Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Benagiano | -- | 3405 | 2024-01-26 09:42:19 | | | |
| 2 | Lindsay Dong | Meta information modification | 3405 | 2024-01-29 02:50:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Benagiano, G.; Mancuso, S.; Guo, S.; Di Renzo, G.C. The Establishment of Pregnancy and Placental Formation. Encyclopedia. Available online: https://encyclopedia.pub/entry/54403 (accessed on 07 February 2026).
Benagiano G, Mancuso S, Guo S, Di Renzo GC. The Establishment of Pregnancy and Placental Formation. Encyclopedia. Available at: https://encyclopedia.pub/entry/54403. Accessed February 07, 2026.
Benagiano, Giuseppe, Salvatore Mancuso, Sun-Wei Guo, Gian Carlo Di Renzo. "The Establishment of Pregnancy and Placental Formation" Encyclopedia, https://encyclopedia.pub/entry/54403 (accessed February 07, 2026).
Benagiano, G., Mancuso, S., Guo, S., & Di Renzo, G.C. (2024, January 26). The Establishment of Pregnancy and Placental Formation. In Encyclopedia. https://encyclopedia.pub/entry/54403
Benagiano, Giuseppe, et al. "The Establishment of Pregnancy and Placental Formation." Encyclopedia. Web. 26 January, 2024.
Copy Citation
The continuing progress in our understanding of the complexity of interactions between the maternal organism and the early embryo is changing the overall outlook on the initial steps in establishing a pregnancy through placental formation. The first two weeks after fertilization must today be viewed as the critical period during which a major embryo selection process takes place in which a proportion that may surpass 50% of them is physiologically eliminated because they are unfit to progress toward birth.
early embryonic loss
embryo-derived platelet-activating factor
early pregnancy factor
gestation
pregnancy
pre-implantation factor
1. Evidence of Early Embryonic Loss
In 1975, Roberts and Lowe were the first to attempt a statistical estimation of pregnancy wastage, placing emphasis on early losses. Their conclusions were somewhat shocking: they estimated that at least 75% of all conceptions do not become a viable fetus and proceed to term [1]. A few years later, Shepard and Fantel quoted an early embryonic/fetal loss of approximately one out of two pregnancies and attributed it to ‘karyotype deviation’ [2].
The availability of the so-called ‘Rosette Inhibition test’ to evidence the presence of a pregnancy-specific protein named Early Pregnancy Factor (EPF) [3], enabled Rolfe to monitor fertilization and early gestation in a group of 13 nulliparous women during 28 cycles. In 18 subjects the presence of EPF was detected in maternal serum within 48 h of the presumed fertilization, but EPF production continued for more than 14 days in only 4 cases and successful pregnancy was maintained in only 2. In the remaining 14 cases, EPF disappeared from the serum before the presumed time of onset of menstruation [4]. A similar investigation was conducted on 18 healthy women during 21 menstrual cycles. EPF was present in 14 cycles, with 6 showing only a transient activity over a 5–10-day period following ovulation [5]. Once again, these investigations showed a surprisingly high proportion of early embryonic loss.
In 1988, Wilcox et al. [6] utilized a highly specific immunoradiometric assay with a sensitivity to detect urinary human chorionic gonadotrophin (hCG) of 0.01 ng/mL, to evaluate the risk of early pregnancy loss in 221 healthy women attempting to conceive over a total of 707 menstrual cycles. They identified 198 pregnancies with an increase in the hCG level near the expected time of implantation and observed that 22% of them ended before pregnancy could be detected clinically. Of relevance, most of the women with unrecognized early pregnancy losses had normal fertility, and, indeed, 95% of them became clinically pregnant within two years.
Demographic and epidemiological evidence also calls for the presence of a major early wastage. Indeed, human fecundity, i.e., the capacity to bear live children, defined as ‘the probability to produce a vital term newborn per menstrual cycle during which there was normal sexual activity’ [7], rarely exceeds 35–40% [8]. In fact, Woods estimated that apparent fecundability varies from 0.14 to 0.31 (0.17–0.38 when adjusted for fetal loss) [9].
Reasons for the reported wide variations in evaluating human fecundity have been critically examined by Smarr et al. [10] who pointed out that there is no ‘population (bio)marker’ that can be used and, as such, fecundity can only be assessed indirectly utilizing a variety of individual- or couple-based endpoints, defaulting both evaluation and monitoring to rely on rates of births (fertility) or adverse outcomes.
