
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aron Berger Syversen | -- | 4421 | 2024-01-23 16:43:09 | | | |
| 2 | Camila Xu | -25 word(s) | 4396 | 2024-01-24 01:57:05 | | | | |
| 3 | Aron Berger Syversen | Meta information modification | 4396 | 2024-01-24 15:22:49 | | |
Video Upload Options
Surgery is a common first-line treatment for many types of disease, including cancer. Mortality rates after major abdominal surgery have seen significant decreases whilst postoperative complications remain a frequent occurrence. Preoperative assessment tools are used to support patient risk stratification but do not always provide a precise and accessible assessment. Wearable sensors (WS) provide an accessible alternative that offers continuous monitoring in a non-clinical setting. Pre-processing involves all changes to data that are made in order to prepare the data for analysis. Pre-processing can be the most vital stage in data processing and has a large impact on the inferences that can be made from a data set.
1. Introduction
1.1. Preoperative Assessment
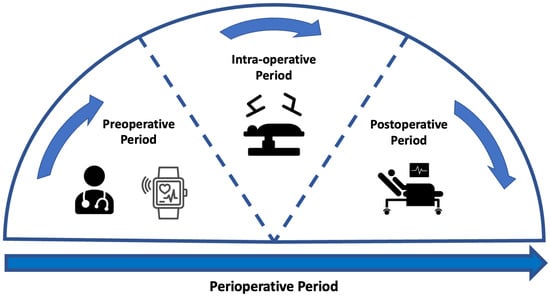
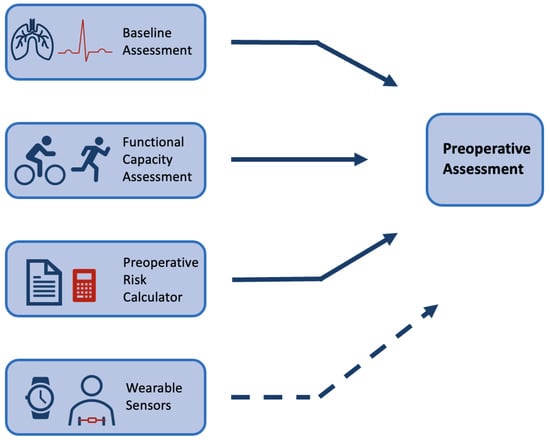
1.2. Wearable Sensors
2. Pre-Processing of Signals
2.1. Missing Data
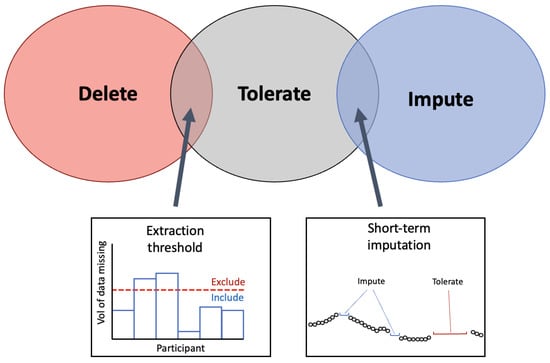
2.1.1. Extraction Threshold
3.1.2. Selecting an Extraction Threshold
2.1.3. Imputation
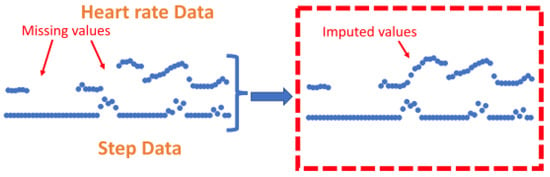
2.1.4. Feature Level Imputation
2.2. Noise
2.3. Encoding Time

References
- Liu, J.H.; Etzioni, D.A.; O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. The Increasing Workload of General Surgery. Arch. Surg. 2004, 139, 423–428.
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon Med. Sch. 2022, 89, 246–254.
- Brunner, M.; Wu, Z.; Krautz, C.; Pilarsky, C.; Grützmann, R.; Weber, G.F. Current Clinical Strategies of Pancreatic Cancer Treatment and Open Molecular Questions. Int. J. Mol. Sci. 2019, 20, 4543.
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. Wjg 2014, 20, 1635.
- World Cancer Research Fund. Colorectal Cancer Statistics; World Cancer Research Fund: London, UK, 2023.
- Cutsem, E.V.; Borràs, J.M.; Castells, A.; Ciardiello, F.; Ducreux, M.; Haq, A.; Schmoll, H.J.; Tabernero, J. Improving outcomes in colorectal cancer: Where do we go from here? Eur. J. Cancer 2013, 49, 2476–2485.
