
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guy Boivin | -- | 3201 | 2024-01-24 20:04:50 | | | |
| 2 | Camila Xu | + 1 word(s) | 3202 | 2024-01-25 04:09:31 | | |
Video Upload Options
Cytomegalovirus (CMV) infections may increase morbidity and mortality in immunocompromised patients. Until recently, standard antiviral drugs against CMV were limited to viral DNA polymerase inhibitors (val)ganciclovir, foscarnet and cidofovir with a risk for cross-resistance. These drugs may also cause serious side effects.
1. Introduction
2. Diagnosis of CMV Infection
3. DNA Polymerase Inhibitors
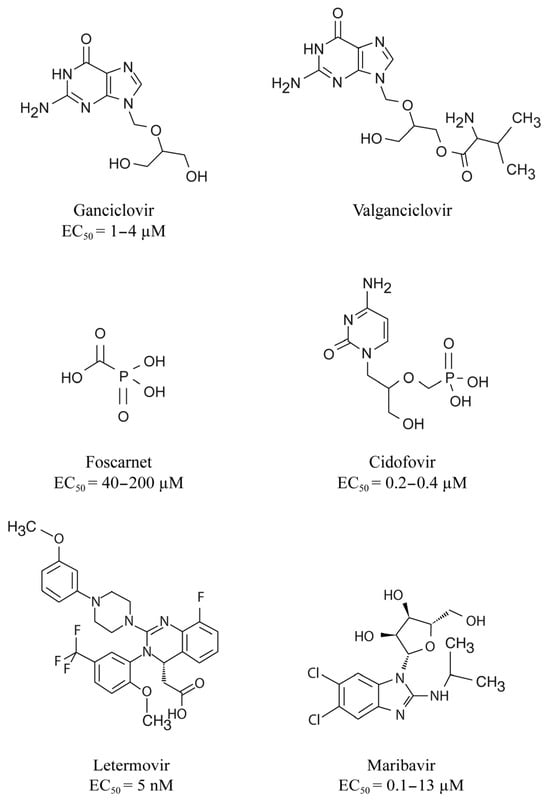
4. Indications for DNA Polymerase Inhibitors
5. Prevention and Treatment of CMV Infection
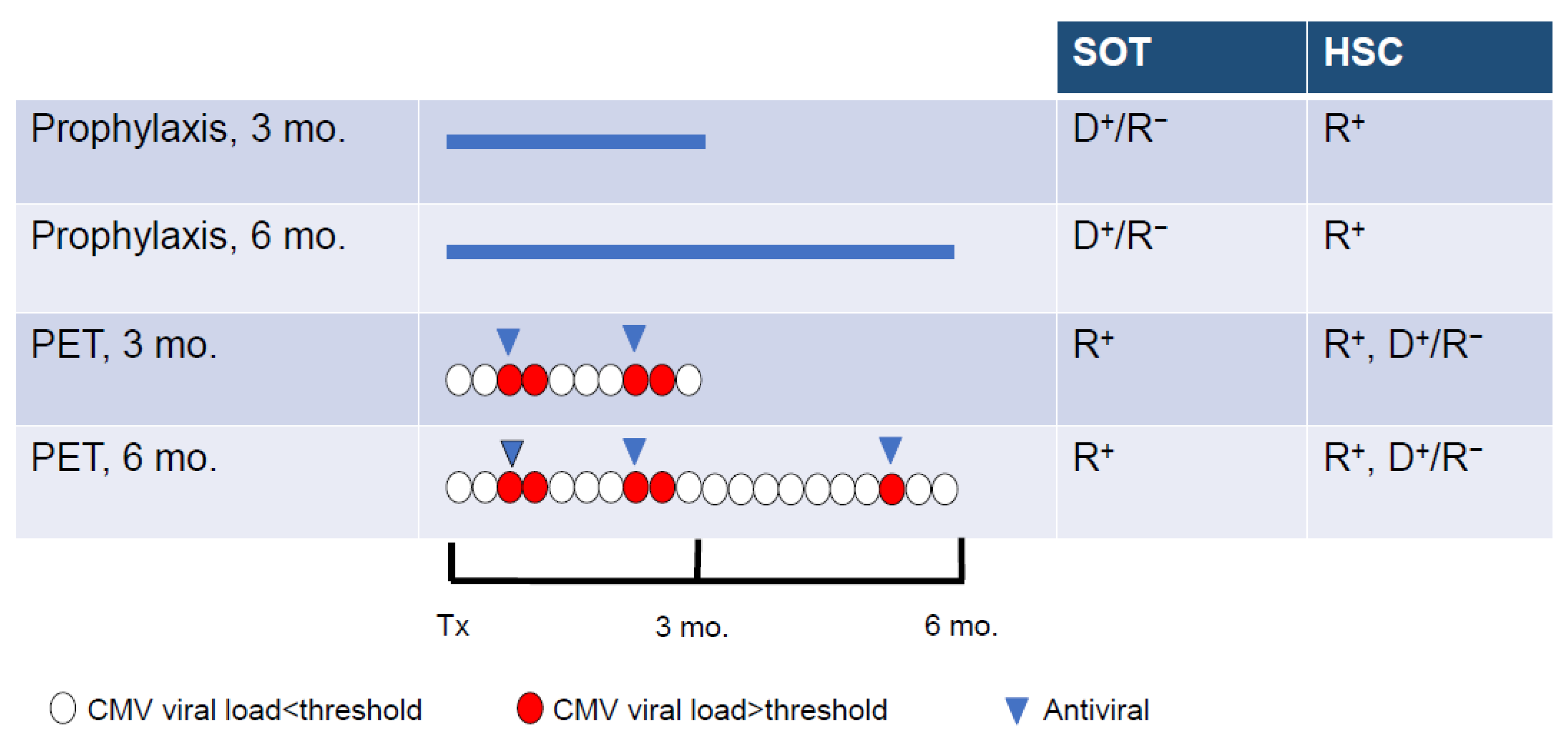
6. When to Suspect CMV Resistance to Antiviral Drugs?
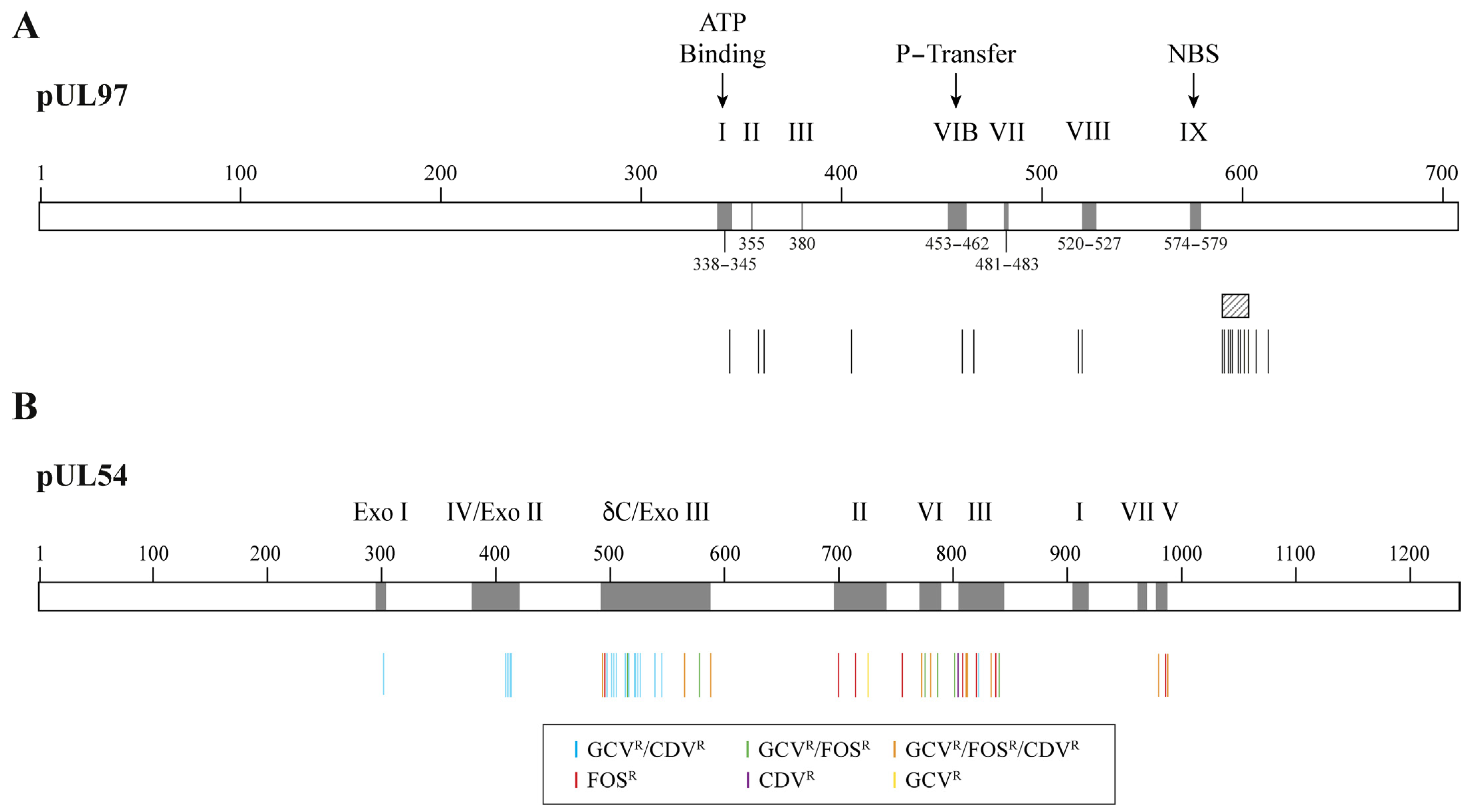
