
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhonghua Sun | -- | 4574 | 2024-01-24 09:47:17 |
Video Upload Options
The recent emergence of photon-counting computed tomography (CT) has further enhanced CT performance in clinical applications, providing improved spatial and contrast resolution. CT-derived fractional flow reserve is superior to standard CT-based anatomical assessment for the detection of lesion-specific myocardial ischemia. CT-derived 3D-printed patient-specific models are also superior to standard CT, offering advantages in terms of educational value, surgical planning, and the simulation of cardiovascular disease treatment, as well as enhancing doctor–patient communication. Three-dimensional visualization tools including virtual reality, augmented reality, and mixed reality are further advancing the clinical value of cardiovascular CT in cardiovascular disease.
1. Introduction
2. Cardiovascular CT: Diagnostic Value Based on Standard Imaging Approach
3. Photon-Counting CT: The Latest Technological Advancements in Cardiovascular CT
4. Cardiovascular CT: Beyond Lumen Assessment
4.1. Patient-Specific 3D-Printed Models: Medical Education
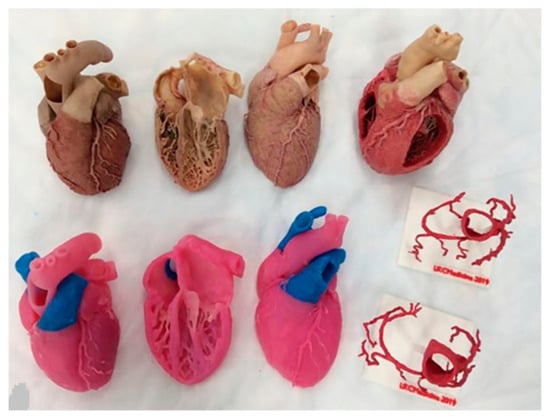
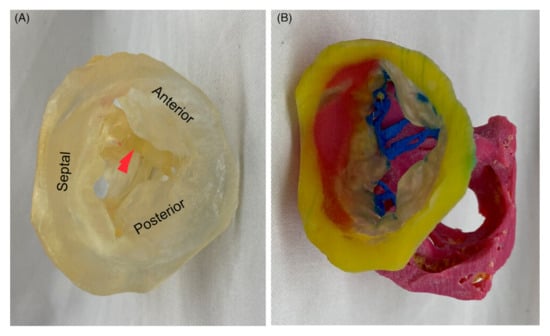
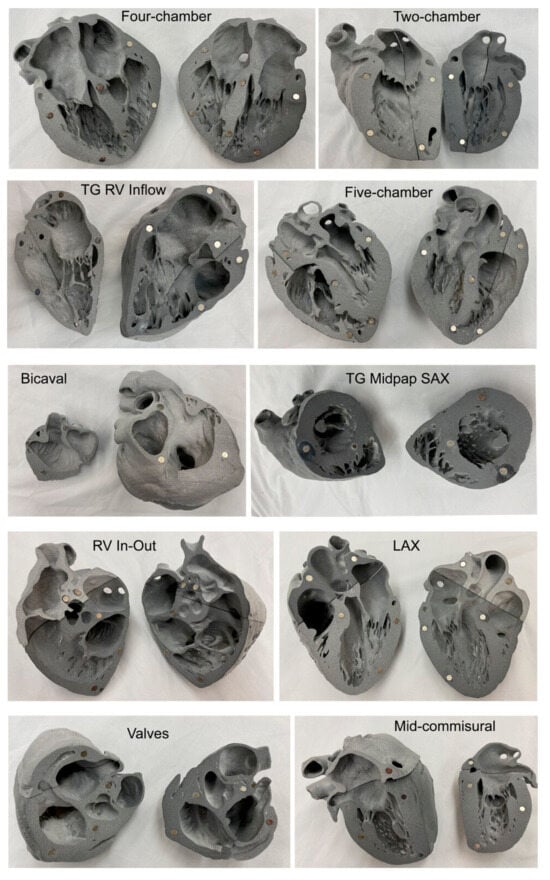
4.2. Patient-Specific 3D-Printed Models: Preoperative Planning and Simulation
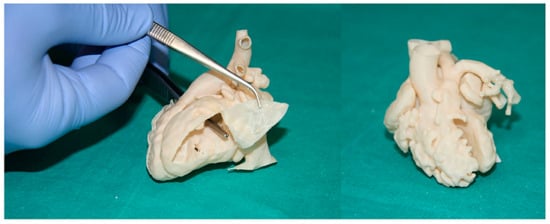
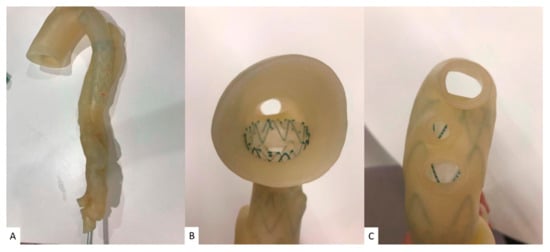
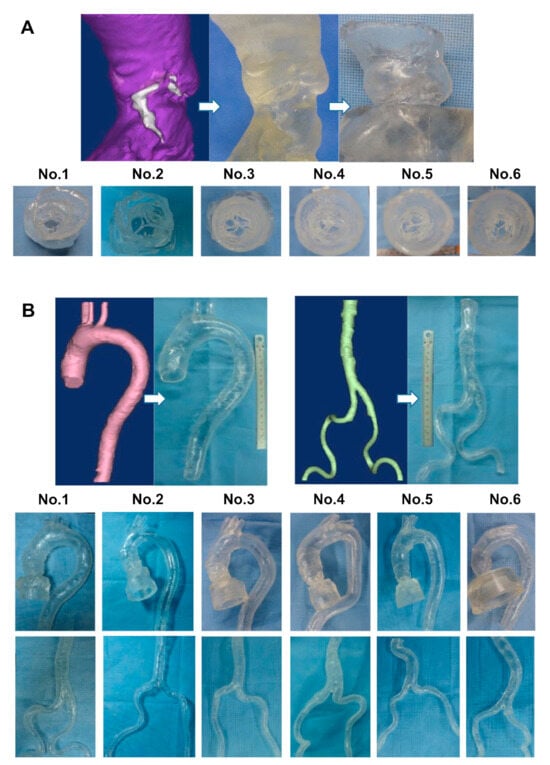
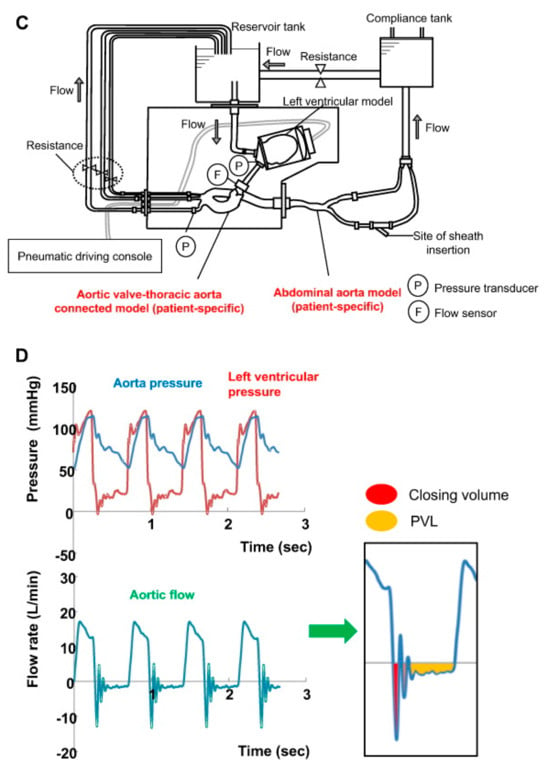
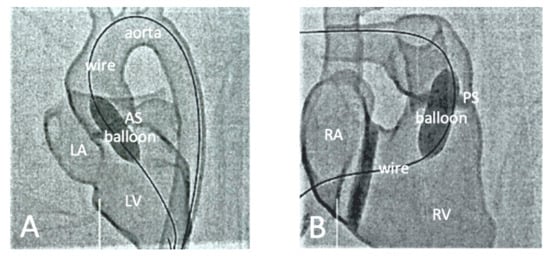