- Cancer Research UK. Bowel Cancer; Cancer Research UK: London, UK, 2023.
- Morris, E.J.; Taylor, E.F.; Thomas, J.D.; Quirke, P.; Finan, P.J.; Coleman, M.P.; Rachet, B.; Forman, D. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011, 60, 806–813.
- Wells, C.I.; Varghese, C.; Boyle, L.J.; McGuinness, M.J.; Keane, C.; O’Grady, G.; Gurney, J.; Koea, J.; Harmston, C.; Bissett, I.P. “Failure to Rescue” following Colorectal Cancer Resection: Variation and Improvements in a National Study of Postoperative Mortality. Ann. Surg. 2023, 278, 87–95.
- Ketelaers, S.H.; Orsini, R.G.; Burger, J.W.; Nieuwenhuijzen, G.A.; Rutten, H.J. Significant improvement in postoperative and 1-year mortality after colorectal cancer surgery in recent years. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 2052–2058.
- Silva, S.D.; Ma, C.; Proulx, M.C.; Crespin, M.; Kaplan, B.S.; Hubbard, J.; Prusinkiewicz, M.; Fong, A.; Panaccione, R.; Ghosh, S.; et al. Postoperative Complications and Mortality Following Colectomy for Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 972–980.
- Alves, A.; Panis, Y.; Mathieu, P.; Mantion, G.; Kwiatkowski, F.; Slim, K. Postoperative Mortality and Morbidity in French Patients Undergoing Colorectal Surgery: Results of a Prospective Multicenter Study. Arch. Surg. 2005, 140, 278–283.
- Tevis, S.E.; Kennedy, G.D. Postoperative complications and implications on patient-centered outcomes. J. Surg. Res. 2013, 181, 106.
- Wick, E.C.; Shore, A.D.; Hirose, K.; Ibrahim, A.M.; Gearhart, S.L.; Efron, J.; Weiner, J.P.; Makary, M.A. Readmission rates and cost following colorectal surgery. Dis. Colon Rectum 2011, 54, 1475–1479.
- Louis, M.; Johnston, S.A.; Churilov, L.; Ma, R.; Christophi, C.; Weinberg, L. Financial burden of postoperative complications following colonic resection: A systematic review. Medicine 2021, 100, e26546.
- Ludbrook, G.L. The Hidden Pandemic: The Cost of Postoperative Complications. Curr. Anesthesiol. Rep. 2022, 12, 1–9.
- Zambouri, A. Preoperative evaluation and preparation for anesthesia and surgery. Hippokratia 2007, 11, 13.
- Royal College of Physicians Developed by the National Guideline Centre. Preoperative Tests (Update) Routine Preoperative Tests for Elective Surgery; National Guideline Centre UK: London, UK, 2016.
- Grocott, M.P.; Pearse, R.M. Perioperative medicine: The future of anaesthesia? Bja Br. J. Anaesth. 2012, 108, 723–726.
- Grocott, M.P.; Edwards, M.; Mythen, M.G.; Aronson, S. Peri-operative care pathways: Re-engineering care to achieve the ‘triple aim’. Anaesthesia 2019, 74, 90–99.
- Noordzij, P.G.; Boersma, E.; Bax, J.J.; Feringa, H.H.; Schreiner, F.; Schouten, O.; Kertai, M.D.; Klein, J.; Urk, H.V.; Elhendy, A.; et al. Prognostic Value of Routine Preoperative Electrocardiography in Patients Undergoing Noncardiac Surgery. Am. J. Cardiol. 2006, 97, 1103–1106.
- Vernooij, J.E.; Koning, N.J.; Geurts, J.W.; Holewijn, S.; Preckel, B.; Kalkman, C.J.; Vernooij, L.M. Performance and usability of pre-operative prediction models for 30-day peri-operative mortality risk: A systematic review. Anaesthesia 2023, 78, 607–619.
- Wagner, D.P.; Draper, E.A. Acute physiology and chronic health evaluation (APACHE II) and Medicare reimbursement. Health Care Financ. Rev. 1984, 1984, 91.
- Jones, H.J.S.; Cossart, L.D. Risk scoring in surgical patients. Br. J. Surg. 1999, 86, 149–157.
- Copeland, G.P. The POSSUM System of Surgical Audit. Arch. Surg. 2002, 137, 15–19.
- Dosis, A.; Helliwell, J.; Syversen, A.; Tiernan, J.; Zhang, Z.; Jayne, D. Estimating postoperative mortality in colorectal surgery—A systematic review of risk prediction models. Int. J. Color. Dis. 2023, 38, 155.
