Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thandiswa Jideani | -- | 3083 | 2024-01-25 22:35:01 | | | |
| 2 | Rita Xu | Meta information modification | 3083 | 2024-01-26 02:40:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jideani, T.; Chukwuchendo, E.; Khotseng, L. Biomass Feedstocks into Biofuel. Encyclopedia. Available online: https://encyclopedia.pub/entry/54366 (accessed on 08 February 2026).
Jideani T, Chukwuchendo E, Khotseng L. Biomass Feedstocks into Biofuel. Encyclopedia. Available at: https://encyclopedia.pub/entry/54366. Accessed February 08, 2026.
Jideani, Thandiswa, Emmanuel Chukwuchendo, Lindiwe Khotseng. "Biomass Feedstocks into Biofuel" Encyclopedia, https://encyclopedia.pub/entry/54366 (accessed February 08, 2026).
Jideani, T., Chukwuchendo, E., & Khotseng, L. (2024, January 25). Biomass Feedstocks into Biofuel. In Encyclopedia. https://encyclopedia.pub/entry/54366
Jideani, Thandiswa, et al. "Biomass Feedstocks into Biofuel." Encyclopedia. Web. 25 January, 2024.
Copy Citation
The conversion of biomass to biofuels as a renewable energy source is continuously gaining momentum due to the environmental concerns associated with using fossil fuels. Biomass is a cost-effective, long-term natural resource that may be converted to biofuels such as biodiesel, biogas, bio-oil, and biohydrogen using a variety of chemical, thermal, and biological methods. Thermochemical processes are one of the most advanced biomass conversion methods, with much potential and room for improvement.
renewable energy
biomass conversion
thermochemical conversion
1. Introduction
As the global economy continues to grow and with increasing population, the demand for the earth’s energy resources is rising. These resources, such as fossil fuels, may be difficult to obtain, expensive, or risky to the environment [1]. Traditional energy sources like coal, petroleum, and natural gas meet primary energy needs. These sources are in danger of becoming extinct. Energy extraction from these sources pollutes the atmosphere, contributing to issues like global warming, acid rain, etc. A switch to non-conventional sources like wind, sunlight, water, biomass, etc., is inevitable, given the rise in energy demands and consideration of pollution [2]. Fossil fuels are regarded as non-renewable resources due to their lengthy formation cycle, and their combustion has resulted in environmental pollution issues, such as the emissions of toxic gases leading to air pollution and irreversible climate change [3].
Production of renewable and CO2-emission-free fuels is essential for sustainability and meeting current energy demands [4]. Among numerous alternatives, biomass has drawn significant interest as a renewable energy resource to replace traditional fossil fuels because of its capacity to provide a constant energy supply and its nature of being environmentally friendly [5]. In this context, agricultural biomass wastes are potential sources of renewable energy for manufacturing biofuels [6].
Energy from biomass can be derived in various forms; the form of determination suggests the conversion process and depends on the availability, type, and quantity of the biomass. The conversion of biomass to biofuels involves the use of various treatment technologies classified as biochemical, physical, and thermochemical. Biochemical conversion involves the use of both anaerobic digestion (production of biogas, a mixture of mainly methane and carbon dioxide) and fermentation (production of ethanol) [7]. The biochemical conversion process generally takes a long time and can only employ biomass’s cellulose and hemicellulose parts.
Physical conversion is a mechanical extraction used to produce liquid biofuels from organic matter containing oils and/or fats such as rapeseeds, oil palm fruits, sunflower seeds, etc. [8]. Despite the fact that it is an environmentally friendly and clean transformation technology with great future energy security, it is still in its infancy and is notably constrained by the high cost of raw materials [3].
Thermochemical conversion involves heating biomass to high temperatures in either an oxygenic or anoxygenic environment to induce structural breakdown [3]. Thermochemical conversion techniques have drawn the attention of researchers because they can be effectively applied to almost any biomass feedstock, not limited by the use of enzymes to break down a limited range of feedstock. There is a relatively higher production of desired products such as oil due to the chemical nature of the reaction, and mostly, the biomass is completely utilised [9]. There are four main thermo-chemical routes to produce fuels, i.e., direct combustion, gasification, pyrolysis, and hydrothermal liquefaction, each differing in the temperature, heating rate, and oxygen level present during the treatment [3]. The three most important process parameters for all thermochemical conversions are temperature, heating rate, and residence time, as they are known to affect the thermochemical processes are illustrated in both Figure 1 and Figure 2, respectively. Figure 1 shows the temperature ranges for thermochemical processes, and Figure 2 shows typical ranges of the heating rate and residence time for pyrolysis. Figure 1 shows that the higher the temperature of your conversion reaction, the more gaseous the products will be.
