Peach fruit has a high respiration rate and quick ripening process because of its climacteric nature, so it has a shorter shelf life at ambient temperature. The main reasons associated with postharvest life reduction include firmness loss, reduction in fruit quality, and decay. With time, the quality of fruit is reduced because of postharvest factors such as softening of fruit and rot development; that is why peaches are marketed soon after harvesting
[5]. Because of high respiration, peach fruit has a fast ripening process, so it cannot be stored for a long period at ambient temperature. It has a shorter storage life of 3–5 days at room temperature
[6]. There are approximately 40% postharvest losses in peach fruit
[7]. As a result, huge financial losses are faced because there are abundant peaches in the market during peak season, and a huge portion goes wasted. At ambient temperature, peach fruit quickly ripens and spoil, so storing peach fruit at a cold storage of 0 °C results in the chilling injury of peach fruit; once the fruit moves from cold storage to room temperature, it looks normal but does not ripen well, which results in quality losses such as dry, mealy texture, tissue browning, failure to ripen, abnormal flesh color, and loss of flavor
[8][9][10]. Peaches also have dynamic living tissues like other fruits, so they have a shorter shelf life and are highly perishable
[11]. Physiological disorders, metabolic changes, decay, mechanical damage, and reduced firmness in stored peaches result in postharvest quality loss of peach fruit. Several factors affect these losses, such as handling, storage conditions, and ripeness stage at harvest time
[12]. Without any treatment or low temperature, the storage life of peaches is much shorter at ambient temperature, which results in color and texture degradation of peach fruits during the storage period
[13]. There are about 40–50% postharvest losses of peaches. In third-world nations, this value rises due to a lack of postharvest handling facilities
[14]. According to Sridhar et al.
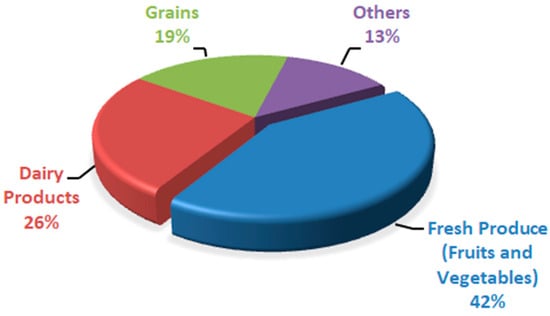
[15], the wastage of different food commodities was expressed in percentages based on rapid spoilage (
Figure 1). Fresh produce (fruits and vegetables) was found to be a less expensive and quickly spoiling commodity, followed by dairy items. Postharvest diseases result in around 20% of peach fruit losses because of Rhizopus rot and brown rot caused by Rhizopus stolonifera and Molinia fructi cola, respectively
[16]. Botrytis cinerea results in grey mold rot in peach fruit during postharvest storage
[17]. Fruit storage life is extended through low-temperature storage or shipping. Still, fruit from some cultivars results in flesh translucency, flesh browning, red pigment (bleeding), black pit cavity, failure to ripen, lack of juiciness/mealiness/wooliness, and loss of flavor after ripening at room temperature or after long-term cold storage
[18]. In developing countries, the world population is increasing at a high rate, and those nations are already facing food safety and food security issues. It is a big challenge for mankind to meet the food requirements of an increasing population. By 2050, it is expected that the population across the globe will exceed 9.1 billion inhabitants, which requires about a 70% increase in fresh produce to meet the needs
[19]. Excluding some plum cultivars like “Sweet Miriam” and cherries, stone fruits are considered climacteric
[20]. During the ripening process, ethylene biosynthesis, which accelerates fruit enzymatic and biochemical reactions, was observed in climacteric fruits
[21]. For those reactions, oxygen serves as substrate; that is why, with a rise in ethylene production, the respiration rate also rises
[22]. During respiration, carbohydrates and other substrates like proteins, organic acids, and fats are metabolized. After metabolism, these substances cannot be replenished once the fruit or vegetable is cut off from the plant
[23]; with the passage of time, the quality of food results in deterioration in terms of color, flavor, weight, and nutritional value. Water loss is the major factor that plays a vital role in quality deterioration in fruits and vegetables, leading to wilting, shriveling, texture loss, flaccidness, and loss of nutritional value
[24]. Over the past few decades, fresh agricultural products and processed foods have benefited greatly from the widespread use of conventional petroleum-based plastic packaging. This is primarily due to the manufacturing simplicity, cheaper cost, ease of use, and favorable physico-chemical properties of the material
[25]. Still, as public awareness grows related to the harmful effects of plastic on the environment, consumers are diverted toward biodegradable, renewable, and environmentally friendly packaging material
[26]. Using edible films and coating is one of the ways to meet the current demand for coatings
[27].