- Pradhan, N.; Dyas, A.R.; Bronsert, M.R.; Lambert-Kerzner, A.; Henderson, W.G.; Qiu, H.; Colborn, K.L.; Mason, N.J.; Meguid, R.A. Attitudes about use of preoperative risk assessment tools: A survey of surgeons and surgical residents in an academic health system. Patient Saf. Surg. 2022, 16, 13.
- Goffi, L.; Saba, V.; Ghiselli, R.; Necozione, S.; Mattei, A.; Carle, F. Preoperative APACHE II and ASA scores in patients having major general surgical operations: Prognostic value and potential clinical applications. Eur. J. Surg. Acta Chir. 1999, 165, 730–735.
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G. Assessment of Functional Capacity in Clinical and Research Settings. Circulation 2007, 116, 329–343.
- Ferreira, V.; Lawson, C.; Ekmekjian, T.; Carli, F.; Scheede-Bergdahl, C.; Chevalier, S. Effects of preoperative nutrition and multimodal prehabilitation on functional capacity and postoperative complications in surgical lung cancer patients: A systematic review. Support. Care Cancer 2021, 29, 5597–5610.
- Mayo, N.E.; Feldman, L.; Scott, S.; Zavorsky, G.; Kim, D.J.; Charlebois, P.; Stein, B.; Carli, F. Impact of preoperative change in physical function on postoperative recovery: Argument supporting prehabilitation for colorectal surgery. Surgery 2011, 150, 505–514.
- Makker, P.G.S.; Koh, C.E.; Solomon, M.J.; Steffens, D.; BMedSc, P.G.S.M.; MBBS, C.E.K.; BCH, M.J.S.M.; BPhyt, D.S. Preoperative functional capacity and postoperative outcomes following abdominal and pelvic cancer surgery: A systematic review and meta-analysis. Anz J. Surg. 2022, 92, 1658–1667.
- Silvapulle, E.; Darvall, J. Objective methods for preoperative assessment of functional capacity. Bja Educ. 2022, 22, 312–320.
- Levett, D.Z.; Jack, S.; Swart, M.; Carlisle, J.; Wilson, J.; Snowden, C.; Riley, M.; Danjoux, G.; Ward, S.A.; Older, P.; et al. Perioperative cardiopulmonary exercise testing (CPET): Consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br. J. Anaesth. 2018, 120, 484–500.
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart 2007, 93, 1285.
- Hennis, P.J.; Meale, P.M.; Grocott, M.P. Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery. Postgrad. Med. J. 2011, 87, 550–557.
- Levett, D.Z.; Grocott, M.P. Cardiopulmonary exercise testing for risk prediction in major abdominal surgery. Anesthesiol. Clin. 2015, 33, 1–16.
- Reeves, T.; Bates, S.; Sharp, T.; Richardson, K.; Bali, S.; Plumb, J.; Anderson, H.; Prentis, J.; Swart, M.; Levett, D.Z.H. Cardiopulmonary exercise testing (CPET) in the United Kingdom-a national survey of the structure, conduct, interpretation and funding. Perioper. Med. 2018, 7, 2.
- Rose, G.A.; Davies, R.G.; Appadurai, I.R.; Williams, I.M.; Bashir, M.; Berg, R.M.; Poole, D.C.; Bailey, D.M. ‘Fit for surgery’: The relationship between cardiorespiratory fitness and postoperative outcomes. Exp. Physiol. 2022, 107, 787–799.
- Jones, L.; Tan, L.; Carey-Jones, S.; Riddell, N.; Davies, R.; Brownsdon, A.; Kelson, M.; Williams-Thomas, R.; Busse, M.; Davies, M.M.; et al. Can wearable technology be used to approximate cardiopulmonary exercise testing metrics? Perioper. Med. 2021, 10, 9.
- Ferreira, J.J.; Fernandes, C.I.; Rammal, H.G.; Veiga, P.M. Wearable technology and consumer interaction: A systematic review and research agenda. Comput. Hum. Behav. 2021, 118, 106710.
- Vijayan, V.; Connolly, J.; Condell, J.; McKelvey, N.; Gardiner, P. Review of Wearable Devices and Data Collection Considerations for Connected Health. Sensors 2021, 21, 5589.
- Smuck, M.; Odonkor, C.A.; Wilt, J.K.; Schmidt, N.; Swiernik, M.A. The emerging clinical role of wearables: Factors for successful implementation in healthcare. npj Digit. Med. 2021, 4, 45.
