
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masaaki Nakayama | -- | 3676 | 2024-01-25 05:19:43 | | | |
| 2 | Sirius Huang | Meta information modification | 3676 | 2024-01-26 01:47:44 | | |
Video Upload Options
Chronic kidney disease (CKD), which is globally on the rise, has become an urgent challenge from the perspective of public health, given its risk factors such as end-stage renal failure, cardiovascular diseases, and infections. The pathophysiology of CKD, including dialysis patients, is deeply associated with enhanced oxidative stress in both the kidneys and the entire body. Therefore, the introduction of a safe and widely applicable antioxidant therapy is expected as a measure against CKD. Electrolyzed hydrogen water (EHW) generated through the electrolysis of water has been confirmed to possess chemical antioxidant capabilities. In Japan, devices producing this water have become popular for household drinking water. In CKD model experiments conducted to date, drinking EHW has been shown to suppress the progression of kidney damage related to hypertension. Furthermore, clinical studies have reported that systemic oxidative stress in patients undergoing dialysis treatment using EHW is suppressed, leading to a reduction in the incidence of cardiovascular complications.
1. Introduction
1.1. Historical Background of Electrolyzed Hydrogen Water
1.2. Cross over with Hydrogen Medicine
2. Latest Insights into H2 Biology Research—Brief Summary
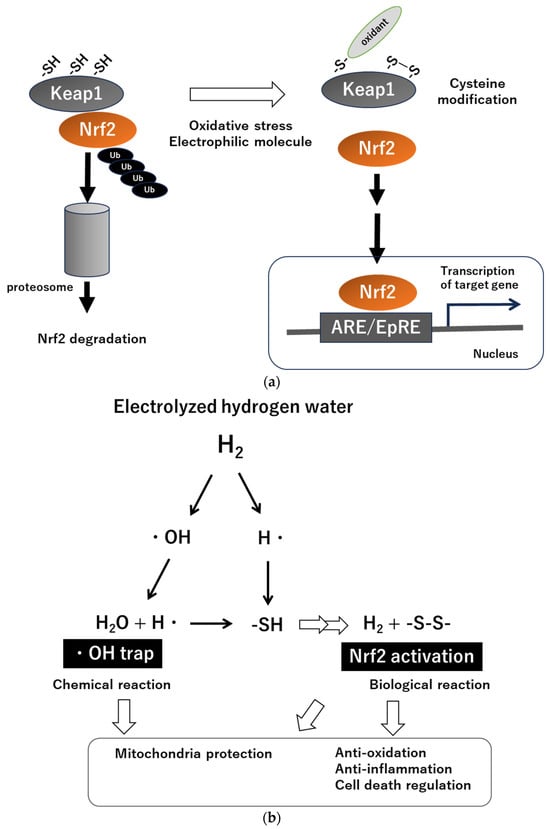
3. H2 Intervention for CKD and Hemodialysis
3.1. Pre-Clinical Studies of H2 in CKD Models
| CKD Model | H2 Load | Intervention | Observation | Main Findings | Oxidative Stress Marker |
|---|---|---|---|---|---|
| Dahl salt-sensitive rat (4 weeks old) [21] |
Ad libitum drinking in respective groups (n = 30 each) EHW (H2: 0.49 mg/L) DW (H2: 0.003 mg/L) FW (H2: <0.001 mg/L) |
N/A (fed by low sodium diet) |
48 weeks | No striking differences in BP among 3 groups. Lower in EHW than DW and FW in cardiac remodeling, glomerular sclerosis with tubulointerstitial fibrosis in the kidney, and increased cardiomyocyte diameter with interstitial fibrosis in the heart. |
Kidney: fewer nitrotyrosine, malondialdehyde, and ED1 cells in EHW than FW. Heart: less malondialdehyde in EW than FW, significantly higher Nrf2, and lower NADPH oxidase expression in EHW than FW. |
| Spontaneous hypertensive rat (8 weeks old) [66] |
Ad libitum drinking in respective groups (n = 72 each) HW (H2: 0.8–1.3 mg/L) vehicle |
N/A | 12 weeks | No striking differences in BP between two groups. Lower glomerular sclerosis score and higher renal blood flow and glomerular filtration rate in HW than vehicle. |
Lowered reactive oxygen species formation; upregulated the activities of superoxide dismutase, glutathione peroxidase, glutathione-S-epoxide transferase, and catalase, and suppressed NADPH oxidase in HW. Depressed pro-inflammatory cytokine expression in HW. Protective effect on mitochondrial function in HW. |
| Dahl salt-sensitive rat (7 weeks old) [67] |
Ad libitum drinking in respective groups (n = 18 in EW, 17 in FW) EHW (H2: 0.35 mg/L) FW (H2: 0.0 mg/L) |