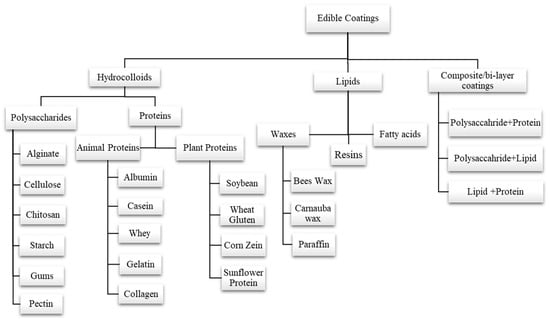
The formulation of edible coatings can be made from different materials with desired properties. Polysaccharides (pectin, starch, gums, alginate, chitosan, cellulose, etc.), proteins (gelatin, egg albumin, wheat gluten, zein, whey protein, casein, soy protein, etc.), and lipid compounds (fatty acids, waxes, etc.) are three major categories that can be considered to classify the basic materials that are used to make edible coatings. Another coating is called composite coating, which results from combining more than one substance or material
[29][30][31][32]. Classification of various edible coatings is depicted in (
Figure 2). Edible coatings also enhance functional properties by incorporating antioxidants, antimicrobial compounds, vitamins, and minerals into a polymer matrix
[33].
Figure 2. Classification of various edible coatings into different types on the basis of formulation.
2. Lipids
The lipid-based edible coating has low water affinity as it is hydrophobic and has excellent moisture barrier properties
[34]. However, it has been reported that lipid-based edible coatings have poor gas barriers and mechanical properties
[35]. Apart from preventing water loss, lipid-based edible coatings reduce respiration rate, prolong postharvest life, and enhance the aesthetic of fresh produce by imparting shine on the surface
[28]. Coatings made of lipids have been used for more than 800 years. In the past, coatings made of lipids were used to coat confectionery items and waxing fruits. Beeswax and paraffin wax were considered the most effective. Lipids prevent the chilling injuries that often occur in cold storage
[36]. In various edible coatings, carnauba wax and beeswax (natural waxes) have been utilized as lipid components
[37]. Because of their hydrophobic nature, these compounds help to prevent weight loss caused by dehydration during storage and impart gloss to the fruit’s surface. Compared to protein and polysaccharide coating, a lipid-based coating has excellent barrier properties and the best compatibility with other coating agents. The lipid-based coating leads to unacceptable organoleptic properties because of its greasy nature and lipid rancidity
[38]. Widely used materials for coatings include beeswax, acetylated monoglycerides, carnauba wax, mineral oil, vegetable oil, surfactants, and paraffin wax
[36].