- Ontario, H.Q. Long-Term Continuous Ambulatory ECG Monitors and External Cardiac Loop Recorders for Cardiac Arrhythmia: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2017, 17, 1–56.
- Turakhia, M.P.; Hoang, D.D.; Zimetbaum, P.; Miller, J.D.; Froelicher, V.F.; Kumar, U.N.; Xu, X.; Yang, F.; Heidenreich, P.A. Diagnostic Utility of a Novel Leadless Arrhythmia Monitoring Device. Am. J. Cardiol. 2013, 112, 520–524.
- Yao, J.; Tan, C.S.; Lim, N.; Tan, J.; Chen, C.; Müller-Riemenschneider, F. Number of daily measurements needed to estimate habitual step count levels using wrist-worn trackers and smartphones in 212,048 adults. Sci. Rep. 2021, 11, 9633.
- Ricardo, L.I.C.; Wendt, A.; Galliano, L.M.; Muller, W.D.A.; Cruz, G.I.N.; Wehrmeister, F.; Brage, S.; Ekelund, U.; Silva, I.C.M. Number of days required to estimate physical activity constructs objectively measured in different age groups: Findings from three Brazilian (Pelotas) population-based birth cohorts. PLoS ONE 2020, 15, e0216017.
- Böhmer, A.B.; Wappler, F.; Zwilßer, B. Preoperative Risk Assessment—From Routine Tests to Individualized Investigation. Dtsch. Ärzteblatt Int. 2014, 111, 437.
- Lequeux, B.; Uzan, C.; Rehman, M.B. Does resting heart rate measured by the physician reflect the patient’s true resting heart rate? White-coat heart rate. Indian Heart J. 2018, 70, 93–98.
- Deshmukh, S.D.; Shilaskar, S.N. Wearable sensors and patient monitoring system: A Review. In Proceedings of the 2015 International Conference on Pervasive Computing (ICPC), Pune, India, 8–10 January 2015; pp. 1–3.
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637.
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens. Actuators Chem. 2020, 321, 128549.
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med. 2020, 27, 73–77.
- Kakria, P.; Tripathi, N.K.; Kitipawang, P. A real-time health monitoring system for remote cardiac patients using smartphone and wearable sensors. Int. J. Telemed. Appl. 2015, 2015, 373474.
- Huang, J.D.; Wang, J.; Ramsey, E.; Leavey, G.; Chico, T.J.A.; Huang, J.D.; Wang, J.; Ramsey, E.; Leavey, G.; Chico, T.J.A.; et al. Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review. Sensors 2022, 22, 8002.
- Wells, C.I.; Xu, W.; Penfold, J.A.; Keane, C.; Gharibans, A.A.; Bissett, I.P.; O’Grady, G. Wearable devices to monitor recovery after abdominal surgery: Scoping review. BJS Open 2022, 6, zrac031.
- van den Eijnden, M.A.C.; van der Stam, J.A.; Bouwman, R.A.; Mestrom, E.H.J.; Verhaegh, W.F.J.; van Riel, N.A.W.; Cox, L.G.E. Machine Learning for Postoperative Continuous Recovery Scores of Oncology Patients in Perioperative Care with Data from Wearables. Sensors 2023, 23, 4455.
- Daskivich, T.J.; Houman, J.; Lopez, M.; Luu, M.; Fleshner, P.; Zaghiyan, K.; Cunneen, S.; Burch, M.; Walsh, C.; Paiement, G.; et al. Association of Wearable Activity Monitors With Assessment of Daily Ambulation and Length of Stay Among Patients Undergoing Major Surgery. JAMA Netw. Open 2019, 2, e187673.
- Esteban, P.A.; Hernández, N.; Novoa, N.; Varela, G. Evaluating patients’ walking capacity during hospitalization for lung cancer resection. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 268–271.
- Rinne, J.K.; Miri, S.; Oksala, N.; Vehkaoja, A.; Kössi, J. Evaluation of a wrist-worn photoplethysmography monitor for heart rate variability estimation in patients recovering from laparoscopic colon resection. J. Clin. Monit. Comput. 2023, 37, 45–53.
- Breteler, M.J.; Huizinga, E.; Loon, K.V.; Leenen, L.P.; Dohmen, D.A.; Kalkman, C.J.; Blokhuis, T.J. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: A clinical validation study. BMJ Open 2018, 8, e020162.
- Waller, E.; Sutton, P.; Rahman, S.; Allen, J.; Saxton, J.; Aziz, O. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: A randomised controlled pilot study (trial registration: NCT04047524). Surg. Endosc. 2022, 36, 1008.