Unilateral kidney I/R (fed a low sodium diet) |
6 weeks of preconditioning followed by intervention and 1 week post observation | Contralateral kidney and heart: less glomerular adhesion, cardiac fibrosis in EHW. |
Lower mRNA expression of NADPH oxidase 4 in heart in HW. Smaller number of ED-1 positive cells and nitrotyrosine in kidney and heart in EHW. |
3.2. Clinical Studies of H2 Intervention in Related to CKD Pathologies
3.3. Clinical Studies Using EHW for Hemodialysis
| Year (Ref.) | Study Design | H2 Level of HD Solution | Number of Patients | Duration | Outcome |
|---|---|---|---|---|---|
| 2009 [82] | Single-arm | ~99.0 ppb | 8 | 1 month | Significant decrease of methylguanidine |
| 2010 [83] | Single-arm | 49 ppb (average) | 21 | 6 months | BP reduction before and after HD Decrease of plasma MCP-1 and MPO (3rd tertile group) |
| 2016 [84] | Parallel-arm | 47–196 ppb | 12 in EHD 38 in CHD |
7 months | Significant elevation in serum reduced albumin fraction pre- and post-HD in EW-HD No differences between EHD (post) and healthy subjects |
| 2017 [85] | Parallel-arm | 30–80 ppb | 140 in EHD 122 in CHD |
12 months | Reduction of anti-hypertensive agents and subjective symptoms such as fatigue and pruritus |
| 2018 [86] | Parallel-arm | 30–80 ppb | 161 in EHD 148 in CHD |
3.28 years (average) | Reduction of post-HD BP in EHD Hazard ratio of EHD 0.59 for composite of all-cause mortality and non-lethal cardio-cerebrovascular events after adjusting for confounding factors |
| 2021 [30] | Single-arm | 41–81 ppb | 63 | 2 months | Elevations of plasma MPO and thioredoxin at post-HD, elevation of plasma malondialdehyde at pre-HD and decrease at post-HD Decrease of VAS of fatigue |
| 2021 [87] | Single-arm | 120–163 ppb | 95 | 2 months | Decrease of VAS of fatigue |
| 2022 [88] | Single-arm | Basal 47 ppb (average) to 154 ppb (average) ppb |
105 | 2 months | Decrease of plasma MPO pre-HD Decrease of NRS of fatigue |
References
- Itokawa, Y. An overview on researches of portable alkaline water by electrolysis. Kinousuikenkyu 2004, 2, 59–64. (In Japanese)
- Alkaline Ionized Water Apparatus Market Trends. Available online: https://www.3aaa.gr.jp/english/markettrend.html (accessed on 1 December 2023).
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274.
- Li, Y.; Hamasaki, T.; Nakamichi, N.; Kashiwagi, T.; Komatsu, T.; Ye, J.; Teruya, K.; Abe, M.; Yan, H.; Kinjo, T.; et al. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2011, 63, 119–131.
- Yan, H.; Kinjo, T.; Tian, H.; Hamasaki, T.; Teruya, K.; Kabayama, S.; Shirahata, S. Mechanism of the lifespan extension of Caenorhabditis elegans by electrolyzed reduced water—participation of Pt nanoparticles. Biosci. Biotechnol. Biochem. 2011, 75, 1295–1299.
- Wang, Y.; Fugetsu, B.; Sakata, I.; Fujisue, C.; Kabayama, S.; Tahara, N.; Morisawa, S. Monolayered Platinum Nanoparticles as Efficient Electrocatalysts for the Mass Production of Electrolyzed Hydrogen Water. Sci. Rep. 2020, 10, 10126.
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen—comprehensive review of 321 original articles. Med. Gas. Res. 2015, 5, 12.