7. CMV Mutations Conferring Resistance to DNA Polymerase Inhibitors
8. Management of Refractory/Resistant CMV Disease in the DNA Polymerase Inhibitors Era
| Genotype Frequency | Relative Increase in EC50 Value Compared to Wild Type | ||
|---|---|---|---|
| <2× (Insignificant) | 2–5× (Low-Grade) | 5–15× (Moderate) | |
| Most common | C592G | M460I/V, H520Q, A594V, L595S, C603W | |
| Less common at codons 460, 590–607 | E596D, N597D, K599E/R, L600I, T601M, C603S, D605E, C607F | A591V, A594E/T/S, E596G/Q, C603S, E596G, 600del2, C607F | M460T, A594G/P, 595del, L595F/W/del, E596Y, 597del2, 599del, K599T, 600del, 601del, 601del2, C603R, C607Y, del(≥3) |
| Atypical loci | M615V, Y617H, A619V, L634Q, E655K, A674T | K359E/N/Q, E362D, L405P, I610T, A613V | F342S/Y, K355M, V356G, V466G, C480R, C518Y, P521L |
9. Letermovir and Maribavir, Two Novel Antiviral Players
There have been important advances in the prevention and treatment of CMV infections over the last 5 years with the approval of LMV and MBV. The administration of both drugs is not associated with myelotoxicity or other serious side effects as seen with DNA polymerase inhibitors. LMV and MBV target other viral proteins (the CMV terminase complex and the pUL97 kinase, respectively) than the pUL54 DNA polymerase with low risk for cross-resistance between antiviral agents, especially with LMV.
LMV is a dihydroxyquinazoline derivative (Figure 1) that demonstrates in vitro activity against CMV with an EC50 value in the nanomolar range but it is not active against other herpesviruses [62][63]. LMV is a specific inhibitor of the CMV terminase complex and shows activity against isolates resistant to DNA polymerase inhibitors [63][64][65]. LMV interferes with the cleavage of the viral DNA and its packaging into capsids [64].