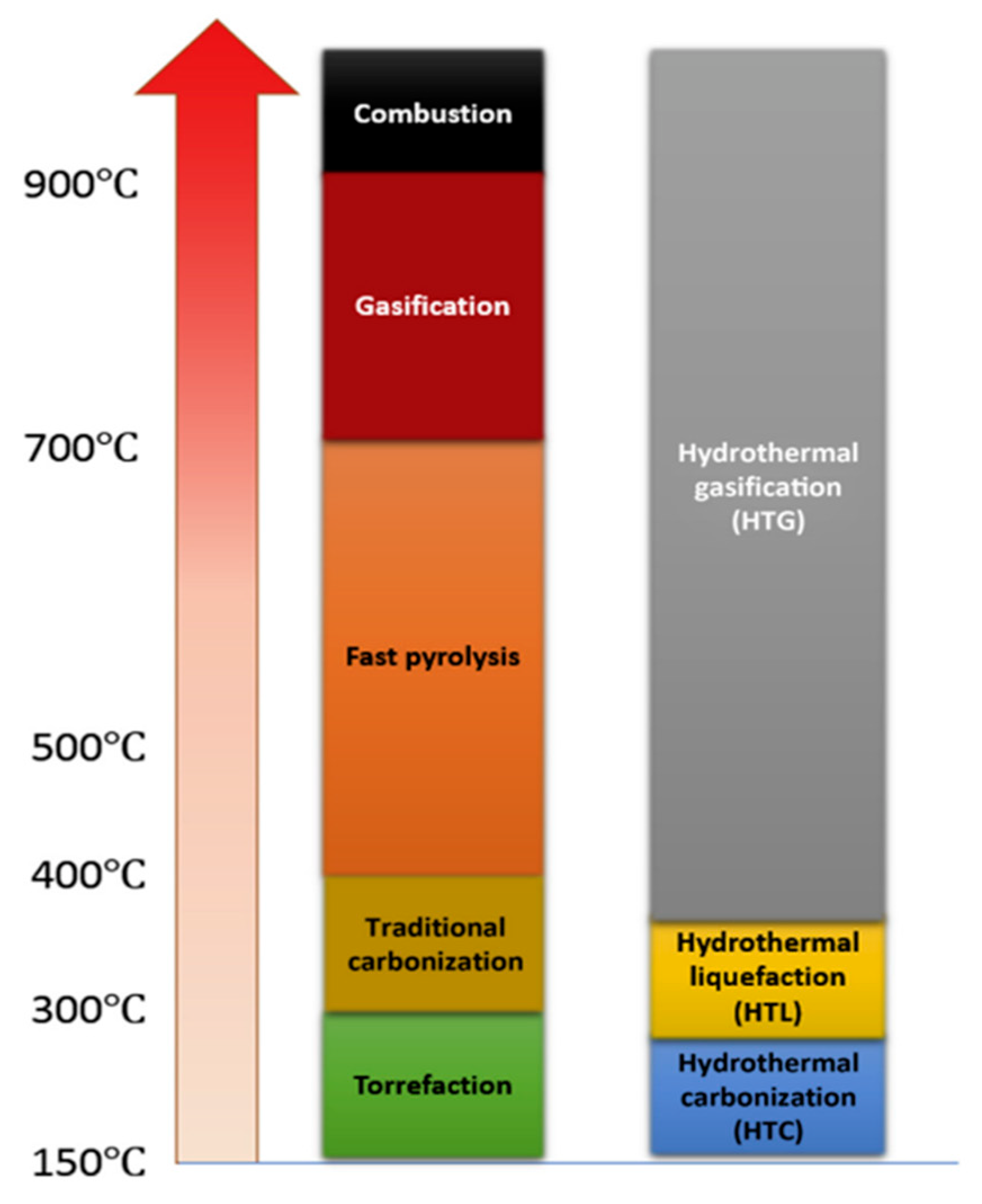
Figure 1. Standard temperature ranges for different thermochemical conversion techniques.