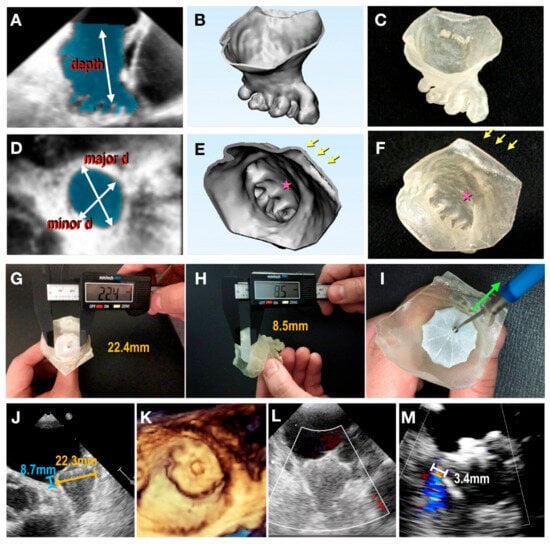
4.3. Patient-Specific 3D-Printed Models: Clinical Communication
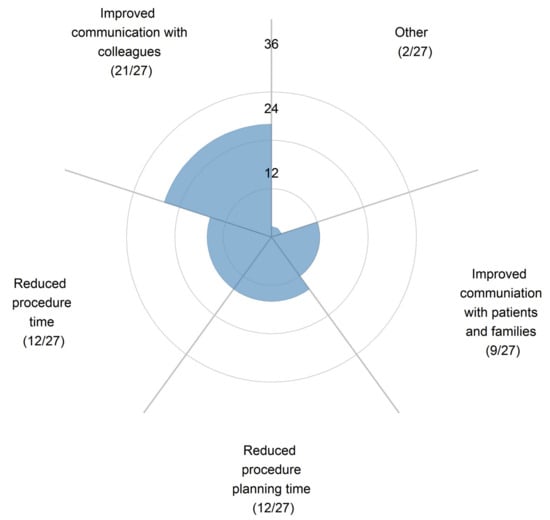
4.4. Patient-Specific 3D-Printed Models: Optimizing CT Protocols
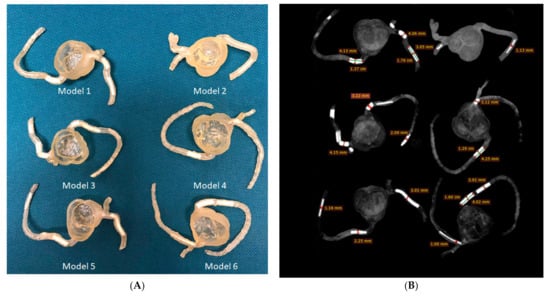
4.5. The Use of 3D-Printed Devices in Treating Cardiovascular Disease
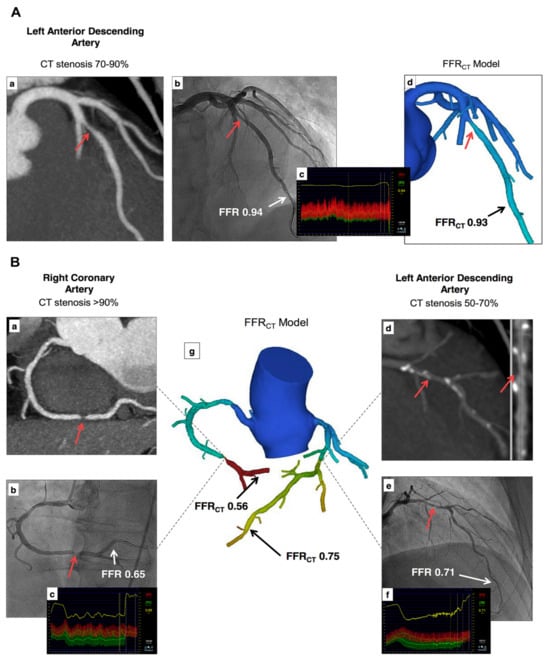
4.6. Cardiac CT: CT-Derived FFR
4.7. Cardiovascular CT: VR, AR, and MR
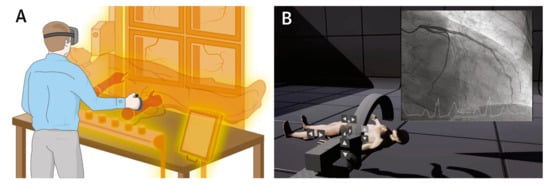
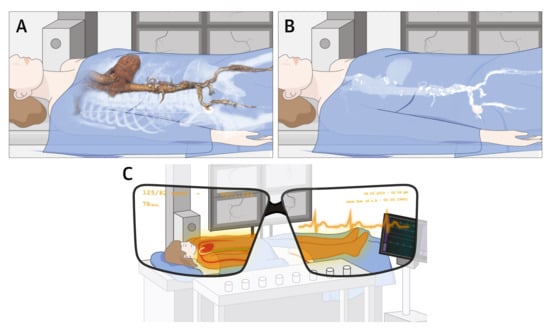
5. Cardiovascular CT: AI/ML/DL
5.1. AI/ML/DL in Coronary Calcium Scoring
5.2. AI/ML/DL in Coronary Artery Disease
5.3. AI/ML/DL in Abdominal Aortic Aneurysm and Aortic Dissection
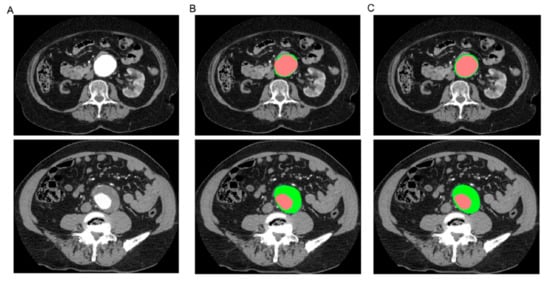
5.4. AI/ML/DL in Pulmonary Artery Disease
6. Summary
References
- Gulsin, G.S.; McVeigh, N.; Leipsic, J.A.; Dodd, J.D. Cardiovascular CT and MRI in 2020: Review of key articles. Radiology 2021, 301, 263–277.
- Sayed, A.; Munir, M.; Bahbah, E.I. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr. Cardiol. Rev. 2021, 17, e230421186875.
- Abbas, A.; Brown, I.; Peebles, C.; Harden, S.; Shambrook, J. The role of multidetector-row CT in the diagnosis, classification and management of acute aortic syndrome. Br. J. Radiol. 2014, 87, 20140354.
- Seitun, S.; Clemente, A.; Maffei, E.; Toia, P.; La Grutta, L.; Cademartiri, F. Prognostic value of cardiac CT. Radiol. Medica 2020, 125, 1135–1147.
- Sun, Z. Cardiac CT imaging in coronary artery disease: Current status and future directions. Quant. Imaging Med. Surg. 2012, 2, 98–105.