In terms of possible causes for the relatively low human fecundity, there is once again increasing evidence that it is due to pre-clinical pregnancy loss, suggestive of spontaneous failure of implantation. Therefore, the mechanisms underlying this phenomenon need to be critically analyzed.
In a 1996 clinical study, Zinaman et al. found a maximal fertility rate of approximately 30% per cycle in the first two cycles, with a pregnancy wastage of 31%. Importantly, 40% of these losses occurred so early after fertilization that the presence of gestation could be identified only through the measurement of urinary hCG [11]. Another clinical investigation followed a cohort of 217 women attempting to become pregnant, observing early loss rates ranging from a low estimate of 11.0% to a high one of 26.9% [12]. More recently, Leridon provided an average figure of 20–25% (at ages 20–30) and pointed out that, although human fetal mortality is high, amounting to 12–15% of confirmed pregnancies, “an even higher proportion of ‘products of conception’ do not develop normally and are evacuated within a few weeks, before the woman becomes aware of her pregnancy”. He cites a figure of around 50% of all conceptions and states that in their great majority these failures are due to severe genetic abnormalities, concluding that “human reproduction has a high error rate, but most of these errors are corrected by eliminating the products of conception” [13].
At the turn of the millennium, Simon et al. [14] recruited 145 subjects undergoing IVF and 92 undergoing oocyte donations. In subjects undergoing IVF, positive implantation was documented in 60.7% of embryo transfer (ET) cycles, but only 20.7% resulted in viable pregnancies, whereas the remaining miscarried; this occurred at an early pre-clinical stage in 72.4% of the failed ET cycles. In ovum donation cycles, positive implantation was recorded in 69.6% of ET cycles. Of these, 37.0% miscarried; among them, pre-clinical losses accounted for 70.6% of the total.
Boomsma et al. [15] utilized rates of rising urinary hCG (indicating initiation of implantation) that did not lead to a subsequent positive pregnancy test. They found that in over 50% of women undergoing ET, a rise in hCG could be documented, indicating an implanting embryo. However, in approximately one-third of these implanting embryos, a pre-clinical pregnancy loss occurred.
Finally, in 2020, Wilcox et al. [16], after combining data from epidemiologic, demographic, laboratory, and in vitro fertilization investigations, constructed an empirical framework aimed at producing plausible estimates of fecundability, sterility, transient anovulation, patterns of intercourse, and the proportion of ova fertilized in the presence of sperm. After combining all this information, they generated an estimation of preimplantation loss, to be considered an average for fertile couples, concluding that “under a plausible range of assumptions… 40 to 50% of fertilized ova fail to implant” even in normally fertile couples.
2. Mechanisms Involved in Early Embryo Selection
The first week of development is characterized by daily changes that can be summarized as follows:
Day 1: Fertilization takes place with the fusion of the sperm and egg to form the zygote.
Day 2: The division of the zygote begins, and two cells are formed.
Day 3: A solid spheric ‘ball’ of cells, the morula, is generated by the further division of the zygote.
Day 4: The morula continues to divide, while in its center a small cavity begins to form as the morula transforms into the blastocyst.
Day 5: The blastocyst begins to implant in the uterus.
Day 6: The implantation process continues, and the blastocyst begins to differentiate with the formation of an outer layer: the trophoblast.
Day 7: Implantation is complete, with the blastocyst fully embedded in the decidua; at this stage, the process of placental formation begins and is characterized by the formation of the two layers: cytotrophoblast and syncytiotrophoblast.
In 2017, Makrigiannakis et al. [17] summarized the steps involved in the process of implantation as follows:
-
The blastocyst moves towards the uterine cavity and contemporarily the decidualized endometrium evolves to a receptive phenotype, resulting in a biochemical cross-talk with the embryo.
-
The pre-implantation embryo begins to secrete factors capable of modulating the implantation site while, in turn, the decidua secretes cytokines and growth factors modulating embryonic differentiation and development.
-
In the presence of a proper biochemical environment, the embryo and the decidua jointly promote trophoblast invasion. This process can be altered in a number of ways resulting in early embryonic loss.
A schematic view of the major phases of the implantation process is presented in Figure 1.