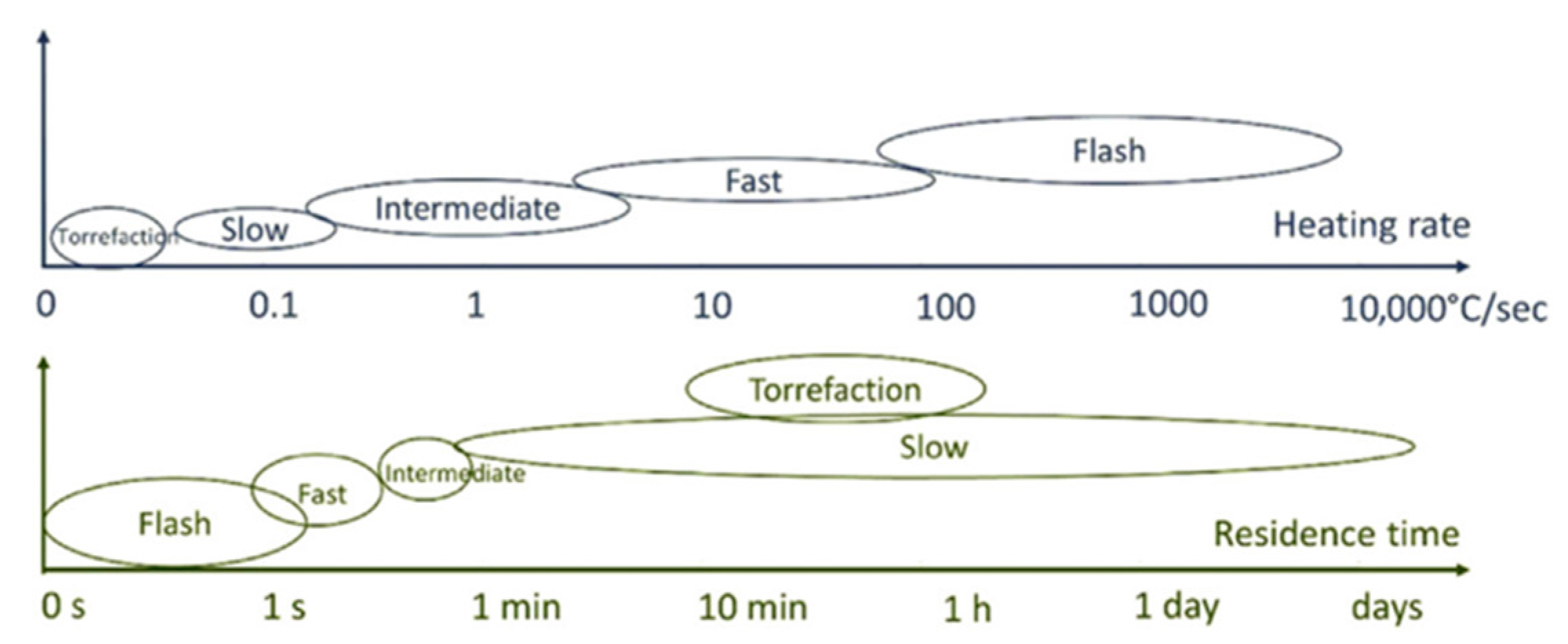
Figure 2. Heating rate and residence time for biomass using different thermochemical techniques. Slow: slow pyrolysis; fast: fast pyrolysis; intermediate: intermediate pyrolysis; flash: flash pyrolysis.
Generally, hydrothermal liquefaction (HTL) is the most promising process over direct combustion, gasification, and pyrolysis because it is a potential method for turning raw materials into liquid biofuels due to its various benefits, such as transforming wet feedstocks directly into bio-oil or crude bio-oil to save energy on drying or pre-treatment. As a consequence, results in higher energy recovery efficiency and decreased energy usage during biofuel production [10].
The use of a catalyst during thermochemical processes reduces the required reaction temperature by improving reaction kinetics and, consequently, the yield of the desired products; it also increases process efficiency by lowering the formation of tar and char and by removing heteroatoms like oxygen, nitrogen, and sulphur [11].