LMV was approved under the trade name, Prevymis®, for the prophylaxis of CMV infection in adult R+ allogeneic HSC recipients [66]. LMV can be administered orally or intravenously (480 mg once daily for up to 12 weeks or 240 mg if given with cyclosporin). In contrast to GCV, the administration of LMV is not associated with myelotoxicity, which allows its use in prophylaxis strategy for the prevention of CMV infection in HSC recipients. LMV could be also an option for CMV prophylaxis in SOT. The efficacy and safety of LMV for CMV prophylaxis in SOT recipients is thus further evaluated in clinical trials.
As LMV targets the viral terminase complex, there is no risk of cross-resistance with other antiviral drugs [67]. LMV has a potentially low genetic barrier to the emergence of resistance, with single mutations that can be associated with very high levels of resistance [68]. The emergence of resistance to LMV should be thus monitored early in patients with a virologic failure. The rate of LMV resistance after prophylaxis is low and comparable to those of DNA polymerase inhibitors. However, it is anticipated that the rate of LMV resistance could be higher when used in treatment. Therefore, LMV is not currently investigated as a treatment option.
Maribavir is a benzimidazole-L-riboside derivative (Figure 1) that demonstrated in vitro activity against CMV including strains resistant to GCV [69], Epstein–Barr virus [70] and human herpesvirus 6 but not against herpes simplex virus and varicella zoster virus. MBV is a selective inhibitor of the pUL97 kinase [71][72]. It prevents the phosphorylation of viral and host proteins and the nuclear egress of virions [73].
MBV was approved under the trade name, Livtencity®, for the treatment of adult and pediatric patients with post-transplant CMV infection/disease refractory/resistant to treatment with DNA polymerase inhibitors [74]. In contrast to FOS and CDV, MBV is available as an oral formulation, which may thus facilitate the treatment of patients with refractory/resistant CMV diseases. The dosage of oral MBV is 400 mg twice daily. MBV is safe and well tolerated. It could be administered to patients with an underlying kidney dysfunction and/or myelosuppression. Due to its lack of myelotoxicity, MBV may have some advantages over VGCV for use as CMV prophylaxis.
The use of MBV is limited by the possible cross-resistance phenotype with GCV. MBV seems to possess an intermediate genetic barrier to resistance compared to LMV (lower) and DNA polymerase inhibitors (higher) but further investigations are still needed. The efficacy and safety of MBV as a primary treatment option for CMV diseases in SOT and HSC recipients need to be further evaluated in clinical trials, especially the risk for cross-resistance with GCV and the genetic barrier to resistance.
References
- Roizman, B.; Knipe, D.M.; Whitley, R.J. Herpes simplex viruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013; Volume 2, pp. 1823–1897.
- Boivin, G.; Limaye, A.P. Cytomegalovirus. In Goldman-Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2023.
- Aldè, M.; Binda, S.; Primache, V.; Pellegrinelli, L.; Pariani, E.; Pregliasco, F.; Di Berardino, F.; Cantarella, G.; Ambrosetti, U. Congenital Cytomegalovirus and Hearing Loss: The State of the Art. J. Clin. Med. 2023, 12, 4465.
- Arthurs, S.K.; Eid, A.J.; Pedersen, R.A.; Kremers, W.K.; Cosio, F.G.; Patel, R.; Razonable, R.R. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin. Infect. Dis. 2008, 46, 840–846.
- Limaye, A.P.; Babu, T.M.; Boeckh, M. Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation. Clin. Microbiol. Rev. 2020, 34, 10–1128.
- Piret, J.; Boivin, G. DNA polymerases of herpesviruses and their inhibitors. Enzymes 2021, 50, 79–132.
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80.
- Ljungman, P.; Deliliers, G.L.; Platzbecker, U.; Matthes-Martin, S.; Bacigalupo, A.; Einsele, H.; Ullmann, J.; Musso, M.; Trenschel, R.; Ribaud, P.; et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 2001, 97, 388–392.