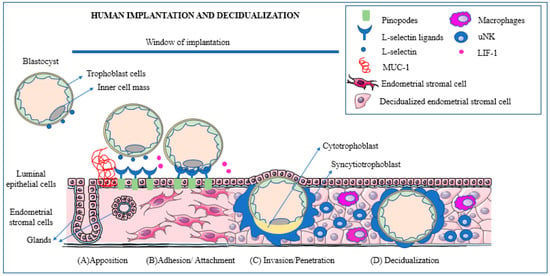
Figure 1. Schematic representation of the main phases of human implantation: apposition, adhesion/attachment, invasion/penetration, and decidualization. (A) (Apposition): the blastocyst expresses L-selectin that reacts with its ligands. The presence of Mucin-1 repels the blastocyst and prevents it from attaching outside of the window of receptivity. (B) (Adhesion): the blastocyst promotes cleavage of Mucin-1 at the implantation site to ensure successful attachment. (C) (Invasion): blastocyst trophoblast cells penetrate the endometrial epithelium and reach the stroma. As soon as implantation is initiated and the embryo reaches the stromal cells surrounding the embryo, these transform into decidualized cells. (D) (Decidualization): during the decidualization process, immune cells, such as macrophages and uterine natural killer (uNK) cells, play an important role promoting an environment conducive to successful implantation. Reprinted with permission from: Ochoa-Bernal and Fazleabas (2020) [18].
Over the last decades, information has been obtained concerning the mechanisms leading to this very early embryonic demise. These have been clearly summarized by Macklon and Brosens [19], who proposed that maternal systems are in place to prevent the implantation of poorly viable embryos. To achieve this, the decidualization of the endometrium acts as a biosensor through which signals from the early embryos are converted into a ‘go’ or ‘no-go’ endometrial response and therefore successful implantation.
2.1. Factors Secreted by the Pre-Implantation Embryo
Fundamental new knowledge has been gained thanks to assisted reproduction techniques. An early investigation by Shutt and Lopata [20] of embryos cultured in vitro over a period of 3–4 days, found that the corona cells surrounding the fertilized ovum could secrete daily a mean amount of 50 ng of progesterone and approximately 100 pg each of estradiol, prostaglandin-E2 (PGE2), and PGF2α, respectively. This experiment provided the first evidence of how the early embryo is supplied with vital substances for its survival before the maternal organism intervenes.
After the blastocyst reaches the uterine cavity, several substances of embryonic origin become involved in the intense cross-talk between the early embryo and endometrium, initiating before and conditioning the process of implantation and of the placenta’s formation.
2.1.1. The Early Pregnancy Factor
In the 1970s, Morton et al. [21], starting from the observation that human lymphocytes showed a depression in their activity when incubated in serum from pregnant women, identified the above-mentioned EPF. Biochemically, EPF is a homolog of chaperonin 10 and belongs to the heat shock family of proteins with immunosuppressive and growth factor properties [22] and seems involved in the suppression of the maternal response, thereby allowing the continued viability of the early embryo. Nahhas and Barnea [23] believe that EPF represents a link between fertilization and immunomodulation and that during the pre-implantation period, EPF is of maternal origin, whereas after nidation it becomes of embryonic origin.
2.1.2. The Pre-Implantation Factor
A second early-secreted substance, coined Pre-Implantation Factor (PIF), was identified by Barnea et al. [24] in 1994. Starting from the observation that in men and non-pregnant women, the proportion of lymphocytes bound by platelets is significantly different from that of pregnant women (p < 0.0001), they developed an assay to measure the presence of PIF. This was detected in subjects who had successfully undergone IVF-ET, followed by a normal pregnancy by 4 days after transfer. PIF is a linear peptide molecule consisting of 15 amino acids, which exhibits a self-protective and antitoxic action and is present in maternal blood as early as 7 days after conception and absent in the presence of non-viable embryos [25].
2.1.3. The Embryo-Derived Platelet-Activating Factor
A third early factor, the embryo-derived platelet-activating factor (PAF) (1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine) was identified by O’Neill et al. [26]. Subsequently, Roudebush et al. [27] proved a correlation between PAF levels in human embryo culture media and pregnancy outcome: as PAF levels increase, so does the corresponding pregnancy rate.