- Al’Aref, S.J.; Min, J.K. Cardiac CT: Current practice and emerging applications. Heart 2019, 105, 1597–1605.
- Mayo, J.; Thakur, Y. Pulmonary CT Angiography as First-Line Imaging for PE: Image Quality and Radiation Dose Considerations. AJR Am. J. Roentgenol. 2013, 200, 522–528.
- Mayo, J.; Thakur, Y. Acute Pulmonary Embolism: From Morphology to Function. Semin. Respir. Crit. Care Med. 2014, 35, 041–049.
- Corballis, N.; Tsampasian, V.; Merinopoulis, I.; Gunawardena, T.; Bhalraam, U.; Eccleshall, S.; Dweck, M.R.; Vassiliou, V. CT angiography compared to invasive angiography for stable coronary disease as predictors of major adverse cardiovascular events—A systematic review and meta-analysis. Heart Lung 2023, 57, 207–213.
- Counseller, Q.; Aboelkassem, Y. Recent technologies in cardiac imaging. Front. Med. Technol. 2023, 4, 984492.
- Newby, D.; Williams, M.; Hunter, A.; Pawade, T.; Shah, A.; Flapan, A.; Forbes, J.; Hargreaves, A.; Stephen, L.; Lewis, S. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): An open-label, parallel-group, multicentre trial. Lancet 2015, 385, 2383–2391.
- Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; Šakalyte, G. CT or invasive coronary angiography in stable chest pain. N. Engl. J. Med. 2022, 386, 1591–1602.
- Sun, Z. Diagnostic Accuracy of Multislice CT Angiography in Peripheral Arterial Disease. J. Vasc. Interv. Radiol. 2006, 17, 1915–1921.
- Walls, M.C.; Thavendiranathan, P.; Rajagopalan, S. Advances in CT angiography for peripheral arterial disease. Cardiol. Clin. 2011, 29, 331–340.
- Kim, J.W.; Choo, K.S.; Jeon, U.B.; Kim, T.U.; Hwang, J.Y.; Yeom, J.A.; Jeong, H.S.; Choi, Y.Y.; Nam, K.J.; Kim, C.W.; et al. Diagnostic performance and radiation dose of lower extremity CT angiography using a 128-slice dual source CT at 80 kVp and high pitch. Acta. Radiol. 2016, 57, 822–828.
- Kalisz, K.; Halliburton, S.; Abbara, S.; Leipsic, J.A.; Albrecht, M.H.; Schoepf, U.J.; Rajiah, P. Update on cardiovascular applications of multienergy CT. Radiographics 2017, 37, 1955–1974.
- Machida, H.; Tanaka, I.; Fukui, R.; Shen, Y.; Ishikawa, T.; Tate, E.; Ueno, E. Dual-energy spectral CT: Various clinical vascular applications. Radiographics 2016, 36, 1215–1232.
- Litmanovich, D.E.; Tack, D.M.; Shahrzad, M.; Bankier, A.A. Dose reduction in cardiothoracic CT: Review of currently available methods. Radiographics 2014, 34, 1469–1489.
- Usai, M.V.; Gerwing, M.; Gottschalk, A.; Sporns, P.; Heindel, W.; Oberhuber, A.; Wildgruber, M.; Köhler, M. Intra-arterial catheter-directed CT angiography for assessment of endovascular aortic aneurysm repair. PLoS ONE 2019, 14, e0221375.
- Schuijf, J.D.; Lima, J.A.C.; Boedeker, K.L.; Takagi, H.; Tanaka, R.; Yoshioka, K.; Arbab-Zadeh, A. CT imaging with ultra-high-resolution: Opportunities for cardiovascular imaging in clinical practice. J. Cardiovasc. Comput. Tomogr. 2022, 16, 388–396.
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112.
- Cademartiri, F.; Meloni, A.; Pistoia, L.; Degiorgi, G.; Clemente, A.; Gori, C.D.; Positano, V.; Celi, S.; Berti, S.; Emdin, M.; et al. Dual-Source Photon-Counting Computed Tomography— Part I: Clinical Overview of Cardiac CT and Coronary CT Angiography Applications. J. Clin. Med. 2023, 12, 3627.
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T. Coronary CT angiography with photon-counting CT: First-in-human results. Radiology 2022, 303, 303–313.
- Flohr, T.; Schmidt, B.; Ulzheimer, S.; Alkadhi, H. Cardiac imaging with photon counting CT. Br. J. Radiol. 2023, 96, 20230407.
- Allmendinger, T.; Nowak, T.; Flohr, T.; Klotz, E.; Hagenauer, J.; Alkadhi, H.; Schmidt, B. Photon-Counting Detector CT-Based Vascular Calcium Removal Algorithm: Assessment Using a Cardiac Motion Phantom. Investig. Radiol. 2022, 57, 399–405.
- Boccalini, S.; Si-Mohamed, S.A.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Villien, M.; Sigovan, M.; Dessouky, R.; Coulon, P.; et al. First in-Human Results of Computed Tomography Angiography for Coronary Stent Assessment with a Spectral Photon Counting Computed Tomography. Investig. Radiol. 2022, 57, 212–221.
- Koons, E.; VanMeter, P.; Rajendran, K.; Yu, L.; McCollough, C.; Leng, S. Improved quantification of coronary artery luminal stenosis in the presence of heavy calcifications using photon-counting detector CT. Proc. SPIE. Int. Soc. Opt. Eng. 2022, 12031, 120311A.
- Meloni, A.; Frijia, F.; Panetta, D.; Degiorgi, G.; De Gori, C.; Maffei, E.; Clemente, A.; Positano, V.; Cademartiri, F. Photon-counting computed tomography (pcct): Technical background and cardio-vascular applications. Diagnostics 2023, 13, 645.
- Bech, G.J.W.; De Bruyne, B.; Pijls, N.H.; De Muinck, E.D.; Hoorntje, J.C.; Escaned, J.; Stella, P.R.; Boersma, E.; Bartunek, J.; Koolen, J.J. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation 2001, 103, 2928–2934.
- Tavoosi, A.; Kadoya, Y.; Chong, A.Y.; Small, G.R.; Chow, B.J.W. Utility of FFRCT in Patients with Chest Pain. Curr. Atheroscler. Rep. 2023, 25, 427–434.
- Chen, J.; Wetzel, L.H.; Pope, K.L.; Meek, L.J.; Rosamond, T.; Walker, C.M. FFRCT: Current Status. AJR Am. J. Roentgenol. 2020, 216, 640–648.
- Ihdayhid, A.R.; Norgaard, B.L.; Gaur, S.; Leipsic, J.; Nerlekar, N.; Osawa, K.; Miyoshi, T.; Jensen, J.M.; Kimura, T.; Shiomi, H. Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology 2019, 292, 343–351.
- Kawaji, T.; Shiomi, H.; Morishita, H.; Morimoto, T.; Taylor, C.A.; Kanao, S.; Koizumi, K.; Kozawa, S.; Morihiro, K.; Watanabe, H.; et al. Feasibility and diagnostic performance of fractional flow reserve measurement derived from coronary computed tomography angiography in real clinical practice. Int. J. Cardiovasc. Imaging 2017, 33, 271–281.
- Nørgaard, B.L.; Jensen, J.M.; Blanke, P.; Sand, N.P.; Rabbat, M.; Leipsic, J. Coronary CT Angiography Derived Fractional Flow Reserve: The Game Changer in Noninvasive Testing. Curr. Cardiol. Rep. 2017, 19, 112.