In thermochemical processes, catalysts can be used to increase yield, decrease solid waste production, and minimise reaction pressure and temperature [12]. Both homogeneous and heterogeneous catalysts can facilitate the conversion of biomass to biofuels via thermochemical processes. Various classes of catalysts have been studied to efficiently convert biomass into biofuels. The conventional homogenous catalysts have been used over the years, but they pose a problem with separation as it is difficult to separate them from the product, making the process more energy-intensive and more costly [13]. The use of homogenous catalysts results in wastewater generation, difficulty in catalyst separation, and non-recyclable. However, the limitations of the homogeneous catalysis process can be solved using heterogeneous catalysts. Heterogeneous catalysts are more environmentally friendly than homogenous catalysts, and they are more efficient and easier to separate from products [14]. Compared to homogeneous catalysts, heterogeneous catalysts are more desirable than homogenous catalysts to produce fuels from biomass. Additional advantages of heterogeneous catalysts include easy recyclability, non-toxic, not naturally corrode, economically viable, and not producing soap [12][15].
The unique function of different heterogeneous catalysts can change product yields and process selectivity, which in turn affects the composition of the bio-oil and its physical and chemical properties [16]. Several heterogeneous catalysts have been studied by researchers for biomass conversion via suitable thermochemical processes. Alkali and alkaline earth metals (AAEMs) and nickel-based catalysts are some of the catalysts that have been used in biomass conversion. These catalysts have been studied, and AAEMs have been found to have promising results in terms of biochar formation and non-condensable gases. Na, K, Mg, and Ca’s effects were investigated. Compared to K, Na, and Ca, Mg was relatively inert. The carbon yield of aromatics and olefins was decreased as a result of AAEM catalysts, while the rate at which biochar was formed thermally increased [17].
Studies on the feasibility of employing commercial steam-reforming Ni catalysts in biomass gasification were conducted by Aznar et al. [18] and Caballero et al. [19]. The syngas composition (CO and H2) was improved, and CH4 and CO2 concentrations were decreased as a result of the catalysts’ success in lowering tar content. Conventional heterogeneous catalyst types such as zeolites have been studied as catalysts for biomass conversion or for the upgrading of bio-oil. Zeolite catalysts are extremely acidic; ZSM-5’s small/medium pore size and two-dimensional channel-like pore system also make it very shape-selective. Due to their distinct characteristics, zeolites are a form of heterogeneous catalyst that is interesting for biomass conversion. Zeolites can operate as molecular sieves by selectively trapping some molecules while letting others pass through due to their porous structure. They can therefore be used to catalyse reactions involving big molecules, such as those in biomass. Because the pores of a given zeolite are a defined size and shape, zeolite catalysts can act only on specific compounds. This is crucial for biomass conversion since the reactants and products may contain intricate molecule mixtures [20]. Zeolites are extremely active in a wide range of catalytic processes because of their adjustable acidity. This is crucial for the conversion of biomass since the reactants and products can both be quite acidic or basic [21]. Zeolites can survive high temperatures without degrading since they are thermally stable. This is crucial for biomass conversion since complicated compounds are frequently broken down at high temperatures. Zeolites are economical and environmentally friendly because they may be reused. This is crucial for biomass conversion because it frequently calls for significant quantities of catalysts [22].
H-ZSM-5 causes several processes during petroleum oil cracking or pyrolysis, including protolytic cracking or β-scission, alkylation, isomerisation, cyclisation, oligomerisation, and aromatisation. The same reactions can occur during biomass conversion, such as HTL; however, before these hydrocarbon-based reactions, the holocellulose and lignin fragments are deoxygenated via dehydration and decarbonylation or decarboxylation reactions [23][24]. Among zeolites, ZSM-5 has primarily been studied as a catalyst for biomass conversion and has been found to significantly alter the composition of the bio-oils by simultaneously increasing the aromatic species and decreasing the amounts of oxygenated compounds via deoxygenation reactions, resulting in an organic fraction (bio-oil) that can be upgraded to gasoline and diesel type fuels [25].
2. Thermochemical Processes
2.1. Direct Combustion
In direct combustion, biomass is directly burnt in the presence of oxygen from the surrounding air to convert the energy stored in biomass into heat, mechanical power, electricity, etc. [2]. The combustion process consists of an exothermic chemical reaction. Chemical energy is released when biomass is burned in the presence of air. Combustion takes place inside combustion chambers at temperatures between 800 and 1000 °C. It is important to point out that the biomass burned to produce biofuels by combustion must have a humidity level lower than 50% [26]. The combustion of biomass has several advantages, such as volume reduction, controlled emissions, use of heat for power generation, and it can be silent and odourless; however, there are even more disadvantages of using combustion as a form of biomass conversion. The disadvantages of combustion include the possibility of producing health-harmful products (dioxins, furans, and heavy metals), potential conflicts with initiatives to cut waste production, and more energy enquired to handle waste with high humidity and high costs to prevent pollution by emissions [26].