2.1.4. The Human Chorionic Gonadotrophin
In spite of the progress made in the biology of these pre-implantation factors, the best known and the most important among them still remains hCG, indispensable for maintaining the corpus luteum. Fishel et al. [28] were the first to detect its presence in the medium surrounding two embryos cultured for more than 7 days after IVF. This places hCG among the pre-implantation factors. In fact, its mRNA is transcribed as early as the 8-cell stage [29].
2.2. Factors Produced by the Endometrium
Successful implantation requires the exquisitely coordinated migration and invasion of trophoblast cells from the outer capsule of the blastocyst into the endometrium [18]. Over and above the described substances produced by the early embryo, the process is also guided by additional signals from cells in the decidualized endometrium. There are numerous recent descriptions of the mechanisms involved in successful implantation, e.g., [13][15][18][19][27][30][31][32][33][34].
Briefly, however, for the implantation process to be successfully achieved, a well-orchestrated interplay of various cell types is necessary. These consist of epithelial, stromal, and immune cells, and trophoblasts, producing a number of cellular molecules, such as cytokines, chemokines, growth factors, and adhesion molecules [18][34]. In addition, endometrial stroma cells have been shown to promote trophoblast invasion through the generation of an inflammatory environment modulated by TNF-α (tumor necrosis factor α) [35]. Furthermore, through the production of IL-17 (interleukine-17), stromal cells promote trophoblast migration [36]. Finally, adequate glycolysis also appears to be necessary to provide all the energy and macromolecules needed for implantation and early pregnancy [37].
2.3. The Role of the Immune System in Early Pregnancy Wastage
Some twenty years ago, Clark, starting from the fact that embryos bear paternal and embryonic antigens foreign to the maternal immune system, asked the question: could some otherwise normal embryos be ‘rejected’? [38]. In a subsequent review [39], he searched and critically analyzed the evidence in animals and humans, finding that various treatments may improve the live birth rate. Unfortunately, not enough evidence exists to indicate when in gestation such mechanisms may be active.
Early investigations by Haddad et al. [40][41] provided some experimental evidence in a murine model of the presence of activated macrophages at implantation sites before overt embryo damage occurs. In terms of mechanism, they showed that increased nitric oxide production by decidual macrophages is involved in early murine embryo loss.
Vento-Tormo et al. [42] have determined the cellular composition of human decidua, documenting the existence of subsets of perivascular and stromal cells located in distinct decidual layers. These include immune cells, principally uNK cells (~70%), macrophages (~20%), and T cells (~10%). In a mouse model, the influx and expansion of these immune cells are controlled by decidual cells, since effector T cells cannot accumulate within the decidua, thanks to the epigenetic silencing of key T cell-attracting inflammatory chemokine genes in decidual stromal cells [43].
In addition, regulatory T cells (Tregs) also seem to play a vital role in implantation and sustaining pregnancy [44]. The fact that a healthy woman can successfully carry her genetically disparate fetus to term without immune rejection strongly indicates that immune cells are actively involved in pregnancy. Tregs are thymus-derived and can be recruited to non-lymphoid tissues to curtail inflammation and maintain immunological self-tolerance and homeostasis [45]. Tregs may also differentiate and proliferate from CD4+ naive T cells when stimulated with immunosuppressive cytokines such as TGF-β and IL-10 [46]. The potent immunosuppressive activity of Tregs comes from their ability to influence the polarization, expansion, and effector function of T effector cells [47]. Tregs can inhibit effector immunity, restrict inflammation, and support maternal vascular adaptations, and, as such, facilitate trophoblast invasion and placental access to the maternal blood supply. Ample data have shown that insufficient Treg numbers or inadequate functional competence are involved in idiopathic infertility and recurrent miscarriage, as well as later-onset pregnancy complications, ranging from preeclampsia and fetal growth restriction [48].
3. Factors Predictive of Embryonic Loss
The Embryo–Maternal Dialogue
Barnea [49] pointed out that the embryo–maternal dialogue starts shortly after fertilization and is exerted through both local and systemic signaling. First comes the PIF, secreted in humans already at the 2–4-cell stage, that initiates the modulation of cellular immunity. Then comes a class of novel proteins/peptides coined developmental proteins (DPs), present in the embryo before a mature immune system has developed. They promote normal proliferation while controlling any abnormal one and seemingly acting through specific receptors. When the development of an embryo becomes incompatible with life, DPs may lead to growth arrest, a decline of pre-implantation factors, reactivation of the immune system, and, ultimately, pregnancy rejection. Drawing attention to the fact that, despite the use of adjuvant therapies, the cumulative rates of live births following IVF-ET remains at ~40%, Yang et al. [50] stressed that the low pregnancy rates, even in the presence of high fertility rates, are due to implantation failure.