- Xu, L.; Sun, Z.; Fan, Z. Noninvasive Physiologic Assessment of Coronary Stenoses Using Cardiac CT. BioMed Res. Int. 2015, 2015, 435737.
- Gao, X.; Wang, R.; Sun, Z.; Zhang, H.; Bo, K.; Xue, X.; Yang, J.; Xu, L. A Novel CT Perfusion-Based Fractional Flow Reserve Algorithm for Detecting Coronary Artery Disease. J. Clin. Med. 2023, 12, 2154.
- Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Nørgaard, B.L.; Berman, D.S.; Raff, G.; Hurwitz-Koweek, L.M.; Pontone, G.; Kawasaki, T.; Sand, N.P.; et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: Lessons from the ADVANCE Registry. Eur. Heart J. 2018, 39, 3701–3711.
- Xue, X.; Liu, X.; Gao, Z.; Wang, R.; Xu, L.; Ghista, D.; Zhang, H. Personalized coronary blood flow model based on CT perfusion to non-invasively calculate fractional flow reserve. Comput. Methods Appl. Mech. Eng. 2023, 404, 115789.
- Donnelly, P.M.; Kolossváry, M.; Karády, J.; Ball, P.A.; Kelly, S.; Fitzsimons, D.; Spence, M.S.; Celeng, C.; Horváth, T.; Szilveszter, B.; et al. Experience with an on-Site Coronary Computed Tomography-Derived Fractional Flow Reserve Algorithm for the Assessment of Intermediate Coronary Stenoses. Am. J. Cardiol. 2018, 121, 9–13.
- Yang, D.H.; Kim, Y.-H.; Roh, J.H.; Kang, J.-W.; Ahn, J.-M.; Kweon, J.; Lee, J.B.; Choi, S.H.; Shin, E.-S.; Park, D.-W.; et al. Diagnostic performance of on-site CT-derived fractional flow reserve versus CT perfusion. Eur. Heart J.—Cardiovasc. Imaging 2016, 18, 432–440.
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; David, V.D.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed from Coronary Computed Tomographic Angiograms. J. Am. Coll. Cardiol. 2011, 58, 1989–1997.
- Yoon, Y.E.; Choi, J.-H.; Kim, J.-H.; Park, K.-W.; Doh, J.-H.; Kim, Y.-J.; Koo, B.-K.; Min, J.K.; Erglis, A.; Gwon, H.-C.; et al. Noninvasive Diagnosis of Ischemia-Causing Coronary Stenosis Using CT Angiography. JACC Cardiovasc. Imaging 2012, 5, 1088–1096.
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic Performance of Noninvasive Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography in Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 63, 1145–1155.
- Colleran, R.; Douglas, P.S.; Hadamitzky, M.; Gutberlet, M.; Lehmkuhl, L.; Foldyna, B.; Woinke, M.; Hink, U.; Nadjiri, J.; Wilk, A. An FFRCT diagnostic strategy versus usual care in patients with suspected coronary artery disease planned for invasive coronary angiography at German sites: One-year results of a subgroup analysis of the PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts) study. Open Heart 2017, 4, e000526.
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFRCT: Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367.
- Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Raff, G.L.; Koweek, L.M.H.; Pontone, G.; Kawasaki, T.; et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT. JACC Cardiovasc. Imaging 2020, 13, 97–105.
- Curzen, N.P.; Nolan, J.; Zaman, A.G.; Nørgaard, B.L.; Rajani, R. Does the Routine Availability of CT–Derived FFR Influence Management of Patients with Stable Chest Pain Compared to CT Angiography Alone?: The FFRCT RIPCORD Study. JACC Cardiovasc. Imaging 2016, 9, 1188–1194.
- Curzen, N.; Nicholas, Z.; Stuart, B.; Wilding, S.; Hill, K.; Shambrook, J.; Eminton, Z.; Ball, D.; Barrett, C.; Johnson, L.; et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: The FORECAST randomized trial. Eur. Heart J. 2021, 42, 3844–3852.
- Giannopoulos, A.A.; Steigner, M.L.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic Applications of 3-dimensional Printing. J. Thorac. Imaging 2016, 31, 253–272.
- Costello, J.P.; Olivieri, L.J.; Krieger, A.; Thabit, O.; Marshall, M.B.; Yoo, S.-J.; Kim, P.C.; Jonas, R.A.; Nath, D.S. Utilizing Three-Dimensional Printing Technology to Assess the Feasibility of High-Fidelity Synthetic Ventricular Septal Defect Models for Simulation in Medical Education. World. J. Pediatr. Congenit. Heart Surg. 2014, 5, 421–426.
- Costello, J.P.; Olivieri, L.J.; Su, L.; Krieger, A.; Alfares, F.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart Dis. 2015, 10, 185–190.
- Sun, Z.; Lau, I.; Wong, Y.H.; Yeong, C.H. Personalized three-dimensional printed models in congenital heart disease. J. Clin. Med. 2019, 8, 522.
- Sun, Z.; Shen-Yuan, L. A systematic review of 3-D printing in cardiovascular and cerebrovascular diseases. Anatol. J. Cardiol. 2017, 17, 423–435.
- Shabbak, A.; Masoumkhani, F.; Fallah, A.; Amani-Beni, R.; Mohammadpour, H.; Shahbazi, T.; Bakhshi, A. 3D printing for cardiovascular surgery and intervention: A review article. Curr. Probl. Cardiol. 2024, 49, 102086.
- Lau, I.; Sun, Z. Three-dimensional printing in congenital heart disease: A systematic review. J. Med. Radiat. Sci. 2018, 65, 226–236.
- Verghi, E.; Catanese, V.; Nenna, A.; Montelione, N.; Mastroianni, C.; Lusini, M.; Stilo, F.; Chello, M. 3D printing in cardiovascular disease: Current appplications an future perspectives. Surg. Technol. Int. 2021, 38, 314–324.
- Lau, I.W.W.; Sun, Z. Dimensional Accuracy and Clinical Value of 3D Printed Models in Congenital Heart Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1483.
- Sun, Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2020, 10, 1577.
- Gómez-Ciriza, G.; Gómez-Cía, T.; Rivas-González, J.A.; Forte, M.N.V.; Valverde, I. Affordable three-dimensional printed heart models. Front. Cardiovasc. Med. 2021, 8, 642011.
- Moore, R.A.; Riggs, K.W.; Kourtidou, S.; Schneider, K.; Szugye, N.; Troja, W.; D’Souza, G.; Rattan, M.; Bryant, R., III; Taylor, M.D. Three-dimensional printing and virtual surgery for congenital heart procedural planning. Birth Defects Res. 2018, 110, 1082–1090.
- Garas, M.; Vaccarezza, M.; Newland, G.; McVay-Doornbusch, K.; Hasani, J. 3D-Printed specimens as a valuable tool in anatomy education: A pilot study. Ann. Anat. 2018, 219, 57–64.
- Anwar, S.; Rockefeller, T.; Raptis, D.A.; Woodard, P.K.; Eghtesady, P. 3D Printing Provides a Precise Approach in the Treatment of Tetralogy of Fallot, Pulmonary Atresia with Major Aortopulmonary Collateral Arteries. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 5.
- Loke, Y.-H.; Harahsheh, A.S.; Krieger, A.; Olivieri, L.J. Usage of 3D models of tetralogy of Fallot for medical education: Impact on learning congenital heart disease. BMC Med. Educ. 2017, 17, 54.
- Bouraghi, H.; Mohammadpour, A.; Khodaveisi, T.; Ghazisaeedi, M.; Saeedi, S.; Familgarosian, S. Virtual Reality and Cardiac Diseases: A Systematic Review of Applications and Effects. J. Healthc. Eng. 2023, 2023, 8171057.