2.2. Gasification
In gasification, biomass is oxidised at high temperatures with gasification media such as oxygen to produce a combustible gaseous mixture [3]. Air, pure oxygen, steam, carbon dioxide, or mixes are all suitable gasifying agents. Air is the most affordable and popular agent, but it contains a significant amount of nitrogen, which slows down the heating rate of the synthesis gas obtained. Higher heating rates are produced using pure oxygen; however, the method is less economically profitable due to the cost of producing oxygen [27]. There are discrete types of catalysts, such as AAEMs (alkali and alkaline earth metals) and Ni-based catalysts, which have been subjected to the gasification process and have reported positive results. The reaction time and temperature required to produce useful gases can be significantly decreased using these distinct catalyst types [11]. Gasifiers are divided into three categories based on configuration: fixed bed, fluidised bed, and entrained flow [28]. Fixed-bed gasifiers are perfect for using biomass on a small scale. Biomass and fuel derived from waste (FDW) are treated using fluidised bed gasifiers. These fluidised bed gasifiers are further separated into bubbling and circulating types. While bubbling-type beds are utilised to treat FDW, circulating-type beds are more frequently employed for biomass. Finally, entrained flow gasifiers are used to treat coal and, in certain situations, biomass. They require raw materials with a micrometric order of magnitude [29]. Some advantages of gasification include the formation of a synthesis gas with a range of applications (production of electricity, fuel, and the production of chemicals). It also prevents the production of harmful nitrogenous, halogenated and sulphur compounds. On the other hand, biomass gasification has limited large-scale use. It also requires a lot of energy if waste with high humidity percentages is handled [26].
2.3. Pyrolysis
Biomass pyrolysis is a thermochemical decomposition process that converts organic material/biomass in the absence of oxygen with possible products of liquid (bio-oil), solid (biochar) and gases [30]. Pyrolysis is made up of numerous spontaneous reactions whose efficiency is affected by a number of variables, including temperature, heating rate, residence time, particle size, pressure, type of biomass, moisture content, and the pre-treatment process of the biomass [26]. The pyrolysis conditions have the biggest impact on product yields. For instance, when the reaction temperature is below 450 °C, the main product is biochar, and when the reaction temperature is between 450 and 800 °C, the main product is bio-oil. At temperatures greater than 800 °C, gases are then formed [31]. Pyrolysis can be divided into three types: slow, fast and flash pyrolysis and the division is based on experimental conditions such as temperature, residence time, heating rate, and particle size [32].
When compared to alternative methods, the pyrolysis process has a number of advantages. It may yield a variety of useful products from solid waste streams (such as liquid fuels, fertilisers, activated carbon, and H2, CH4, and CO); it can be incorporated into microturbine, fuel cell, and thermophotovoltaic (TPV) systems for power generation. The product stream is more complicated for pyrolysis processing than for many alternative treatments, which is its main drawback [33].
2.4. Hydrothermal Liquefaction
Liquefaction is a thermochemical process that converts biomass into liquid fuels by breaking down the polymer structure into liquid components in a high-temperature pressured environment [11]. The process is usually carried out at pressures of 1 to 25 Mpa and temperatures of 250 to 450 °C [34]. Liquefaction improves the quality of bio-oil, such as HHV, bio-oil yield, and oxygen and nitrogen contents. Compared to pyrolysis, which generates a highly oxygenated bio-oil, liquefaction produces a bio-oil with a lower oxygen content [33].
The liquefaction process has several advantages over pyrolysis, including higher energy efficiency, lower operating temperature, and reduced coke formation than fast pyrolysis. Furthermore, the bio-crude produced from HTL conversion has higher quality and energy density, as well as excellent thermal and storage stability [35]. Studies have shown that temperature, residence time, heating rate, feedstock particle size, and type of solvent media all affect the HTL process, and temperature, pressure, and solvent media are crucial parameters [29]. Hydrothermal liquefaction is one of the most promising thermochemical liquefaction techniques (HTL) as it is a method that can effectively treat both wet and dry biomass from lignocellulosic to organic waste without limiting the amount of lipid present. The product produced in this process is known as bio-crude, a renewable alternative to oil since it is an energy-dense intermediate that can be converted into various liquid fuels [26]. The flow diagram in Figure 3 shows the reaction process for dry lignocellulose biomass. Dry biomass usually needs pre-treatment, especially woody biomass, to reduce particle size, remove contaminants and alkaline treatment to obtain a stable slurry for easy pumping [36].