3. Proteins
Proteins are classified into two groups based on the source from which they are obtained, i.e., globular protein and fibrous protein. Globular proteins are attained from plant sources (e.g., cotton seed protein, wheat gluten, cotton seed protein, corn-zein, peanut protein, soy protein) and are soluble in bases, salts, and aqueous solutions of acids or water, while fibrous proteins are attained from animal sources (e.g., collagen, whey protein, keratin, gelatin, casein) and are insoluble in water
[39]. The coatings obtained from animal sources (such as milk protein) and plant sources (such as zein, wheat gluten, and soy protein) have excellent lipid, oxygen, and carbon dioxide barrier properties, especially at low RH
[40]. Edible coatings from protein sources possess excellent gas barrier and mechanical properties, but their use is limited due to allergenic risks and ethical or religious beliefs
[35]. Additionally, edible coatings obtained from protein sources were susceptible to cracking and brittle
[38]. Various fruits and vegetables can be coated with proteins based on edible coatings derived from gelatin, milk, corn, soybeans, wheat, peanuts, or gelatin. Most protein-based films have good results on hydrophilic surfaces, but they rarely resist water vapor diffusion. Coatings derived from proteins have poor mechanical and water barrier properties but excellent oxygen and carbon dioxide barrier properties
[41]. Compared to polysaccharide films, the protein coating has good mechanical and excellent gas barrier properties. Still, as it is hydrophile, like polysaccharide films, the protein coating is considered to have a poor moisture barrier
[42]. It has been assumed that it has a great potential for aromatic and organoleptic attribute retention, develops a barrier against mechanical strength, and has a high oxygen permeability, but because of its hydrophilic nature, it is not a good moisture barrier that can be strengthened by the addition of hydrophobic substance like lipids
[43].
4. Polysaccharides
Polysaccharides are biodegradable macromolecules that are safe, non-toxic, and highly stable
[28]. Polysaccharides are naturally occurring polymers used in the production of edible coatings. Chitosan, starch, and gums are important ingredients used for food preservation in polysaccharide-based edible natural coatings. This coating has many advantages, like easy availability and cheap cost. However, different polysaccharides were found to have a lower water barrier quality. Some polysaccharides like carrageenan and alginate are thick-filmed and have a hygroscopic nature. Polysaccharide-based edible coatings possess antimicrobial and antioxidant characteristics, which are considered efficient in keeping fruits and vegetables fresh and improving quality. Because of its hydrophilic nature, it cannot act as a barrier to moisture
[44]. Throughout the literature, polysaccharides, such as starches, gums, cellulose derivatives, and pectins, are widely mentioned and highly favored for use as an edible coating in all types of stone fruit
[45]. Polysaccharides are excellent coating materials because of their easy availability, allergen-free nature, and typically water-soluble nature. Their strong hydrogen-bonded and orderly network structure leads to a good gas barrier and mechanical properties
[35]. A variety of polysaccharides can be utilized in the production of edible coating. The most often available polymers are extracted from agricultural plants, animals, and marine sources. During short-term storage, these coatings have been used to prevent some food moisture loss
[36].
5. Composites or Bi-Layer Natural Edible Coatings
For improved functional qualities in coatings, the development of bi-layer and composite coatings containing proteins, lipids, and polysaccharides has received much attention in the past few years. Multiple coating materials are combined in a composite edible coating, resulting in the improved functionality of the coating due to several advantageous properties
[35]. Because each coating material serves a distinct but limited function, the functionality can be enhanced by combining two distinct coating materials. The main goal of composites is to maximize the benefits of the mixture’s highest possible performance while maintaining the quality as stable as an individual component. Combining multiple materials can create composite edible coatings with distinctive properties
[43]. Multi-component or composite films possess both features of hydrocolloid (proteins or polysaccharides) and lipid components; hydrocolloid serves as a selective barrier to oxygen and carbon dioxide, while lipid components serve as an excellent barrier to water vapor
[46]. A coating composed of just one material, such as a protein, polysaccharide, or lipid, may be effective in exhibiting a specific property but may not possess several properties or barriers simultaneously. For example, hydrocolloids (proteins and polysaccharides) are excellent film-forming materials with good structural and mechanical properties. Still, they often have a poor moisture barrier, so to enhance the hydrophobicity, lipids are often added to the edible coating to create a barrier to postharvest moisture loss. As a result, the hydrophobic nature of the lipids may play a significant role by acting as an excellent barrier to water. In other words, bi-layer or composite coatings are designed to integrate the benefits of lipids and hydrocolloid coatings to reduce or mask each limitation
[41]. Edible coatings comprised of protein, polysaccharides, and lipid blends may vary in nature. This strategy assists an individual in taking advantage of the different functional properties of each film class
[47].