Brosens et al. [51] have identified multiple decidual checkpoints conditioning the implantation of the blastocyst. The first is appropriate timing since a delayed rise in hCG levels beyond the putative implantation window is strongly associated with the interruption of gestation in the first two weeks of pregnancy. The second is the action of migratory decidual cells encapsulating the implanting embryo, serving as biosensors, and acting in both negative and positive selection. In the presence of low-quality embryos, these cells engage in a stress response inhibiting the secretion of implantation factors and hindering embryo encapsulation, while normal embryos secrete factors enhancing the expression of maternal implantation and metabolic genes, thus actively promoting the completion of the implantation process. The third is represented by a sufficient secretion of hCG to rescue ovarian progesterone production until around 8 to 10 gestation weeks when the placenta takes over progesterone production [52]. Brosens et al. [51] concluded that at the time of implantation, both positive and negative selection mechanisms are active in a process that is intrinsically dynamic and adaptable.
Information is also available on the mechanisms through which the early embryo identifies its own defects, such as, first and foremost, apoptosis which begins to appear at the blastocyst level [53][54]. The starting point has been the observation that many embryos grown in vitro contain unequal-sized blastomeres and multiple cellular fragments and, when fragmentation becomes excessive, their developmental potential both in vitro and in vivo is severely limited [54].
Recently, a study by Haouzi et al. [55] of genes involved in the regulation of the apoptotic and survival pathways of mouse and human embryos found that components of the major apoptotic and survival signaling pathways were expressed during early human and mouse embryonic development in a species-specific manner.
In an attempt to identify visual markers of early pregnancy loss in women undergoing IVF, Amitai et al. assembled an expansive set of 314 morphological, morphokinetic, and dynamic features derived from measurable static and dynamic properties of preimplantation embryo development [56]. They identified a subset of six non-redundant morphodynamical features possessing high predictive capacity. Among them, of particular interest were features that account for the distribution of the nucleolus precursor bodies within the small pronucleus and pronuclei dynamics. Using these features, they developed a “decision-support tool” for prioritizing embryos for transfer based on their predicted implantation potential.
4. The Need for New Terminology
As summarized here, human preimplantation embryos exhibit in vitro high levels of apoptotic and high rates of developmental arrest during the first week [54]. In fact, in vitro and in vivo, errors and failures of the first and the next three cleavage divisions frequently cause immediate embryo death or lead to aberrant subsequent development. At the same time, the ability of human embryos to eliminate/expel abnormal blastomeres as cell debris/fragments and carry out—whenever possible—self-correction or quality control has been also documented [57]. Finally, Monsivais et al. [58] have shown that for the correct regulation of uterine receptivity, a convergence is necessary of bone morphogenetic proteins (BMPs, members of the TGF-β family that regulate the post-implantation and mid-gestation stages of pregnancy) and steroid hormone signaling pathways.
For a number of years, in assisted reproduction, the establishment of a viable pregnancy has been defined by a rise in circulating hCG. The use of this marker shows that in IVF the successful implantation of an embryo represents the major milestone in determining the success of gestation since it has been shown that only 50% of transferred embryos implant and that half of these embryos are subsequently lost [15].
The documented occurrence of massive early/preimplantation embryo loss provides the scientific basis to necessitate a clear distinction between the first 1 or 2 weeks and the subsequent 9 months of gestation. In the first phase, a physiological loss of fertilized oocytes/early embryos, calculated at around 50%, occurs, whereas during the second phase, the pathologic wastage of embryos/fetuses has been estimated to be around 15%, although increasing with maternal age. Given this stark contrast, it seems appropriate to distinguish these two periods also using a different nomenclature.
On the one hand, employing the word gestation to identify the period from fertilization (whether in vitro or in utero) to birth, and, on the other, to utilize the word pregnancy when referring to the period after implantation is completed [59]. The idea is certainly not new in view of the above-mentioned definition of pregnancy in the IVF clinic as the rise of hCG upon embryo implantation [60]. Extending this concept to all pregnancies would create a simple, clear nomenclature.