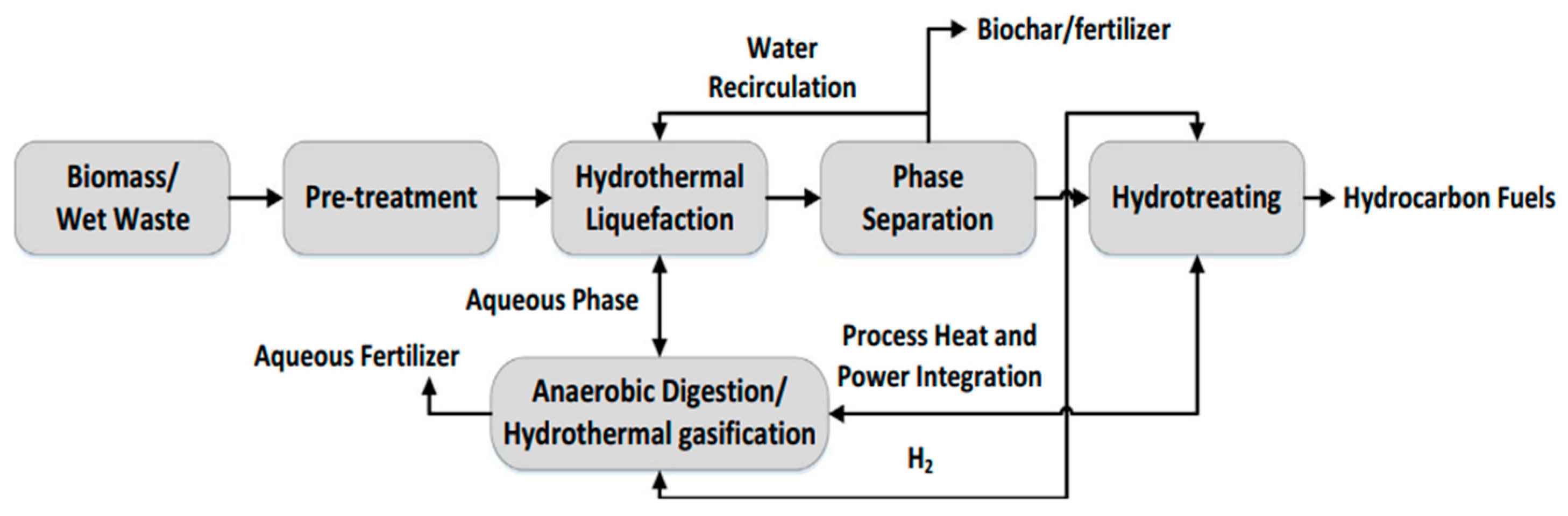
Figure 3. Process flow diagram of HTL process of lignocellulose biomass.
Zeolite Catalysts in HTL Conversion of Biomass
Zeolites, encompassing varieties such as ZSM-5, are integral catalysts in the hydrothermal liquefaction (HTL) process, exerting significant influence via their acidity, confinement effects, and morphology. The tuneable acidity of zeolites, including both Brønsted and Lewis acid sites, is pivotal in promoting the breakdown of complex biomass molecules during HTL [37]. This catalytic activity enhances the production of valuable hydrocarbons while minimising undesired by-products. The unique microporous structure of zeolites contributes to a confinement effect, influencing reaction pathways and product selectivity in HTL. This effect stabilises reactive intermediates, potentially improving catalytic efficiency and controlling molecular interactions within zeolite channels [21]. The morphology of zeolites, including particle size and shape, is a critical factor influencing catalytic performance. Smaller particles with higher surface areas may enhance accessibility to active sites and impact reaction kinetics. Moreover, the stability of zeolites under harsh HTL conditions is crucial for sustained catalytic activity [38]. Researchers often modify zeolites using strategies like ion exchange or metal impregnation to tailor their properties, further enhancing acidity, selectivity, and stability. In summary, zeolites, as catalysts in HTL, offer a versatile platform for efficient and selective biomass conversion into biofuels, with their unique acidity, confinement effects, and morphology playing key roles in optimising catalytic performance [39].