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed-Reduced Water: Review I. Molecular Hydrogen Is the Exclusive Agent Responsible for the Therapeutic Effects. Int. J. Mol. Sci. 2022, 23, 14750.
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular Hydrogen Therapy-A Review on Clinical Studies and Outcomes. Molecules 2023, 28, 7785.
- Mizuno, K.; Watanabe, K.; Yamano, E.; Ebisu, K.; Tajima, K.; Nojima, J.; Ohsaki, Y.; Kabayama, S.; Watanabe, Y. Antioxidant effects of continuous intake of electrolyzed hydrogen water in healthy adults. Heliyon 2022, 8, e11853.
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11.
- Zhang, Y.; Tan, S.; Xu, J.; Wang, T. Hydrogen Therapy in Cardiovascular and Metabolic Diseases: From Bench to Bedside. Cell Physiol. Biochem. 2018, 47, 1–10.
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076.
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8384742.
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507.
- Rahman, M.H.; Jeong, E.-S.; You, H.S.; Kim, C.-S.; Lee, K.-J. Redox-Mechanisms of Molecular Hydrogen Promote Healthful Longevity. Antioxidants 2023, 12, 988.
- Saengsin, K.; Sittiwangkul, R.; Chattipakorn, S.C.; Chattipakorn, N. Hydrogen therapy as a potential therapeutic intervention in heart disease: From the past evidence to future application. Cell Mol. Life Sci. 2023, 80, 174.
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824.
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells. 2023, 46, 133–141.
- Zhu, W.J.; Nakayama, M.; Mori, T.; Hao, K.; Terawaki, H.; Katoh, J.; Kabayama, S.; Ito, S. Amelioration of cardio-renal injury with aging in dahl salt-sensitive rats by H2-enriched electrolyzed water. Med. Gas. Res. 2013, 3, 26.
- Yuan, J.; Wang, D.; Liu, Y.; Chen, X.; Zhang, H.; Shen, F.; Liu, X.; Fu, J. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J. Surg. Res. 2018, 228, 238–246.
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharmacol. 2019, 97, 287–292.
- Yu, Y.; Feng, J.; Lian, N.; Yang, M.; Xie, K.; Wang, G.; Wang, C.; Yu, Y. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. Int. Immunopharmacol. 2020, 85, 106585.
- Lu, Y.; Li, C.F.; Ping, N.N.; Sun, Y.Y.; Wang, Z.; Zhao, G.X.; Yuan, S.H.; Zibrila, A.I.; Soong, L.; Liu, J.J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22467.
- Hu, Y.; Wang, P.; Han, K. Hydrogen Attenuated Inflammation Response and Oxidative in Hypoxic Ischemic Encephalopathy via Nrf2 Mediated the Inhibition of NLRP3 and NF-κB. Neuroscience 2022, 485, 23–36.
- Peng, J.; He, Q.; Li, S.; Liu, T.; Zhang, J. Hydrogen-Rich Water Mitigates LPS-Induced Chronic Intestinal Inflammatory Response in Rats via Nrf-2 and NF-κB Signaling Pathways. Vet. Sci. 2022, 9, 621.
- Murakami, Y.; Ito, M.; Ohsawa, I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS ONE 2017, 12, e0176992.
- Hirayama, M.; Ito, M.; Minato, T.; Yoritaka, A.; LeBaron, T.W.; Ohno, K. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2’-deoxyguanine in Parkinson’s disease. Med. Gas. Res. 2019, 8, 144–149.
- Satta, H.; Iwamoto, T.; Kawai, Y.; Koguchi, N.; Shibata, K.; Kobayashi, N.; Yoshida, M.; Nakayama, M. Amelioration of hemodialysis-induced oxidative stress and fatigue with a hemodialysis system employing electrolyzed water containing molecular hydrogen. Ren. Replace. Ther. 2021, 7, 37.
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes 1. Can J. Physiol. Pharmacol. 2019, 97, 797–807.
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013, 65, 645–657.
- Satoh, T.; Lipton, S.A. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007, 30, 37–45.
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811.
- Shingu, C.; Koga, H.; Hagiwara, S.; Matsumoto, S.; Goto, K.; Yokoi, I.; Noguchi, T. Hydrogen-rich saline solution attenuates renal ischemia-reperfusion injury. J. Anesth. 2010, 24, 569–574.