This seems the position taken by several organizations, which—in practice—affirm that there is no pregnancy before implantation: among them is the World Health Organization [61], which, referring to “Medicines for Reproductive Health and Perinatal Care” (Item 22), under ‘Oral Hormonal Contraceptives’ (22.1.1), lists hormonal emergency contraceptives levonorgestrel and ulipristal; and the other is the American College of Obstetricians and Gynecologists [62].
References
- Roberts, C.J.; Lowe, C.R. Where have all the conceptions gone? Lancet 1975, 305, 498–499.
- Shepard, T.H.; Fantel, A.G. Embryonic and early fetal loss. Clin. Perinatol. 1979, 6, 219–243.
- Morton, H.; Tinneberg, H.R.; Rolfe, B.; Wolf, M.; Mettler, L. Rosette inhibition test: A multicentre investigation of early pregnancy factor in humans. J. Reprod. Immunol. 1982, 4, 251–261.
- Rolfe, B.E. Detection of fetal wastage. Fertil. Steril. 1982, 37, 655–660.
- Smart, Y.C.; Fraser, I.S.; Roberts, T.K.; Clancy, R.L.; Cripps, A.W. Fertilization and early pregnancy loss in healthy women attempting conception. Clin. Reprod. Fertil. 1982, 1, 177–184.
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194.
- Olsen, J.; Rachootin, P. Invited commentary: Monitoring fecundity over time—If we do it, then let’s do it right. Am. J. Epidemiol. 2003, 157, 94–97.
- Benagiano, G.; Farris, M.; Grudzinskas, G. Fate of fertilized human oocytes. Reprod. Biomed. Online 2010, 21, 732–741.
- Wood, J.W. Fecundity and natural fertility in humans. Oxf. Rev. Reprod. Biol. 1989, 11, 61–109.
- Smarr, M.M.; Sapra, K.J.; Gemmill, A.; Kahn, L.G.; Wise, L.A.; Lynch, C.D.; Factor-Litvak, P.; Mumford, S.L.; Skakkebaek, N.E.; Slama, R.; et al. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum. Reprod. 2017, 32, 499–504.
- Zinaman, M.J.; Clegg, E.D.; Brown, C.C.; O’Connor, J.; Selevan, S.G. Estimates of human fertility and pregnancy loss. Fertil. Steril. 1996, 65, 503–509.
- Ellish, N.J.; Saboda, K.; O’Connor, J.; Nasca, P.C.; Stanek, E.J.; Boyle, C. A prospective study of early pregnancy loss. Hum. Reprod. 1996, 11, 406–412.
- Leridon, H. Human fecundity: Situation and outlook. Popul. Soc. 2010, 471, 1–4.
- Simon, C.; Landeras, J.; Zuzuarregui, J.L.; Martin, J.C.; Remohi, J.; Pellicer, A. Early pregnancy losses in in vitro fertilization and oocyte donation. Fertil. Steril. 1999, 72, 1061–1065.
- Boomsma, C.M.; Kavelaars, A.; Eijkemans, M.J.; Lentjes, E.G.; Fauser, B.C.; Heijnen, C.J.; Macklon, N.S. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. 2009, 24, 1427–1435.
- Wilcox, A.J.; Harmon, Q.; Doody, K.; Wolf, D.P.; Adashi, E.Y. Preimplantation loss of fertilized human ova: Estimating the unobservable. Hum. Reprod. 2020, 35, 743–750.
- Makrigiannakis, A.; Vrekoussis, T.; Zoumakis, E.; Kalantaridou, S.N.; Jeschke, U. The Role of HCG in Implantation: A Mini-Review of Molecular and Clinical Evidence. Int. J. Mol. Sci. 2017, 18, 1305.
- Kim, S.M.; Kim, J.S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359.
- Macklon, N.S.; Brosens, J.J. The human endometrium as a sensor of embryo quality. Biol. Reprod. 2014, 91, 98.
- Shutt, D.A.; Lopata, A. The secretion of hormones during the culture of human preimplantation embryos with corona cells. Fertil. Steril. 1981, 35, 413–416.
- Morton, H.; Rolfe, B.; Clunie, G.J. An early pregnancy factor detected in human serum by the rosette inhibition test. Lancet 1977, 1, 394–397.