Catalysts play a significant role in the reaction by lowering the activation energy, which has various benefits, including increased bio-oil yield and biomass conversion efficiency. The bio-oil yield was reported to have increased by 50 to 60% when a suitable catalyst was used in an HTL process [40]. Catalysts can improve bio-oil flow characteristics while lowering heteroatom content; furthermore, suitable catalysts can improve the higher heating value (HHV) of bio-crude [40]. Numerous studies have been conducted on the utilisation of acidic and alkaline homogeneous catalysts (HCl, H3PO4, Na2CO3, K2CO3, KOH, NaOH, Ca(OH)2, etc). The results revealed that their addition significantly increased the yield and quality of bio-crude, but because these catalysts are homogeneous, expensive separation procedures to remove them at the conclusion of the reaction and corrosion-resistant equipment are needed [41]. Since recovering the catalyst in homogeneous catalysis is difficult and expensive, the catalyst will be discharged with the water phase at the end of the process, and it should request the appropriate neutralising treatments. Recently, heterogeneous catalysts have received a lot of attention due to their high activity and ease of recovery from liquid products. This allows them to be reused, reducing the cost associated with bio-crude production and encouraging large-scale production. Heterogenous catalysts are also not corrosive and have higher thermal stability. It is also known that heterogeneous catalysts enhance bio-oil stability [42].
For instance, the H form of Zeolite Socony Mobil-5 (HZSM-5) increases the bio-oil stability by reducing the organic acid content and by increasing the fraction of alkene, alkane, and ketones. Reusing catalysts is crucial for the cost-effective generation of HTL bio-oil, especially when using expensive metal catalysts [40].
Data from the literature show that ZSM-5 (Zeolite Socony Mobil-5) is one of the most promising catalysts that can be utilised to produce bio-oil from lignocellulosic feedstock. The presence of such a substance facilitates the production of a liquid fraction with a mixture of organic compounds with a lower oxygen content and a greater contribution from aromatics [43]. A number of techniques, including ion exchange, calcination conditions, and the ratio of silica to alumina (Si/Al), are used to regulate the acidity of the zeolite. They can be employed at high temperatures because of their excellent thermal stability, which frequently leads to higher yields and simpler heat recovery [44]. Various synthetic techniques have been proposed to circumvent the limitation imposed by narrow channel entrances of zeolites while maintaining their chemical features. Making hierarchical materials that combine micro and mesoporosity because these materials have mesopores, the acid sites are more easily accessible while maintaining the zeolites’ acidity. Because mesopores make active sites more easily accessible, faster diffusion of reactants and products is thought to be the cause of the increase in activity. It is expected that the variations in reaction rates along the individual crystals will be lessened when mesopores are present. Such catalysts can be quite successful at converting biomass because of the wide range of pore sizes found in them [45].
References
- Forero, J.A.J.; Tran, T.H.T.; Tana, T.; Baker, A.; Beltramini, J.; Doherty, W.O.S.; Moghaddam, L. Hydrothermal liquefaction of sugarcane bagasse to bio-oils: Effect of liquefaction solvents on bio-oil stability. Fuel 2022, 312, 122793.
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517.
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186.
- Qureshi, M.I.; Rasli, A.M.; Zaman, K. Energy crisis, greenhouse gas emissions and sectoral growth reforms: Repairing the fabricated mosaic. J. Clean. Prod. 2016, 112, 3657–3666.
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63.
- Cao, M.; Long, C.; Sun, S.; Zhao, Y.; Luo, J.; Wu, D. Catalytic hydrothermal liquefaction of peanut shell for the production aromatic rich monomer compounds. J. Energy Inst. 2021, 96, 90–96.
- Deublein, D.; Angelika, S. Biogas from Waste and Renewable Resources; Wiley-VCH Verlag GmmbH & Co. KGaA: Weinheim, Germany, 2008.
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass Conversion Technologies. In Greenhouse Gases Balances of Bioenergy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–139.
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–368.
- Baloch, H.A.; Siddiqui, M.T.; Nizamuddin, S.; Mubarak, N.M.; Khalid, M.; Srinivasan, M.; Griffin, G. Catalytic co-liquefaction of sugarcane bagasse and polyethylene for bio-oil production under supercritical conditions: Effect of catalysts. J. Anal. Appl. Pyrolysis 2021, 153, 104944.
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266.
- Rehman, A.; Noor, T.; Hussain, A.; Iqbal, N.; Jahan, Z. Role of Catalysis in Biofuels Production Process–A Review. ChemBioEng Rev. 2021, 8, 417–438.
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18.