- Bonatti, H.; Sifri, C.D.; Larcher, C.; Schneeberger, S.; Kotton, C.; Geltner, C. Use of Cidofovir for Cytomegalovirus Disease Refractory to Ganciclovir in Solid Organ Recipients. Surg. Infect. 2017, 18, 128–136.
- Mehta Steinke, S.A.; Alfares, M.; Valsamakis, A.; Shoham, S.; Arav-Boger, R.; Lees, L.; Ostrander, D.; Forman, M.S.; Shedeck, A.; Ambinder, R.F.; et al. Outcomes of transplant recipients treated with cidofovir for resistant or refractory cytomegalovirus infection. Transpl. Infect. Dis. 2021, 23, e13521.
- Gourin, C.; Alain, S.; Hantz, S. Anti-CMV therapy, what next? A systematic review. Front. Microbiol. 2023, 14, 1321116.
- Ligat, G.; Cazal, R.; Hantz, S.; Alain, S. The human cytomegalovirus terminase complex as an antiviral target: A close-up view. FEMS Microbiol. Rev. 2018, 42, 137–145.
- Gentry, B.G.; Bogner, E.; Drach, J.C. Targeting the terminase: An important step forward in the treatment and prophylaxis of human cytomegalovirus infections. Antivir. Res. 2019, 161, 116–124.
- Prichard, M.N. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 2009, 19, 215–229.
- Steingruber, M.; Marschall, M. The Cytomegalovirus Protein Kinase pUL97:Host Interactions, Regulatory Mechanisms and Antiviral Drug Targeting. Microorganisms 2020, 8, 515.
- Fryer, J.F.; Heath, A.B.; Minor, P.D.; Collaborative Study, G. A collaborative study to establish the 1st WHO International Standard for human cytomegalovirus for nucleic acid amplification technology. Biologicals 2016, 44, 242–251.
- Cotte, L.; Drouet, E.; Bailly, F.; Vitozzi, S.; Denoyel, G.; Trepo, C. Cytomegalovirus DNA level on biopsy specimens during treatment of cytomegalovirus gastrointestinal disease. Gastroenterology 1996, 111, 439–444.
- Jeong, T.D.; Sung, H.; Choi, S.H.; Lee, S.O.; Yoon, H.K.; Kim, M.N.; Im, H.J. Cytomegalovirus ventriculoencephalitis with compartmentalization of antiviral-resistant cytomegalovirus in a T cell-depleted haploidentical peripheral blood stem cell transplant recipient. Diagn. Microbiol. Infect. Dis. 2012, 74, 307–310.
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018, 102, 900–931.
- Prakash, K.; Chandorkar, A.; Saharia, K.K. Utility of CMV-Specific Immune Monitoring for the Management of CMV in Solid Organ Transplant Recipients: A Clinical Update. Diagnostics 2021, 11, 875.
- Schachtner, T.; Stein, M.; Reinke, P. CMV-Specific T Cell Monitoring Offers Superior Risk Stratification of CMV-Seronegative Kidney Transplant Recipients of a CMV-Seropositive Donor. Transplantation 2017, 101, e315–e325.
- Gliga, S.; Korth, J.; Krawczyk, A.; Wilde, B.; Horn, P.A.; Witzke, O.; Lindemann, M.; Fiedler, M. T-Track-CMV and QuantiFERON-CMV assays for prediction of protection from CMV reactivation in kidney transplant recipients. J. Clin. Virol. 2018, 105, 91–96.
- Sood, S.; Haifer, C.; Yu, L.; Pavlovic, J.; Gow, P.J.; Jones, R.M.; Visvanathan, K.; Angus, P.W.; Testro, A.G. Early viral-specific T-cell testing predicts late cytomegalovirus reactivation following liver transplantation. Transpl. Infect. Dis. 2018, 20, e12934.