- Morton, H. Early pregnancy factor: An extracellular chaperonin 10 homologue. Immunol. Cell Biol. 1998, 76, 483–496.
- Nahhas, F.; Barnea, E. Human embryonic origin early pregnancy factor before and after implantation. Am. J. Reprod. Immunol. 1990, 22, 105–108.
- Barnea, E.R.; Lahijani, K.I.; Roussev, R.; Barnea, J.D.; Coulam, C.B. Use of lymphocyte platelet bindingassay for detecting a preimplantation factor: A quantitative assay. Am. J. Reprod. Immunol. 1994, 32, 133–138.
- Barnea, E.R.; Simon, J.; Levine, S.P.; Coulam, C.B.; Taliadouros, G.S.; Leavis, P.C. Progress in characterization of pre-implantation factor in embryo cultures and in vivo. Am. J. Reprod. Immunol. 1999, 42, 95–99.
- O’Neill, C.; Collier, M.; Ryan, J.P.; Spinks, N.R. Embryo-derived platelet-activating factor. J. Reprod. Fertil. Suppl. 1989, 37, 19–27.
- Roudebush, W.E.; Wininger, J.D.; Jones, A.E.; Wright, G.; Toledo, A.A.; Kort, H.I.; Massey, J.B.; Shapiro, D.B. Embryonic platelet-activating factor: An indicator of embryo viability. Hum. Reprod. 2002, 17, 1306–1310.
- Fishel, S.B.; Edwards, R.G.; Evans, C.J. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science 1984, 223, 816–818.
- Bonduelle, M.L.; Dodd, R.; Liebaers, I.; Van Steirteghem, A.; Williamson, R.; Akhurst, R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum. Reprod. 1988, 3, 909–914.
- Macklon, N.S.; Geraedts, J.P.; Fauser, B.C. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343.
- Kennedy, T.G. Physiology of implantation. In In Vitro Fertilization and Assisted Reproduction; Gomel, V., Leung, P.C.K., Eds.; Monduzzi Editore: Bologna, Italy, 1997; pp. 729–735.
- Short, R.V. When a conception fails to become a pregnancy. Ciba Found. Symp. 1978, 64, 377–394.
- Velasquez, L.A.; Maisey, K.; Fernandez, R.; Valdes, D.; Cardenas, H.; Imarai, M.; Delgado, J.; Aguilera, J.; Croxatto, H.B. PAF receptor and PAF acetylhydrolase expression in the endosalpinx of the human Fallopian tube: Possible role of embryo-derived PAF in the control of embryo transport to the uterus. Hum. Reprod. 2001, 16, 1583–1587.
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973.
- You, Y.; Stelzl, P.; Joseph, D.N.; Aldo, P.B.; Maxwell, A.J.; Dekel, N.; Liao, A.; Whirledge, S.; Mor, G. TNF-alpha Regulated Endometrial Stroma Secretome Promotes Trophoblast Invasion. Front. Immunol. 2021, 12, 737401.
- Zhang, Z.; Yang, Y.; Lv, X.; Liu, H. Interleukin-17 promotes proliferation, migration, and invasion of trophoblasts via regulating PPAR-γ/RXR-α/Wnt signaling. Bioengineered 2022, 13, 1224–1234.
- Chi, F.; Sharpley, M.S.; Nagaraj, R.; Sen Roy, S.; Banerjee, U. Glycolysis-Independent Glucose Metabolism Distinguishes TE from ICM Fate during Mammalian Embryogenesis. Dev. Cell 2020, 53, 9–26.e4.
- Clark, D.A. Is there any evidence for immunologically mediated or immunologically modifiable early pregnancy failure? J. Assist. Reprod. Genet. 2003, 20, 63–72.
- Clark, D.A. Immunological factors in pregnancy wastage: Fact or fiction. Am. J. Reprod. Immunol. 2008, 59, 277–300.
- Haddad, E.K.; Duclos, A.J.; Antecka, E.; Lapp, W.S.; Baines, M.G. Role of interferon-gamma in the priming of decidual macrophages for nitric oxide production and early pregnancy loss. Cell Immunol. 1997, 181, 68–75.
- Haddad, E.K.; Duclos, A.J.; Lapp, W.S.; Baines, M.G. Early embryo loss is associated with the prior expression of macrophage activation markers in the decidua. J. Immunol. 1997, 158, 4886–4892.
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353.