- Saoud, K. Nanocatalyst for Biofuel Production: A Review. In Green Nanotechnology for Biofuel Production, Biofuel and Biorefinery Technologies; Srivastava, N., Srivastava, M., Pandey, H., Mishra, P.K., Ramteke, P.W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 5, pp. 39–62.
- Ibrahim, S.M.; Mustafa, A. Synthesis and characterization of new bifunctional SnZrSi oxide catalysts for biodiesel production. J. Mol. Liq. 2022, 354, 118811.
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290.
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on in-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056.
- Aznar, M.P.; Caballero, M.A.; Gil, J.; Martín, J.A.; Corella, J. Commercial steam reforming catalysts to improve biomass gasification with steam-oxygen mixtures. 2. Catalytic tar removal. Ind. Eng. Chem. Res. 1998, 37, 2668–2680.
- Caballero, M.A.; Aznar, P.; Gil, J.; Martı, J.A.; France, E. 98/03884 Commercial steam reforming catalysts to improve biomass gasification with steam-oxygen mixtures 1. Hot gas upgrading by the catalytic reactor. Fuel Energy Abstr. 1998, 39, 363.
- Kouwenhoven, H.W.; De Kroes, B. Preparation of zeolite catalysts. Stud. Surf. Sci. Catal. 2001, 137, 673–706.
- Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Confinement in a Zeolite and Zeolite Catalysis. Acc. Chem. Res. 2021, 54, 2894–2904.
- Nature Materials Zeolite catalysts come into focus. Nat. Mater. 2020, 19, 1037.
- Wang, S.; Wang, Y.; Cai, Q.; Guo, Z. Production of Bio-gasoline by Co-cracking of Acetic Acid in Bio-oil. Chin. J. Chem. Eng. 2014, 22, 98–103.
- Mortensen, P.M.; Grunwaldt, J.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19.
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals q. Fuel 2016, 179, 124–134.
- Grande, L.; Pedroarena, I.; Korili, S.A.; Gil, A. Hydrothermal liquefaction of biomass as one of the most promising alternatives for the synthesis of advanced liquid biofuels: A review. Materials 2021, 14, 5286.
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581.
- Shahabuddin, M.; Alam, T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Bioresource Technology A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596.
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977.
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597.
- Chen, W.; Lin, B.; Huang, M.; Chang, J. Bioresource Technology Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2014, 184, 314–327.
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409.
- Serio, M.A.; Kroo, E.; Wójtowicz, M.A. Biomass Pyrolysis for Distributed Energy Generation. ACS Div. Fuel Chem. 2003, 48, 589.
- Nanda, S.; Kozinski, J.A.; Dalai, A.K. Lignocellulosic Biomass: A Review of Conversion Technologies and Fuel Products. Curr. Biochem. Eng. 2016, 3, 24–36.
- Rudra, S.; Jayathilake, M. Hydrothermal Liquefaction of Biomass for Biofuel Production. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–186.
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392.
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals. ACS Catal. 2018, 8, 148–187.
- Wang, Y.; Wang, C.; Wang, L.; Wang, L.; Xiao, F. Zeolite Fixed Metal Nanoparticles: New Perspective in Catalysis. Acc. Chem. Res. 2021, 54, 2579–2590.
- Zhang, Z.; Sun, Y.; Zhang, H.; Wei, L. Synthesis, Types, and Applications of Zeolite Capsule Catalysts. Cryst. Res. Technol. 2023, 58, 2200287.
- Nagappan, S.; Bhosale, R.R.; Duc, D.; Thuy, N.; Chi, L. Catalytic hydrothermal liquefaction of biomass into bio-oils and other value- added products–A review. Fuel 2021, 285, 119053.
- Perego, C.; Bianchi, D. Biomass upgrading through acid–base catalysis. Chem. Eng. J. 2010, 161, 314–322.
- Scarsella, M.; de Caprariis, B.; Damizia, M.; De Filippis, P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: A review. Biomass Bioenergy 2020, 140, 105662.
- Grams, J.; Ruppert, A. Development of Heterogeneous Catalysts for Thermo-Chemical Conversion of Lignocellulosic Biomass. Energies 2017, 10, 545.
- Balagurumurthy, B.; Singh, R.; Bhaskar, T. Catalysts for Thermochemical Conversion of Biomass; Elsevier B.V.: Amsterdam, The Netherlands, 2015.
- Centi, G.; Perez-pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15.
More
Information
Subjects:
Chemistry, Inorganic & Nuclear
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
661
Revisions:
2 times
(View History)
Update Date:
26 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




