.; Bressac-de Paillerets, B.; Nagore, E.; Avril, M.F.; et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 2014, 46, 482–486.
- Simonin-Wilmer, I.; Ossio, R.; Leddin, E.M.; Harland, M.; Pooley, K.A.; Martil De La Garza, M.G.; Obolenski, S.; Hewinson, J.; Wong, C.C.; Iyer, V.; et al. Population-based analysis of POT1 variants in a cutaneous melanoma case–control cohort. J. Med. Genet. 2022, 60, 692–696.
- Robles-Espinoza, C.D.; Harland, M.; Ramsay, A.J.; Aoude, L.G.; Quesada, V.; Ding, Z.; Pooley, K.A.; Pritchard, A.L.; Tiffen, J.C.; Petljak, M.; et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 2014, 46, 478–481.
- Herrera-Mullar, J.; Fulk, K.; Brannan, T.; Yussuf, A.; Polfus, L.; Richardson, M.E.; Horton, C. Characterization of tumor predisposition syndrome: Tumor prevalence in a clinically diverse hereditary cancer cohort. Genet. Med. 2023, 25, 100937.
- Wu, Y.; Poulos, R.C.; Reddel, R.R. Role of POT1 in Human Cancer. Cancers 2020, 12, 2739.
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291.
- Gong, Y.; Stock, A.J.; Liu, Y. The enigma of excessively long telomeres in cancer: Lessons learned from rare human POT1 variants. Curr. Opin. Genet. Dev. 2020, 60, 48–55.
- Henry, M.-L.; Osborne, J.; Else, T. POT1 Tumor Predisposition; University of Washington: Seattle, WA, USA, 2022.
- Bainbridge, M.N.; Armstrong, G.N.; Gramatges, M.M.; Bertuch, A.A.; Jhangiani, S.N.; Doddapaneni, H.; Lewis, L.; Tombrello, J.; Tsavachidis, S.; Liu, Y.; et al. Germline mutations in shelterin complex genes are associated with familial glioma. J. Natl. Cancer Inst. 2015, 107, 384.
- Cooke, J.G. RE: Germline Mutations in Shelterin Complex Genes Are Associated with Familial Glioma. J. Natl. Cancer Inst. 2015, 107, djv173.
- Bainbridge, M.; Bondy, M.L. Response. J. Natl. Cancer Inst. 2015, 107, djv174.
- Jalali, A.; Yu, K.; Beechar, V.; Huerta, N.A.B.; Grichuk, A.; Mehra, D.; Lozzi, B.; Kong, K.; Scott, K.L.; Rao, G.; et al. POT1 Regulates Proliferation and Confers Sexual Dimorphism in Glioma. Cancer Res. 2021, 81, 2703–2713.
- Saunders, C.N.; Kinnersley, B.; Culliford, R.; Cornish, A.J.; Law, P.J.; Houlston, R.S. Relationship between genetically determined telomere length and glioma risk. Neuro-Oncology 2022, 24, 171–181.
- Chubb, D.; Broderick, P.; Dobbins, S.E.; Frampton, M.; Kinnersley, B.; Penegar, S.; Price, A.; Ma, Y.P.; Sherborne, A.L.; Palles, C.; et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat. Commun. 2016, 7, 11883.
- Karami, S.; Han, Y.; Pande, M.; Cheng, I.; Rudd, J.; Pierce, B.L.; Nutter, E.L.; Schumacher, F.R.; Kote-Jarai, Z.; Lindstrom, S.; et al. Telomere structure and maintenance gene variants and risk of five cancer types. Int. J. Cancer 2016, 139, 2655–2670.
- Martel-Martel, A.; Corchete, L.A.; Martí, M.; Vidal-Tocino, R.; Hurtado, E.; Álvaro, E.; Jiménez, F.; Jiménez-Toscano, M.; Balaguer, F.; Sanz, G.; et al. Telomere Length as a New Risk Marker of Early-Onset Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 3526.
- Wilson, T.L.-S.; Hattangady, N.; Lerario, A.M.; Williams, C.; Koeppe, E.; Quinonez, S.; Osborne, J.; Cha, K.B.; Else, T. A new POT1 germline mutation—Expanding the spectrum of POT1-associated cancers. Fam. Cancer 2017, 16, 561–566.
- Srivastava, A.; Miao, B.; Skopelitou, D.; Kumar, V.; Kumar, A.; Paramasivam, N.; Bonora, E.; Hemminki, K.; Försti, A.; Bandapalli, O.R. A Germline Mutation in the POT1 Gene Is a Candidate for Familial Non-Medullary Thyroid Cancer. Cancers 2020, 12, 1441.
- Orois, A.; Badenas, C.; Reverter, J.L.; López, V.; Potrony, M.; Mora, M.; Halperin, I.; Oriola, J. Lack of Mutations in POT1 Gene in Selected Families with Familial Non-Medullary Thyroid Cancer. Horm. Cancer 2020, 11, 111–116.
- Richard, M.A.; Lupo, P.J.; Morton, L.M.; Yasui, Y.A.; Sapkota, Y.A.; Arnold, M.A.; Aubert, G.; Neglia, J.P.; Turcotte, L.M.; Leisenring, W.M.; et al. Genetic variation in POT1 and risk of thyroid subsequent malignant neoplasm: A report from the Childhood Cancer Survivor Study. PLoS ONE 2020, 15, e0228887.