- Stephenson, N.; Pushparajah, K.; Wheeler, G.; Deng, S.; Schnabel, J.A.; Simpson, J.M. Extended reality for procedural planning and guidance in structural heart disease—A review of the state-of-the-art. Int. J. Cardiovasc. Imaging 2023, 39, 1405–1419.
- Maresky, H.; Oikonomou, A.; Ali, I.; Ditkofsky, N.; Pakkal, M.; Ballyk, B. Virtual reality and cardiac anatomy: Exploring immersive three-dimensional cardiac imaging, a pilot study in undergraduate medical anatomy education. Clin. Anat. 2019, 32, 238–243.
- Barteit, S.; Lanfermann, L.; Bärnighausen, T.; Neuhann, F.; Beiersmann, C. Augmented, mixed, and virtual reality-based head-mounted devices for medical education: Systematic review. JMIR Serious Games 2021, 9, e29080.
- Dhar, P.; Rocks, T.; Samarasinghe, R.M.; Stephenson, G.; Smith, C. Augmented reality in medical education: Students’ experiences and learning outcomes. Med. Educ. Online 2021, 26, 1953953.
- Park, M.J.; Jung, J.I.; Choi, Y.-S.; Ann, S.H.; Youn, H.-J.; Jeon, G.N.; Choi, H.C. Coronary CT angiography in patients with high calcium score: Evaluation of plaque characteristics and diagnostic accuracy. Int. J. Cardiovasc. Imaging 2011, 27, 43–51.
- Vavere, A.L.; Arbab-Zadeh, A.; Rochitte, C.E.; Dewey, M.; Niinuma, H.; Gottlieb, I.; Clouse, M.E.; Bush, D.E.; Hoe, J.W.; de Roos, A.; et al. Coronary artery stenoses: Accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification—A subanalysis of the CORE-64 trial. Radiology 2011, 261, 100–108.
- Chen, C.-C.; Chen, C.-C.; Hsieh, I.C.; Liu, Y.-C.; Liu, C.-Y.; Chan, T.; Wen, M.-S.; Wan, Y.-L. The effect of calcium score on the diagnostic accuracy of coronary computed tomography angiography. Int. J. Cardiovasc. Imaging 2011, 27, 37–42.
- Meng, L.; Cui, L.; Cheng, Y.; Wu, X.; Tang, Y.; Wang, Y.; Xu, F. Effect of heart rate and coronary calcification on the diagnostic accuracy of the dual-source CT coronary angiography in patients with suspected coronary artery disease. Korean J. Radiol. 2009, 10, 347–354.
- Meijs, M.F.L.; Meijboom, W.B.; Prokop, M.; Mollet, N.R.; van Mieghem, C.A.G.; Doevendans, P.A.; de Feyter, P.J.; Cramer, M.J. Is there a role for CT coronary angiography in patients with symptomatic angina? Effect of coronary calcium score on identification of stenosis. Int. J. Cardiovasc. Imaging 2009, 25, 847–854.
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012.
- Wolf, E.V.; Halfmann, M.C.; Schoepf, U.J.; Zsarnoczay, E.; Fink, N.; Griffith, J.P., III; Aquino, G.J.; Willemink, M.J.; O’Doherty, J.; Hell, M.M. Intra-individual comparison of coronary calcium scoring between photon counting detector-and energy integrating detector-CT: Effects on risk reclassification. Front. Cardiovasc. Med. 2023, 9, 1053398.
- Soschynski, M.; Hagen, F.; Baumann, S.; Hagar, M.T.; Weiss, J.; Krauss, T.; Schlett, C.L.; von zur Mühlen, C.; Bamberg, F.; Nikolaou, K.; et al. High Temporal Resolution Dual-Source Photon-Counting CT for Coronary Artery Disease: Initial Multicenter Clinical Experience. J. Clin. Med. 2022, 11, 6003.
- Karsenty, C.; Guitarte, A.; Dulac, Y.; Briot, J.; Hascoet, S.; Vincent, R.; Delepaul, B.; Vignaud, P.; Djeddai, C.; Hadeed, K.; et al. The usefulness of 3D printed heart models for medical student education in congenital heart disease. BMC Med. Educ. 2021, 21, 480.
- Lim, K.H.A.; Loo, Z.Y.; Goldie, S.J.; Adams, J.W.; McMenamin, P.G. Use of 3D printed models in medical education: A randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat. Sci. Educ. 2016, 9, 213–221.
- Su, W.; Xiao, Y.; He, S.; Huang, P.; Deng, X. Three-dimensional printing models in congenital heart disease education for medical students: A controlled comparative study. BMC Med. Educ. 2018, 18, 178.
- Smith, C.F.; Tollemache, N.; Covill, D.; Johnston, M. Take away body parts! An investigation into the use of 3D-printed anatomical models in undergraduate anatomy education. Anat. Sci. Educ. 2018, 11, 44–53.
- Yi, X.; Ding, C.; Xu, H.; Huang, T.; Kang, D.; Wang, D. Three-Dimensional Printed Models in Anatomy Education of the Ventricular System: A Randomized Controlled Study. World Neurosurg. 2019, 125, e891–e901.
- Mogali, S.R.; Chandrasekaran, R.; Radzi, S.; Peh, Z.K.; Tan, G.J.S.; Rajalingam, P.; Yeong, W.Y. Investigating the effectiveness of three-dimensionally printed anatomical models compared with plastinated human specimens in learning cardiac and neck anatomy: A randomized crossover study. Anat. Sci. Educ. 2022, 15, 1007–1017.
- Arango, S.; Gorbaty, B.; Brigham, J.; Iaizzo, P.A.; Perry, T.E. A role for ultra-high resolution three-dimensional printed human heart models. Echocardiography 2023, 40, 703–710.
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordoñez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148.
- Cen, J.; Liufu, R.; Wen, S.; Qiu, H.; Liu, X.; Chen, X.; Yuan, H.; Huang, M.; Zhuang, J. Three-Dimensional Printing, Virtual Reality and Mixed Reality for Pulmonary Atresia: Early Surgical Outcomes Evaluation. Heart Lung. Circ. 2021, 30, 296–302.
- Guo, H.C.; Wang, Y.; Dai, J.; Ren, C.W.; Li, J.H.; Lai, Y.Q. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: A preliminary study. J. Thorac. Dis. 2018, 10, 867–873.
- Ryan, J.; Plasencia, J.; Richardson, R.; Velez, D.; Nigro, J.J.; Pophal, S.; Frakes, D. 3D printing for congenital heart disease: A single site’s initial three-yearexperience. 3D Print. Med. 2018, 4, 10.
- Zhao, L.; Zhou, S.; Fan, T.; Li, B.; Liang, W.; Dong, H. Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. J. Cardiac. Surg. 2018, 33, 24–27.
- Ghosh, R.M.; Jolley, M.A.; Mascio, C.E.; Chen, J.M.; Fuller, S.; Rome, J.J.; Silvestro, E.; Whitehead, K.K. Clinical 3D modeling to guide pediatric cardiothoracic surgery and intervention using 3D printed anatomic models, computer aided design and virtual reality. 3D Print. Med. 2022, 8, 11.
- Hell, M.M.; Achenbach, S.; Yoo, I.S.; Franke, J.; Blachutzik, F.; Roether, J.; Graf, V.; Raaz-Schrauder, D.; Marwan, M.; Schlundt, C. 3D printing for sizing left atrial appendage closure device: Head-to-head comparison with computed tomography and transoesophageal echocardiography. EuroIntervention 2017, 13, 1234–1241.