- Nancy, P.; Tagliani, E.; Tay, C.S.; Asp, P.; Levy, D.E.; Erlebacher, A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012, 336, 1317–1321.
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235.
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811.
- Josefowicz, S.Z.; Rudensky, A. Control of regulatory T cell lineage commitment and maintenance. Immunity 2009, 30, 616–625.
- Vent-Schmidt, J.; Han, J.M.; MacDonald, K.G.; Levings, M.K. The role of FOXP3 in regulating immune responses. Int. Rev. Immunol. 2014, 33, 110–128.
- Sadlon, T.; Brown, C.Y.; Bandara, V.; Hope, C.M.; Schjenken, J.E.; Pederson, S.M.; Breen, J.; Forrest, A.; Beyer, M.; Robertson, S.; et al. Unravelling the molecular basis for regulatory T-cell plasticity and loss of function in disease. Clin. Transl. Immunol. 2018, 7, e1011.
- Barnea, E.R. Embryo maternal dialogue: From pregnancy recognition to proliferation control. Early Pregnancy 2001, 5, 65–66.
- Yang, M.; Yang, Y.; She, S.; Li, S. Proteomic investigation of the effects of preimplantation factor on human embryo implantation. Mol. Med. Rep. 2018, 17, 3481–3488.
- Brosens, J.J.; Bennett, P.R.; Abrahams, V.M.; Ramhorst, R.; Coomarasamy, A.; Quenby, S.; Lucas, E.S.; McCoy, R.C. Maternal selection of human embryos in early gestation: Insights from recurrent miscarriage. Semin. Cell Dev. Biol. 2022, 131, 14–24.
- Csapo, A.I.; Pulkkinen, M.O.; Ruttner, B.; Sauvage, J.P.; Wiest, W.G. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am. J. Obstet. Gynecol. 1972, 112, 1061–1067.
- Jurisicova, A.; Varmuza, S.; Casper, R.F. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 1996, 2, 93–98.
- Cecchele, A.; Cermisoni, G.C.; Giacomini, E.; Pinna, M.; Vigano, P. Cellular and Molecular Nature of Fragmentation of Human Embryos. Int. J. Mol. Sci. 2022, 23, 1349.
- Haouzi, D.; Boumela, I.; Chebli, K.; Hamamah, S. Global, Survival, and Apoptotic Transcriptome during Mouse and Human Early Embryonic Development. Biomed. Res. Int. 2018, 2018, 5895628.
- Amitai, T.; Kan-Tor, Y.; Or, Y.; Shoham, Z.; Shofaro, Y.; Richter, D.; Har-Vardi, I.; Ben-Meir, A.; Srebnik, N.; Buxboim, A. Embryo classification beyond pregnancy: Early prediction of first trimester miscarriage using machine learning. J. Assist. Reprod. Genet. 2023, 40, 309–322.
- Orvieto, R.; Shimon, C.; Rienstein, S.; Jonish-Grossman, A.; Shani, H.; Aizer, A. Do human embryos have the ability of self-correction? Reprod. Biol. Endocrinol. 2020, 18, 98.
- Monsivais, D.; Clementi, C.; Peng, J.; Titus, M.M.; Barrish, J.P.; Creighton, C.J.; Lydon, J.P.; DeMayo, F.J.; Matzuk, M.M. Uterine ALK3 is essential during the window of implantation. Proc. Natl. Acad. Sci. USA 2016, 113, E387–E395.
- Benagiano, G.; Mancuso, S.; Gianaroli, L.; Di Renzo, G.C. Gestation vs. Pregnancy. Am. J. Obstet. Gynecol. 2023, 229, 91–92.
- Chung, K.; Sammel, M.D.; Coutifaris, C.; Chalian, R.; Lin, K.; Castelbaum, A.J.; Freedman, M.F.; Barnhart, K.T. Defining the rise of serum HCG in viable pregnancies achieved through use of IVF. Hum. Reprod. 2006, 21, 823–828.
- World Health Organization. WHO Model List of Essential Medicines—22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 15 September 2023).
- American College of Obstetricians, Gynecologists. Committee on Practice B-G. ACOG Practice Bulletin No. 200: Early Pregnancy Loss. Obstet. Gynecol. 2018, 132, e197–e207.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
639
Revisions:
2 times
(View History)
Update Date:
29 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