- Jones, S.; Anagnostou, V.; Lytle, K.; Parpart-Li, S.; Nesselbush, M.; Riley, D.R.; Shukla, M.; Chesnick, B.; Kadan, M.; Papp, E.; et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci. Transl. Med. 2015, 7, 283ra53.
- Jajosky, A.N.; Mitchell, A.L.; Akgul, M.; Shetty, S.; Yoest, J.M.; Gerson, S.L.; Sadri, N.; Oduro, K.A. Identification of a Cancer-Predisposing Germline POT1 p.Ile49Metfs*7 Variant by Targeted Sequencing of a Splenic Marginal Zone Lymphoma. Genes 2022, 13, 591.
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; de la Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341.
- Derrien, A.-C.; Rodrigues, M.; Eeckhoutte, A.; Dayot, S.; Houy, A.; Mobuchon, L.; Gardrat, S.; Lequin, D.; Ballet, S.; Pierron, G.; et al. Germline MBD4 Mutations and Predisposition to Uveal Melanoma. J. Natl. Cancer Inst. 2021, 113, 80–87.
- Nathan, V.; Palmer, J.M.; Johansson, P.A.; Hamilton, H.R.; Warrier, S.K.; Glasson, W.; McGrath, L.A.; Kahl, V.F.S.; Vasireddy, R.S.; Pickett, H.A.; et al. Loss-of-function variants in POT1 predispose to uveal melanoma. J. Med. Genet. 2021, 58, 234–236.
- Calvete, O.; Martinez, P.; Garcia-Pavia, P.; Benitez-Buelga, C.; Paumard-Hernández, B.; Fernandez, V.; Dominguez, F.; Salas, C.; Romero-Laorden, N.; Garcia-Donas, J.; et al. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li–Fraumeni-like families. Nat. Commun. 2015, 6, 8383.
- Calvete, O.; Garcia-Pavia, P.; Domínguez, F.; Bougeard, G.; Kunze, K.; Braeuninger, A.; Teule, A.; Lasa, A.; Ramón y Cajal, T.; Llort, G.; et al. The wide spectrum of POT1 gene variants correlates with multiple cancer types. Eur. J. Hum. Genet. 2017, 25, 1278–1281.
- Martínez, P.; Sánchez-Vázquez, R.; Ferrara-Romeo, I.; Serrano, R.; Flores, J.M.; Blasco, M.A. A mouse model for Li-Fraumeni-like Syndrome with cardiac angiosarcomas associated to POT1 mutations. PLoS Genet. 2022, 18, e1010260.
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients with Osteosarcoma. JAMA Oncol. 2020, 6, 724–734.
- Li, Y.; Xie, Y.; Wang, D.; Xu, H.; Ye, J.; Yin, J.C.; Chen, J.; Yan, J.; Ye, B.; Chen, C. Whole exome sequencing identified a novel POT1 variant as a candidate pathogenic allele underlying a Li–Fraumeni-like family. Front. Oncol. 2022, 12, 963364.
- Speedy, H.E.; Di Bernardo, M.C.; Sava, G.P.; Dyer, M.J.S.; Holroyd, A.; Wang, Y.; Sunter, N.J.; Mansouri, L.; Juliusson, G.; Smedby, K.E.; et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat. Genet. 2013, 46, 56–60.
- Speedy, H.E.; Kinnersley, B.; Chubb, D.; Broderick, P.; Law, P.J.; Litchfield, K.; Jayne, S.; Dyer, M.J.S.; Dearden, C.; Follows, G.A.; et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016, 128, 2319–2326.
- Ramsay, A.J.; Quesada, V.; Foronda, M.; Conde, L.; Martínez-Trillos, A.; Villamor, N.; Rodríguez, D.; Kwarciak, A.; Garabaya, C.; Gallardo, M.; et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013, 45, 526–530.
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530.
- McMaster, M.L.; Sun, C.; Landi, M.T.; Savage, S.A.; Rotunno, M.; Yang, X.R.; Jones, K.; Vogt, A.; Hutchinson, A.; Zhu, B.; et al. Germline mutations in Protection of Telomeres 1 in two families with Hodgkin lymphoma. Br. J. Haematol. 2018, 181, 372–377.
- Nathan, V.; Johansson, P.A.; Palmer, J.M.; Hamilton, H.R.; Howlie, M.; Brooks, K.M.; Hayward, N.K.; Pritchard, A.L. A rare missense variant in protection of telomeres 1 (POT1) predisposes to a range of haematological malignancies. Br. J. Haematol. 2021, 192, e57–e60.
- Michler, P.; Schedel, A.; Witschas, M.; Friedrich, U.A.; Wagener, R.; Mehtonen, J.; Brozou, T.; Menzel, M.; Walter, C.; Nabi, D.; et al. Germline POT1 Deregulation Can Predispose to Myeloid Malignancies in Childhood. Int. J. Mol. Sci. 2021, 22, 11572.
- Hakkarainen, M.; Koski, J.R.; Heckman, C.A.; Anttila, P.; Silvennoinen, R.; Lievonen, J.; Kilpivaara, O.; Wartiovaara-Kautto, U. A germline exome analysis reveals harmful POT1 variants in multiple myeloma patients and families. eJHaem 2022, 3, 1352–1357.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
496
Revisions:
2 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