- Russo, J.J.; Yuen, T.; Tan, J.; Willson, A.B.; Gurvitch, R. Assessment of Coronary Artery Obstruction Risk during Transcatheter Aortic Valve Replacement Utilising 3D-Printing. Heart Lung Circ. 2022, 31, 1134–1143.
- Fan, Y.; Yang, F.; Cheung, G.S.-H.; Chan, A.K.-Y.; Wang, D.D.; Lam, Y.-Y.; Chow, M.C.-K.; Leong, M.C.-W.; Kam, K.K.-H.; So, K.C.-Y.; et al. Device Sizing Guided by Echocardiography-Based Three-Dimensional Printing Is Associated with Superior Outcome after Percutaneous Left Atrial Appendage Occlusion. J. Am. Soc. Echocardiogr. 2019, 32, 708–719.e701.
- Wu, C.-A.; Squelch, A.; Jansen, S.; Sun, Z. Optimization of computed tomography angiography protocols for follow-up type B aortic dissection patients by using 3D printed model. Appl. Sci. 2021, 11, 6844.
- Xenofontos, P.; Zamani, R.; Akrami, M. The application of 3D printing in preoperative planning for transcatheter aortic valve replacement: A systematic review. Biomed. Eng. Online 2022, 21, 59.
- Tanaka, Y.; Saito, S.; Sasuga, S.; Takahashi, A.; Aoyama, Y.; Obama, K.; Umezu, M.; Iwasaki, K. Quantitative assessment of paravalvular leakage after transcatheter aortic valve replacement using a patient-specific pulsatile flow model. Int. J. Cardiol. 2018, 258, 313–320.
- Brunner, B.S.; Thierij, A.; Jakob, A.; Tengler, A.; Grab, M.; Thierfelder, N.; Leuner, C.J.; Haas, N.A.; Hopfner, C. 3D-printed heart models for hands-on training in pediatric cardiology—The future of modern learning and teaching? GMS J. Med. Educ. 2022, 39, Doc23.
- Li, H.; Qingyao; Bingshen; Shu, M.; Lizhong; Wang, X.; Song, Z. Application of 3D printing technology to left atrial appendage occlusion. Int. J. Cardiol. 2017, 231, 258–263.
- Conti, M.; Marconi, S.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Italiano, G.; Mancini, M.E.; Auricchio, F.; Andreini, D.; Rabbat, M.G.; et al. Left atrial appendage closure guided by 3D computed tomography printing technology: A case control study. J. Cardiovasc. Comput. Tomogr. 2019, 13, 336–339.
- Goitein, O.; Fink, N.; Guetta, V.; Beinart, R.; Brodov, Y.; Konen, E.; Goitein, D.; Di Segni, E.; Grupper, A.; Glikson, M. Printed MDCT 3D models for prediction of left atrial appendage (LAA) occluder device size: A feasibility study. EuroIntervention 2017, 13, e1076–e1079.
- Traynor, G.; Shearn, A.I.; Milano, E.G.; Ordonez, M.V.; Forte, M.N.V.; Caputo, M.; Schievano, S.; Mustard, H.; Wray, J.; Biglino, G. The use of 3D-printed models in patient communication: A scoping review. J. 3D Print. Med. 2022, 6, 13–23.
- Illmann, C.F.; Hosking, M.; Harris, K.C. Utility and Access to 3-Dimensional Printing in the Context of Congenital Heart Disease: An International Physician Survey Study. CJC Open. 2020, 2, 207–213.
- Biglino, G.; Capelli, C.; Leaver, L.-K.; Schievano, S.; Taylor, A.M.; Wray, J. Involving patients, families and medical staff in the evaluation of 3D printing models of congenital heart disease. Commun. Med. 2015, 12, 157–169.
- Lau, I.W.W.; Liu, D.; Xu, L.; Fan, Z.; Sun, Z. Clinical value of patient-specific three-dimensional printing of congenital heart disease: Quantitative and qualitative assessments. PLoS ONE 2018, 13, e0194333.
- Biglino, G.; Koniordou, D.; Gasparini, M.; Capelli, C.; Leaver, L.-K.; Khambadkone, S.; Schievano, S.; Taylor, A.M.; Wray, J. Piloting the Use of Patient-Specific Cardiac Models as a Novel Tool to Facilitate Communication During Cinical Consultations. Pediatr. Cardiol. 2017, 38, 813–818.
- Giovanni, B.; Claudio, C.; Jo, W.; Silvia, S.; Lindsay-Kay, L.; Sachin, K.; Alessandro, G.; Graham, D.; Alexander, J.; Andrew, M.T. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: Feasibility and acceptability. BMJ Open 2015, 5, e007165.
- Biglino, G.; Moharem-Elgamal, S.; Lee, M.; Tulloh, R.; Caputo, M. The Perception of a Three-Dimensional-Printed Heart Model from the Perspective of Different Stakeholders: A Complex Case of Truncus Arteriosus. Front. Pediatr. 2017, 5, 209.
- Wu, C.-A.; Squelch, A.; Sun, Z. Investigation of Three-dimensional Printing Materials for Printing Aorta Model Replicating Type B Aortic Dissection. Curr. Med. Imaging Rev. 2021, 17, 843–849.
- Sun, Z.; Ng, C.K.C.; Wong, Y.H.; Yeong, C.H. 3D-Printed Coronary Plaques to Simulate High Calcification in the Coronary Arteries for Investigation of Blooming Artifacts. Biomolecules 2021, 11, 1307.
- Sun, Z.; Ng, C.K.C.; Squelch, A. Synchrotron radiation computed tomography assessment of calcified plaques and coronary stenosis with different slice thicknesses and beam energies on 3D printed coronary models. Quant. Imaging Med. Surg. 2019, 9, 6–22.
- Sun, Z. 3D printed coronary models offer new opportunities for developing optimal coronary CT angiography protocols in imaging coronary stents. Quant. Imaging Med. Surg. 2019, 9, 1350–1355.
- Sun, Z. 3D printing in medical applications. Curr. Med. Imaging. 2021, 17, 811–813.
- Sommer, K.N.; Iyer, V.; Kumamaru, K.K.; Rava, R.A.; Ionita, C.N. Method to simulate distal flow resistance in coronary arteries in 3D printed patient specific coronary models. 3D Print. Med. 2020, 6, 19.
- Wu, C.-A.; Squelch, A.; Sun, Z. Assessment of optimization of computed tomography angiography protocols for follow-up type B aortic dissection patients by using a 3D-printed model. J. 3D Print. Med. 2022, 6, 117–127.
- Aldosari, S.; Jansen, S.; Sun, Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant. Imaging Med. Surg. 2019, 9, 53–62.
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85.
- Sun, Z.; Jansen, S. Personalized 3D printed coronary models in coronary stenting. Quant. Imaging Med. Surg. 2019, 9, 1356–1367.
- Ripley, B.; Kelil, T.; Cheezum, M.K.; Goncalves, A.; Di Carli, M.F.; Rybicki, F.J.; Steigner, M.; Mitsouras, D.; Blankstein, R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2016, 10, 28–36.
- Kiraly, L.; Shah, N.C.; Abdullah, O.; Al-Ketan, O.; Rowshan, R. Three-Dimensional Virtual and Printed Prototypes in Complex Congenital and Pediatric Cardiac Surgery—A Multidisciplinary Team-Learning Experience. Biomolecules 2021, 11, 1703.