- Paez-Vega, A.; Poyato, A.; Rodriguez-Benot, A.; Guirado, L.; Fortun, J.; Len, O.; Abdala, E.; Farinas, M.C.; Cordero, E.; de Gracia, C.; et al. Analysis of spontaneous resolution of cytomegalovirus replication after transplantation in CMV-seropositive patients with pretransplant CD8+IFNG+ response. Antivir. Res. 2018, 155, 97–105.
- Kumar, D.; Chin-Hong, P.; Kayler, L.; Wojciechowski, D.; Limaye, A.P.; Osama Gaber, A.; Ball, S.; Mehta, A.K.; Cooper, M.; Blanchard, T.; et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 2505–2516.
- Nesher, L.; Shah, D.P.; Ariza-Heredia, E.J.; Azzi, J.M.; Siddiqui, H.K.; Ghantoji, S.S.; Marsh, L.Y.; Michailidis, L.; Makedonas, G.; Rezvani, K.; et al. Utility of the Enzyme-Linked Immunospot Interferon-gamma-Release Assay to Predict the Risk of Cytomegalovirus Infection in Hematopoietic Cell Transplant Recipients. J. Infect. Dis. 2016, 213, 1701–1707.
- El Haddad, L.; Ariza-Heredia, E.; Shah, D.P.; Jiang, Y.; Blanchard, T.; Ghantoji, S.S.; El Chaer, F.; El-Haddad, D.; Prayag, A.; Nesher, L.; et al. The Ability of a Cytomegalovirus ELISPOT Assay to Predict Outcome of Low-Level CMV Reactivation in Hematopoietic Cell Transplant Recipients. J. Infect. Dis. 2019, 219, 898–907.
- Navarro, D.; Amat, P.; de la Camara, R.; Lopez, J.; Vazquez, L.; Serrano, D.; Nieto, J.; Rovira, M.; Pinana, J.L.; Gimenez, E.; et al. Efficacy and Safety of a Preemptive Antiviral Therapy Strategy Based on Combined Virological and Immunological Monitoring for Active Cytomegalovirus Infection in Allogeneic Stem Cell Transplant Recipients. Open Forum Infect. Dis. 2016, 3, ofw107.
- Piret, J.; Boivin, G. Antiviral Drugs Against Herpesviruses. Adv. Exp. Med. Biol. 2021, 1322, 1–30.
- Sullivan, V.; Talarico, C.L.; Stanat, S.C.; Davis, M.; Coen, D.M.; Biron, K.K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 1992, 358, 162–164.
- Derse, D.; Cheng, Y.C.; Furman, P.A.; St Clair, M.H.; Elion, G.B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J. Biol. Chem. 1981, 256, 11447–11451.
- Biron, K.K.; Stanat, S.C.; Sorrell, J.B.; Fyfe, J.A.; Keller, P.M.; Lambe, C.U.; Nelson, D.J. Metabolic activation of the nucleoside analog 9-methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc. Natl. Acad. Sci. USA 1985, 82, 2473–2477.
- Cihlar, T.; Chen, M.S. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 1996, 50, 1502–1510.
- Xiong, X.; Smith, J.L.; Chen, M.S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 1997, 41, 594–599.
- Chrisp, P.; Clissold, S.P. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 1991, 41, 104–129.
- Limaye, A.P.; Bakthavatsalam, R.; Kim, H.W.; Randolph, S.E.; Halldorson, J.B.; Healey, P.J.; Kuhr, C.S.; Levy, A.E.; Perkins, J.D.; Reyes, J.D.; et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006, 81, 1645–1652.
- van der Beek, M.T.; Berger, S.P.; Vossen, A.C.; van der Blij-de Brouwer, C.S.; Press, R.R.; de Fijter, J.W.; Claas, E.C.; Kroes, A.C. Preemptive versus sequential prophylactic-preemptive treatment regimens for cytomegalovirus in renal transplantation: Comparison of treatment failure and antiviral resistance. Transplantation 2010, 89, 320–326.
- Boillat Blanco, N.; Pascual, M.; Venetz, J.P.; Nseir, G.; Meylan, P.R.; Manuel, O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation 2011, 91, 251–255.