- Gvozdjáková, A.; Kucharská, J.; Kura, B.; Vančová, O.; Rausová, Z.; Sumbalová, Z.; Uličná, O.; Slezák, J. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Can. J. Physiol. Pharmacol. 2020, 98, 29–34.
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500.
- Han, B.; Sivaramakrishnan, P.; Lin, C.J.; Neve, I.A.A.; He, J.; Tay, L.W.R.; Sowa, J.N.; Sizovs, A.; Du, G.; Wang, J.; et al. Microbial Genetic Composition Tunes Host Longevity. Cell 2017, 169, 1249–1262.e13.
- Matsuhashi, T.; Sato, T.; Kanno, S.I.; Suzuki, T.; Matsuo, A.; Oba, Y.; Kikusato, M.; Ogasawara, E.; Kudo, T.; Suzuki, K.; et al. Mitochonic Acid 5 (MA-5) Facilitates ATP Synthase Oligomerization and Cell Survival in Various Mitochondrial Diseases. EBioMedicine 2017, 20, 27–38.
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, eaaw3159.
- Wolf, P.G.; Biswas, A.; Morales, S.E.; Greening, C.; Gaskins, H.R. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes 2016, 7, 235–245.
- Xie, F.; Jiang, X.; Yi, Y.; Liu, Z.J.; Ma, C.; He, J.; Xun, Z.M.; Wang, M.; Liu, M.Y.; Mawulikplimi Adzavon, Y.; et al. Different effects of hydrogen-rich water intake and hydrogen gas inhalation on gut microbiome and plasma metabolites of rats in health status. Sci. Rep. 2022, 12, 7231.
- Colombijn, J.M.; Hooft, L.; Jun, M.; Webster, A.C.; Bots, M.L.; Verhaar, M.C.; Vernooij, R.W. Antioxidants for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2023, 11, CD008176.
- Wang, B.; Li, Z.; Mao, L.; Zhao, M.; Yang, B.; Tao, X.; Li, Y.; Yin, G. Hydrogen: A Novel Treatment Strategy in Kidney Disease. Kidney Dis. 2022, 8, 126–136.
- Hirano, S.I.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Clinical Use and Treatment Mechanism of Molecular Hydrogen in the Treatment of Various Kidney Diseases including Diabetic Kidney Disease. Biomedicines. 2023, 11, 2817.
- Yao, W.; Guo, A.; Han, X.; Wu, S.; Chen, C.; Luo, C.; Li, H.; Li, S.; Hei, Z. Aerosol inhalation of a hydrogen-rich solution restored septic renal function. Aging 2019, 11, 12097–12113.
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral administration of Si-based agent attenuates oxidative stress and ischemia-reperfusion injury in a rat model: A novel hydrogen administration method. Front. Med. 2020, 7, 95.
- Li, J.; Hong, Z.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front. Pharmacol. 2016, 7, 106.
- Xu, X.; He, X.; Liu, J.; Qin, J.; Ye, J.; Fan, M. Protective effects of hydrogen-rich saline against renal ischemia-reperfusion injury by increased expression of heme oxygenase-1 in aged rats. Int. J. Clin. Exp. Pathol. 2019, 12, 1488–1496.
- Nishida, T.; Hayashi, T.; Inamoto, T.; Kato, R.; Ibuki, N.; Takahara, K.; Yoshikawa, Y.; Uchimoto, T.; Saito, K.; Tanda, N.; et al. Dual gas treatment with hydrogen and carbon monoxide attenuates oxidative stress and protects from renal ischemia-reperfusion injury. Transplant. Proc. 2018, 50, 250–258.
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-rich saline attenuates acute kidney injury after liver transplantation via activating p53-mediated autophagy. Transplantation. 2016, 100, 563–570.
- Wang, F.; Yu, G.; Liu, S.Y.; Li, J.B.; Wang, J.F.; Bo, L.L.; Qian, L.R.; Sun, X.J.; Deng, X.M. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J. Surg. Res. 2011, 167, e339–e344.
- Chen, J.; Zhang, H.; Hu, J.; Gu, Y.; Shen, Z.; Xu, L.; Jia, X.; Zhang, X.; Ding, X. Hydrogen-rich saline alleviates kidney fibrosis following AKI and retains Klotho expression. Front. Pharmacol. 2017, 8, 499.