- Ullah, M.; Bibi, A.; Wahab, A.; Hamayun, S.; Rehman, M.U.; Khan, S.U.; Awan, U.A.; Riaz, N.-u.-a.; Naeem, M.; Saeed, S.; et al. Shaping the Future of Cardiovascular Disease by 3D Printing Applications in Stent Technology and its Clinical Outcomes. Curr. Probl. Cardiol. 2024, 49, 102039.
- Sun, Z.; Zhao, J.; Leung, E.; Flandes-Iparraguirre, M.; Vernon, M.; Silberstein, J.; De-Juan-Pardo, E.M.; Jansen, S. Three-Dimensional Bioprinting in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2023, 13, 1180.
- Jana, S.; Tefft, B.J.; Spoon, D.B.; Simari, R.D. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014, 10, 2877–2893.
- Mela, P. Subject- and Leaflet-Specific Remodeling of Polymeric Heart Valves for In Situ Tissue Engineering. JACC Basic. Transl. Sci. 2020, 5, 32–34.
- Vesely, I. Heart Valve Tissue Engineering. Circ. Res. 2005, 97, 743–755.
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A.I.P.M. Biomaterial-driven in situ cardiovascular tissue engineering—A multi-disciplinary perspective. npj Regen. Med. 2017, 2, 18.
- Butcher, J.T. The root problem of heart valve engineering. Sci. Transl. Med. 2018, 10, eaat5850.
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701.
- Seymour, A.J.; Westerfield, A.D.; Cornelius, V.C.; Skylar-Scott, M.A.; Heilshorn, S.C. Bioprinted microvasculature: Progressing from structure to function. Biofabrication 2022, 14, 22002.
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969.
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672.
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Tissue Engineering: Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352.
- Erdem, A.; Darabi, M.A.; Nasiri, R.; Sangabathuni, S.; Ertas, Y.N.; Alem, H.; Hosseini, V.; Shamloo, A.; Nasr, A.S.; Ahadian, S.; et al. 3D Bioprinting of Oxygenated Cell-Laden Gelatin Methacryloyl Constructs. Adv. Healthc. Mater. 2020, 9, e1901794.
- Ahrens, J.H.; Uzel, S.G.M.; Skylar-Scott, M.; Mata, M.M.; Lu, A.; Kroll, K.T.; Lewis, J.A. Programming Cellular Alignment in Engineered Cardiac Tissue via Bioprinting Anisotropic Organ Building Blocks. Adv. Mater. 2022, 34, e2200217.
- Asulin, M.; Michael, I.; Shapira, A.; Dvir, T. One-Step 3D Printing of Heart Patches with Built-in Electronics for Performance Regulation. Adv. Sci. 2021, 8, 2004205.
- Häneke, T.; Sahara, M. Progress in Bioengineering Strategies for Heart Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3482.
- Zhou, Z.; Tang, W.; Yang, J.; Fan, C. Application of 4D printing and bioprinting in cardiovascular tissue engineering. Biomater. Sci. 2023, 11, 6403–6420.
- Pijls, N.H.J.; Van Schaardenburgh, P.; De Bruyne, B.; Manoharan, G.; Boersma, E.; Bech, J.-W.; Vant Veer, M.; BÄR, F.; Hoorntje, J.; Koolen, J.; et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111.
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.-C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188.
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N. Eng. J. Med. 2009, 360, 213–224.
- Pijls, N.H.J.; Fearon, W.F.; Oldroyd, K.G.; Ver Lee, P.N.; Maccarthy, P.A.; De Bruyne, B.; Tonino, P.A.L.; Siebert, U.; Ikeno, F.; Bornschein, B.; et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease: 2-Year Follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Study. J. Am. Coll. Cardiol. 2010, 56, 177–184.
- Van Nunen, L.X.M.D.; Zimmermann, F.M.M.D.; Tonino, P.A.L.P.; Barbato, E.P.; Baumbach, A.P.; Engstrøm, T.P.; Klauss, V.P.; MacCarthy, P.A.P.; Manoharan, G.M.D.; Oldroyd, K.G.P.; et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015, 386, 1853–1860.
- Fearon, W.F.; Tonino, P.A.; De Bruyne, B.; Siebert, U.; Pijls, N.H. Rationale and design of the fractional flow reserve versus angiography for multivessel evaluation (FAME) study. Am. Heart J. 2007, 154, 632–636.
- Fearon, W.F.; Bornschein, B.; Tonino, P.A.L.; Gothe, R.M.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U. Economic Evaluation of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients With Multivessel Disease. Circulation 2010, 122, 2545–2550.
- De Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.A.L.; Piroth, Z.; Jagic, N.; Mobius-Winckler, S.; Rioufol, G.; Witt, N.; et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Eng. J. Med. 2012, 367, 991–1001.
- Jung, C.; Wolff, G.; Wernly, B.; Bruno, R.R.; Franz, M.; Schulze, P.C.; Silva, J.N.A.; Silva, J.R.; Bhatt, D.L.; Kelm, M. Virtual and Augmented Reality in Cardiovascular Care: State-of-the-Art and Future Perspectives. JACC Cardiovasc. Imaging 2022, 15, 519–532.
- Mitsuno, D.; Ueda, K.; Hirota, Y.; Ogino, M. Effective Application of Mixed Reality Device HoloLens: Simple Manual Alignment of Surgical Field and Holograms. Plast. Reconstr. Surg. 2019, 143, 647–651.
- Moro, C.; Phelps, C.; Redmond, P.; Stromberga, Z. HoloLens and mobile augmented reality in medical and health science education: A randomised controlled trial. Br. J. Educ. Technol. 2021, 52, 680–694.
- Gehrsitz, P.; Rompel, O.; Schöber, M.; Cesnjevar, R.; Purbojo, A.; Uder, M.; Dittrich, S.; Alkassar, M. Cinematic Rendering in Mixed-Reality Holograms: A New 3D Preoperative Planning Tool in Pediatric Heart Surgery. Front. Cardiovasc. Med. 2021, 8, 633611.
- Soulami, R.B.; Verhoye, J.-P.; Duc, H.N.; Castro, M.; Auffret, V.; Anselmi, A.; Haigron, P.; Ruggieri, V.G. Computer-Assisted Transcatheter Heart Valve Implantation in Valve-in-Valve Procedures. Innovations 2016, 11, 193–200.
- Opolski, M.P.; Debski, A.; Borucki, B.A.; Staruch, A.D.; Kepka, C.; Rokicki, J.K.; Sieradzki, B.; Witkowski, A. Feasibility and safety of augmented-reality glass for computed tomography-assisted percutaneous revascularization of coronary chronic total occlusion: A single center prospective pilot study. J. Cardiovasc. Comput. Tomogr. 2017, 11, 489–496.
- Ye, W.; Zhang, X.; Li, T.; Luo, C.; Yang, L. Mixed-reality hologram for diagnosis and surgical planning of double outlet of the right ventricle: A pilot study. Clin. Radiol. 2021, 76, 237.e1–237.e7.
- Kumar, R.P.; Pelanis, E.; Bugge, R.; Brun, H.; Palomar, R.; Aghayan, D.L.; Fretland, Å.A.; Edwin, B.; Elle, O.J. Use of mixed reality for surgery planning: Assessment and development workflow. J. Biomed. Inform. 2020, 112, 100077.
- Brun, H.; Bugge, R.A.B.; Suther, L.K.R.; Birkeland, S.; Kumar, R.; Pelanis, E.; Elle, O.J. Mixed reality holograms for heart surgery planning: First user experience in congenital heart disease. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 883–888.