- Asberg, A.; Humar, A.; Jardine, A.G.; Rollag, H.; Pescovitz, M.D.; Mouas, H.; Bignamini, A.; Toz, H.; Dittmer, I.; Montejo, M.; et al. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am. J. Transplant. 2009, 9, 1205–1213.
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712.
- Limaye, A.P.; Corey, L.; Koelle, D.M.; Davis, C.L.; Boeckh, M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 2000, 356, 645–649.
- Limaye, A.P. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin. Infect. Dis. 2002, 35, 866–872.
- Fisher, C.E.; Knudsen, J.L.; Lease, E.D.; Jerome, K.R.; Rakita, R.M.; Boeckh, M.; Limaye, A.P. Risk Factors and Outcomes of Ganciclovir-Resistant Cytomegalovirus Infection in Solid Organ Transplant Recipients. Clin. Infect. Dis. 2017, 65, 57–63.
- Campos, A.B.; Ribeiro, J.; Boutolleau, D.; Sousa, H. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: Current state of the art. Rev. Med. Virol. 2016, 26, 161–182.
- Limaye, A.P.; Raghu, G.; Koelle, D.M.; Ferrenberg, J.; Huang, M.L.; Boeckh, M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J. Infect. Dis. 2002, 185, 20–27.
- Lurain, N.S.; Bhorade, S.M.; Pursell, K.J.; Avery, R.K.; Yeldandi, V.V.; Isada, C.M.; Robert, E.S.; Kohn, D.J.; Arens, M.Q.; Garrity, E.R.; et al. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J. Infect. Dis. 2002, 186, 760–768.
- Ambrose, T.; Sharkey, L.M.; Louis-Auguste, J.; Rutter, C.S.; Duncan, S.; English, S.; Gkrania-Klotsas, E.; Carmichael, A.; Woodward, J.M.; Russell, N.; et al. Cytomegalovirus Infection and Rates of Antiviral Resistance Following Intestinal and Multivisceral Transplantation. Transplant. Proc. 2016, 48, 492–496.
- Timpone, J.G.; Yimen, M.; Cox, S.; Teran, R.; Ajluni, S.; Goldstein, D.; Fishbein, T.; Kumar, P.N.; Matsumoto, C. Resistant cytomegalovirus in intestinal and multivisceral transplant recipients. Transpl. Infect. Dis. 2016, 18, 202–209.
- Allice, T.; Busca, A.; Locatelli, F.; Falda, M.; Pittaluga, F.; Ghisetti, V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post-allogenic stem cell transplantation: Implications for the emergence of drug-resistant cytomegalovirus. J. Antimicrob. Chemother. 2009, 63, 600–608.
- van der Beek, M.T.; Marijt, E.W.; Vossen, A.C.; van der Blij-de Brouwer, C.S.; Wolterbeek, R.; Halkes, C.J.; Claas, E.C.; Kroes, A.C. Failure of pre-emptive treatment of cytomegalovirus infections and antiviral resistance in stem cell transplant recipients. Antivir. Ther. 2012, 17, 45–51.
- Shmueli, E.; Or, R.; Shapira, M.Y.; Resnick, I.B.; Caplan, O.; Bdolah-Abram, T.; Wolf, D.G. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J. Infect. Dis. 2014, 209, 557–561.
- Jabs, D.A.; Enger, C.; Forman, M.; Dunn, J.P. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. The Cytomegalovirus Retinitis and Viral Resistance Study Group. Antimicrob. Agents Chemother. 1998, 42, 2240–2244.
- Weinberg, A.; Jabs, D.A.; Chou, S.; Martin, B.K.; Lurain, N.S.; Forman, M.S.; Crumpacker, C.; Cytomegalovirus Retinitis Viral Resistance Study Group; Adult AIDS Clinical Trials Group Cytomegalovirus Laboratories. Mutations conferring foscarnet resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J. Infect. Dis. 2003, 187, 777–784.
- Erice, A. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 1999, 12, 286–297.