- Liu, W.; Dong, X.S.; Sun, Y.Q.; Liu, Z. A novel fluid resuscitation protocol: Provide more protection on acute kidney injury during septic shock in rats. Int. J. Clin. Exp. Med. 2014, 15, 919–926.
- Shi, Q.; Liao, K.S.; Zhao, K.L.; Wang, W.X.; Zuo, T.; Deng, W.H.; Chen, C.; Yu, J.; Guo, W.Y.; He, X.B.; et al. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators Inflamm. 2015, 2015, 685043.
- Guan, P.; Sun, Z.M.; Luo, L.F.; Zhou, J.; Yang, S.; Zhao, Y.S.; Yu, F.Y.; An, J.R.; Wang, N.; Ji, E.S. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019, 225, 46–54.
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109.
- Abe, T.; Li, X.K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H.; et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012, 94, 14–21.
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761.
- Li, F.Y.; Zhu, S.X.; Wang, Z.P.; Wang, H.; Zhao, Y.; Chen, G.P. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem. Toxicol. 2013, 61, 248–254.
- Peng, Z.; Chen, W.; Wang, L.; Ye, Z.; Gao, S.; Sun, X.; Guo, Z. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 2680–2689.
- Lu, H.; Ding, J.; Liu, W.; Peng, Z.; Chen, W.; Sun, X.; Guo, Z. UPLC/MS-based metabolomics investigation of the protective effect of hydrogen gas inhalation on mice with calcium oxalate-induced renal injury. Biol. Pharm. Bull. 2018, 41, 1652–1658.
- Xu, B.; Zhang, Y.B.; Li, Z.Z.; Yang, M.W.; Wang, S.; Jiang, D.P. Hydrogen-rich saline ameliorates renal injury induced by unilateral ureteral obstruction in rats. Int. Immunopharmacol. 2013, 17, 447–452.
- Xing, Z.; Pan, W.; Zhang, J.; Xu, X.; Zhang, X.; He, X.; Fan, M. Hydrogen rich water attenuates renal injury and fibrosis by regulation transforming growth factor-β induced Sirt1. Biol. Pharm. Bull. 2017, 40, 610–615.
- Mizutani, A.; Endo, A.A.; Saito, M.; Hara, T.; Nakagawa, M.; Sakuraya, K.; Murano, Y.; Nishizaki, N.; Hirano, D.; Fujinaga, S.; et al. Hydrogen-rich water reduced oxidative stress and renal fibrosis in rats with unilateral ureteral obstruction. Pediatr. Res. 2022, 91, 1695–1702.
- Xin, H.G.; Zhang, B.B.; Wu, Z.Q.; Hang, X.F.; Xu, W.S.; Ni, W.; Zhang, R.Q.; Miao, X.H. Consumption of hydrogen-rich water alleviates renal injury in spontaneous hypertensive rats. Mol. Cell Biochem. 2014, 392, 117–124.
- Zhu, W.J.; Nakayama, M.; Mori, T.; Nakayama, K.; Katoh, J.; Murata, Y.; Sato, T.; Kabayama, S.; Ito, S. Intake of water with high levels of dissolved hydrogen (H2) suppresses ischemia-induced cardio-renal injury in Dahl salt-sensitive rats. Nephrol. Dial. Transplant. 2011, 26, 2112–2118.
- Kelly, K.J. Distant effects of experimental renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2003, 14, 1549–1558.
- Sugai, K.; Tamura, T.; Sano, M.; Uemura, S.; Fujisawa, M.; Katsumata, Y.; Endo, J.; Yoshizawa, J.; Homma, K.; Suzuki, M.; et al. Daily inhalation of hydrogen gas has a blood pressure-lowering effect in a rat model of hypertension. Sci. Rep. 2020, 10, 20173.
- Inoue, T. Neuroimmune system-mediated renal protection mechanisms. Clin. Exp. Nephrol. 2021, 25, 915–924.
- Xie, F.; Song, Y.; Yi, Y.; Jiang, X.; Ma, S.; Ma, C.; Li, J.; Zhanghuang, Z.; Liu, M.; Zhao, P.; et al. Therapeutic Potential of Molecular Hydrogen in Metabolic Diseases from Bench to Bedside. Pharmaceuticals 2023, 16, 541.