- Lau, I.; Gupta, A.; Sun, Z. Clinical Value of Virtual Reality versus 3D Printing in Congenital Heart Disease. Biomolecules 2021, 11, 884.
- Yang, J.; Shan, D.; Wang, X.; Sun, X.; Shao, M.; Wang, K.; Pan, Y.; Wang, Z.; Schoepf, U.J.; Savage, R.H.; et al. On-Site Computed Tomography-Derived Fractional Flow Reserve to Guide Management of Patients with Stable Coronary Artery Disease: The TARGET Randomized Trial. Circulation 2023, 147, 1369–1381.
- Wang, H.; Wang, R.; Li, Y.; Zhou, Z.; Gao, Y.; Bo, K.; Yu, M.; Sun, Z.; Xu, L. Assessment of Image Quality of Coronary Computed Tomography Angiography in Obese Patients by Comparing Deep Learning Image Reconstruction with Adaptive Statistical Iterative Reconstruction Veo. J. Comput. Assist. Tomogr. 2022, 46, 34–40.
- Wang, W.; Wang, H.; Chen, Q.; Zhou, Z.; Wang, R.; Wang, H.; Zhang, N.; Chen, Y.; Sun, Z.; Xu, L. Coronary artery calcium score quantification using a deep-learning algorithm. Clin. Radiol. 2020, 75, e211–e237.
- Han, D.; Liu, J.; Sun, Z.; Cui, Y.; He, Y.; Yang, Z. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput. Methods Programs. Biomed. 2020, 196, 105651.
- Gao, Y.; Wang, W.; Wang, H.; Zhou, Z.; Xu, P.; Jiang, M.; Yang, L.; Wang, H.; Wen, H.; Sun, Z.; et al. Impact of Sublingual Nitroglycerin on the Assessment of Computed Tomography–derived Fractional Flow Reserve: An Intraindividual Comparison Study. J. Comput. Assist. Tomogr. 2022, 46, 23–28.
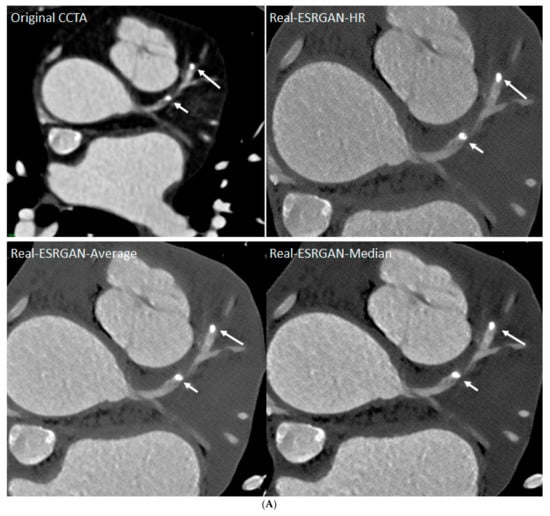
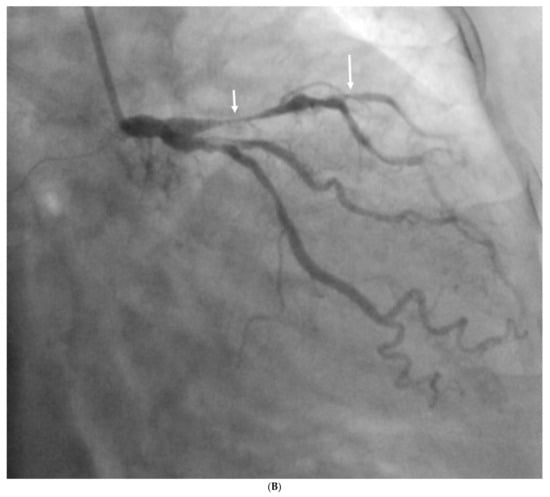
- Sun, Z.; Ng, C.K. Artificial intelligence (enhanced super-resolution generative adversarial network) for calcium deblooming in coronary computed tomography angiography: A feasibility study. Diagnostics 2022, 12, 991.
- Sun, Z.; Ng, C.K. Finetuned super-resolution generative adversarial network (artificial intelligence) model for calcium deblooming in coronary computed tomography angiography. J. Pers. Med. 2022, 12, 1354.
- Li, P.; Xu, L.; Yang, L.; Wang, R.; Hsieh, J.; Sun, Z.; Fan, Z.; Leipsic, J.A. Blooming Artifact Reduction in Coronary Artery Calcification by a New De-blooming Algorithm: Initial Study. Sci. Rep. 2018, 8, 6945.
- Alskaf, E.; Dutta, U.; Scannell, C.M.; Chiribiri, A. Deep learning applications in coronary anatomy imaging: A systematic review and meta-analysis. J. Med. Artif. Intell. 2022, 5, 11.
- Lin, A.; Manral, N.; McElhinney, P.; Killekar, A.; Matsumoto, H.; Kwiecinski, J.; Pieszko, K.; Razipour, A.; Grodecki, K.; Park, C.; et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: An international multicentre study. Lancet 2022, 4, e256–e265.
- Raffort, J.; Adam, C.; Carrier, M.; Ballaith, A.; Coscas, R.; Jean-Baptiste, E.; Hassen-Khodja, R.; Chakfé, N.; Lareyre, F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020, 72, 321–333.e321.
- Lareyre, F.; Adam, C.; Carrier, M.; Dommerc, C.; Mialhe, C.; Raffort, J. A fully automated pipeline for mining abdominal aortic aneurysm using image segmentation. Sci. Rep. 2019, 9, 13750.
- Spinella, G.; Fantazzini, A.; Finotello, A.; Vincenzi, E.; Boschetti, G.A.; Brutti, F.; Magliocco, M.; Pane, B.; Basso, C.; Conti, M. Artificial Intelligence Application to Screen Abdominal Aortic Aneurysm Using Computed tomography Angiography. J. Digit. Imaging 2023, 36, 2125–2137.
- Chandra, S.; Sarkar, P.K.; Chandra, D.; Ginsberg, N.E.; Cohen, R.I. Finding an alternative diagnosis does not justify increased use of CT-pulmonary angiography. BMC Pulm. Med. 2013, 13, 9.
- Soffer, S.; Klang, E.; Shimon, O.; Barash, Y.; Cahan, N.; Greenspana, H.; Konen, E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15814.
- Huhtanen, H.; Nyman, M.; Mohsen, T.; Virkki, A.; Karlsson, A.; Hirvonen, J. Automated detection of pulmonary embolism from CT-angiograms using deep learning. BMC Med. Imaging 2022, 22, 43.
- Ma, X.; Ferguson, E.C.; Jiang, X.; Savitz, S.I.; Shams, S. A multitask deep learning approach for pulmonary embolism detection and identification. Sci. Rep. 2022, 12, 13087.
- Grenier, P.A.; Ayobi, A.; Quenet, S.; Tassy, M.; Marx, M.; Chow, D.S.; Weinberg, B.D.; Chang, P.D.; Chaibi, Y. Deep Learning-Based Algorithm for Automatic Detection of Pulmonary Embolism in Chest CT Angiograms. Diagnostics 2023, 13, 1324.
- Colak, E.; Kitamura, F.C.; Hobbs, S.B.; Wu, C.C.; Lungren, M.P.; Prevedello, L.M.; Kalpathy-Cramer, J.; Ball, R.L.; Shih, G.; Stein, A.; et al. The RSNA Pulmonary Embolism CT Dataset. Radiol. Artif. Intell. 2021, 3, e200254.
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and application of artificial intelligence in cardiac imaging. Br. J. Radiol. 2020, 93, 20190812.