- Piret, J.; Boivin, G. Herpesvirus resistance to antiviral drugs. In Antimicrobial Drug Resistance, 2nd ed.; Mayers, D.L., Sobel, J., Ouellette, M., Kaye, K., Marchaim, D., Eds.; Springer: New York, NY, USA, 2017; pp. 1185–1211.
- Smith, I.L.; Cherrington, J.M.; Jiles, R.E.; Fuller, M.D.; Freeman, W.R.; Spector, S.A. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 1997, 176, 69–77.
- Chou, S.; Marousek, G.I.; Van Wechel, L.C.; Li, S.; Weinberg, A. Growth and drug resistance phenotypes resulting from cytomegalovirus DNA polymerase region III mutations observed in clinical specimens. Antimicrob. Agents Chemother. 2007, 51, 4160–4162.
- Drouot, E.; Piret, J.; Boivin, G. Novel method based on “en passant” mutagenesis coupled with a Gaussia luciferase reporter assay for studying the combined effects of human cytomegalovirus mutations. J. Clin. Microbiol. 2013, 51, 3216–3224.
- Drouot, E.; Piret, J.; Lebel, M.H.; Boivin, G. Characterization of multiple cytomegalovirus drug resistance mutations detected in a hematopoietic stem cell transplant recipient by recombinant phenotyping. J. Clin. Microbiol. 2014, 52, 4043–4046.
- Mylonakis, E.; Kallas, W.M.; Fishman, J.A. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin. Infect. Dis. 2002, 34, 1337–1341.
- SOCA. Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS. The Cytomegalovirus Retreatment Trial. The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. Arch. Ophthalmol. 1996, 114, 23–33.
- Lischka, P.; Hewlett, G.; Wunberg, T.; Baumeister, J.; Paulsen, D.; Goldner, T.; Ruebsamen-Schaeff, H.; Zimmermann, H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob. Agents Chemother. 2010, 54, 1290–1297.
- Marschall, M.; Stamminger, T.; Urban, A.; Wildum, S.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrob. Agents Chemother. 2012, 56, 1135–1137.
- Goldner, T.; Hewlett, G.; Ettischer, N.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J. Virol. 2011, 85, 10884–10893.
- Piret, J.; Goyette, N.; Boivin, G. In vitro activity of letermovir against human cytomegalovirus isolates with different drug susceptibility phenotypes. Antivir. Res. 2022, 202, 105328.
- Kim, E.S. Letermovir: First Global Approval. Drugs 2018, 78, 147–152.
- Piret, J.; Boivin, G. Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance. Antivir. Res. 2019, 163, 91–105.
- Chou, S.; Kleiboeker, S. Relative frequency of cytomegalovirus UL56 gene mutations detected in genotypic letermovir resistance testing. Antivir. Res. 2022, 207, 105422.
- Drew, W.L.; Miner, R.C.; Marousek, G.I.; Chou, S. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J. Clin. Virol. 2006, 37, 124–127.
- Whitehurst, C.B.; Sanders, M.K.; Law, M.; Wang, F.Z.; Xiong, J.; Dittmer, D.P.; Pagano, J.S. Maribavir inhibits Epstein-Barr virus transcription through the EBV protein kinase. J. Virol. 2013, 87, 5311–5315.
- Biron, K.K.; Harvey, R.J.; Chamberlain, S.C.; Good, S.S.; Smith, A.A., 3rd; Davis, M.G.; Talarico, C.L.; Miller, W.H.; Ferris, R.; Dornsife, R.E.; et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 2002, 46, 2365–2372.
- Williams, S.L.; Hartline, C.B.; Kushner, N.L.; Harden, E.A.; Bidanset, D.J.; Drach, J.C.; Townsend, L.B.; Underwood, M.R.; Biron, K.K.; Kern, E.R. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 2003, 47, 2186–2192.
- Krosky, P.M.; Baek, M.C.; Coen, D.M. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 2003, 77, 905–914.
- Kang, C. Maribavir: First Approval. Drugs 2022, 82, 335–340.