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143.
- Ogawa, S.; Ohsaki, Y.; Shimizu, M.; Nako, K.; Okamura, M.; Kabayama, S.; Tabata, K.; Tanaka, Y.; Ito, S. Electrolyzed hydrogen-rich water for oxidative stress suppression and improvement of insulin resistance: A multicenter prospective double-blind randomized control trial. Diabetol. Int. 2021, 13, 209–219.
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149.
- Liu, B.; Jiang, X.; Xie, Y.; Jia, X.; Zhang, J.; Xue, Y.; Qin, S. The effect of a low dose hydrogen-oxygen mixture inhalation in midlife/older adults with hypertension: A randomized, placebo-controlled trial. Front. Pharmacol. 2022, 13, 1025487.
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134.
- Cosola, C.; Rocchetti, M.T.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S.; et al. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins 2021, 13, 334.
- Huang, K.C.; Yang, C.C.; Lee, K.T.; Chien, C.T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714.
- Huang, K.C.; Yang, C.C.; Hsu, S.P.; Lee, K.T.; Liu, H.W.; Morisawa, S.; Otsubo, K.; Chien, C.T. Electrolyzed-reduced water reduced hemodialysis-induced erythrocyte impairment in end-stage renal disease patients. Kidney Int. 2006, 70, 391–398.
- Tange, Y.; Takesawa, S.; Yoshitake, S. Dialysate with high dissolved hydrogen facilitates dissociation of indoxyl sulfate from albumin. Nephrourol. Mon. 2015, 7, e26847.
- Nakayama, M.; Kabayama, S.; Ito, S. The hydrogen molecule as antioxidant therapy: Clinical application in hemodialysis and perspectives. Ren. Replace. Ther. 2016, 2, 23.
- Nakayama, M.; Kabayama, S.; Nakano, H.; Zhu, W.J.; Terawaki, H.; Nakayama, K.; Katoh, K.; Satoh, T.; Ito, S. Biological effects of electrolyzed water in hemodialysis. Nephron Clin. Pract. 2009, 112, c9–c15.
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: A clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033.
- Maeda, K.; Yoshizaki, S.; Iida, T.; Terada, T.; Era, S.; Sakashita, K.; Arikawa, H. Improvement of the fraction of human mercaptalbumin on hemodialysis treatment using hydrogen-dissolved hemodialysis fluid: A prospective observational study. Ren. Replace. Ther. 2016, 2, 42.
- Nakayama, M.; Itami, N.; Suzuki, H.; Hamada, H.; Osaka, N.; Yamamoto, R.; Tsunoda, K.; Nakano, H.; Watanabe, K.; Zhu, W.J.; et al. in a 12 month observation. PLoS ONE 2017, 12, e0184535.
- Nakayama, M.; Itami, N.; Suzuki, H.; Hamada, H.; Yamamoto, R.; Tsunoda, K.; Osaka, N.; Nakano, H.; Maruyama, Y.; Kabayama, S.; et al. Novel haemodialysis (HD) treatment employing molecular hydrogen (H2)-enriched dialysis solution improves prognosis of chronic dialysis patients: A prospective observational study. Sci. Rep. 2018, 8, 254.
- Tsujimoto, Y.; Kuratsune, D.; Kabayama, S.; Miyazaki, M.; Watanabe, Y.; Nishizawa, Y.; Nakayama, M. Amelioration of fatigue in chronic dialysis patients with dialysis solution employing electrolyzed water containing molecular hydrogen (H2) and its association with autonomic function balance. Ren. Replace. Ther. 2021, 7, 58.
- Uemura, S.; Kegasa, Y.; Tada, K.; Tsukahara, T.; Kabayama, S.; Yamamoto, T.; Miyazaki, M.; Takada, J.; Nakayama, M. Impact of hemodialysis solutions containing different levels of molecular hydrogen (H2) on the patient-reported outcome of fatigue. Ren. Replace. Ther. 2022, 8, 32.
- Mouzakis, F.L.; Khadka, L.B.; da Silva, M.P.; Mottaghy, K. Quantification of dissolved H2 and continuous monitoring of hydrogen-rich water for haemodialysis applications: An experimental study. Int. J. Artif. Organs 2022, 45, 254–261.