- Beilstein, C.M.; Krutkyte, G.; Vetsch, T.; Eser, P.; Wilhelm, M.; Stanga, Z.; Bally, L.; Verra, M.; Huber, M.; Wuethrich, P.Y.; et al. Multimodal prehabilitation for major surgery in elderly patients to lower complications: Protocol of a randomised, prospective, multicentre, multidisciplinary trial (PREHABIL Trial). BMJ Open 2023, 13, e070253.
- Feeney, C.; Reynolds, J.V.; Hussey, J. Preoperative physical activity levels and postoperative pulmonary complications post-esophagectomy. Dis. Esophagus 2011, 24, 489–494.
- Greco, M.; Angelucci, A.; Avidano, G.; Marelli, G.; Canali, S.; Aceto, R.; Lubian, M.; Oliva, P.; Piccioni, F.; Aliverti, A.; et al. Wearable Health Technology for Preoperative Risk Assessment in Elderly Patients: The WELCOME Study. Diagnostics 2023, 13, 630.
- Spathis, D.; Perez-Pozuelo, I.; Brage, S.; Wareham, N.J.; Mascolo, C. Self-supervised transfer learning of physiological representations from free-living wearable data. In Proceedings of the ACM CHIL 2021–2021 ACM Conference on Health, Inference, and Learning, Virtual, 8–9 April 2021; Volume 21, pp. 69–78.
- Cho, S.; Ensari, I.; Weng, C.; Kahn, M.G.; Natarajan, K. Factors Affecting the Quality of Person-Generated Wearable Device Data and Associated Challenges: Rapid Systematic Review. Jmir Mhealth Uhealth 2021, 9, e20738.
- Cos, H.; Li, D.; Williams, G.; Chininis, J.; Dai, R.; Zhang, J.; Srivastava, R.; Raper, L.; Sanford, D.; Hawkins, W.; et al. Predicting Outcomes in Patients Undergoing Pancreatectomy Using Wearable Technology and Machine Learning: Prospective Cohort Study. J. Med. Internet Res. 2021, 23, e23595.
- Zhang, J.; Li, D.; Dai, R.; Cos, H.; Williams, G.A.; Raper, L.; Hammill, C.W.; Lu, C. Predicting Post-Operative Complications with Wearables. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2022, 6, 1–27.
- Haveman, M.E.; van Melzen, R.; Schuurmann, R.C.; Hermens, H.J.; Tabak, M.; de Vries, J.P.P. Feasibility and patient’s experiences of perioperative telemonitoring in major abdominal surgery: An observational pilot study. Expert Rev. Med. Devices 2022, 19, 515–523.
- Little, R.J.; Rubin, D.B. Statistical Analysis with Missing Data; John Wiley & Sons.: Hoboken, NJ, USA, 2019; pp. 1–449.
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162.
- Darji, J.; Biswas, N.; Jones, L.D.; Ashili, S. Handling missing data in the time-series data from wearables. In Time Series Analysis—Recent Advances, New Perspectives and Applications ; IntechOpen: London, UK, 2023.
- Tackney, M.S.; Cook, D.G.; Stahl, D.; Ismail, K.; Williamson, E.; Carpenter, J. A framework for handling missing accelerometer outcome data in trials. Trials 2021, 22, 379.
- Sun, V.; Dumitra, S.; Ruel, N.; Lee, B.; Melstrom, L.; Melstrom, K.; Woo, Y.; Sentovich, S.; Singh, G.; Fong, Y. Wireless Monitoring Program of Patient-Centered Outcomes and Recovery Before and after Major Abdominal Cancer Surgery. JAMA Surg. 2017, 152, 852–859.
- Billé, A.; Buxton, J.; Viviano, A.; Gammon, D.; Veres, L.; Routledge, T.; Harrison-Phipps, K.; Dixon, A.; Minetto, M.A. Preoperative Physical Activity Predicts Surgical Outcomes Following Lung Cancer Resection. Integr. Cancer Ther. 2021, 20, 1534735420975853.
- Richards, S.J.G.; Jerram, P.M.; Brett, C.; Falloon, M.; Frizelle, F.A. The association between low pre-operative step count and adverse post-operative outcomes in older patients undergoing colorectal cancer surgery. Perioper. Med. 2020, 9, 20.
- Haveman, M.E.; van Melzen, R.; Moumni, M.E.; Schuurmann, R.C.L.; Hermens, H.J.; Tabak, M.; de Vries, J.P.P.M. Determining the Reliable Measurement Period for Preoperative Baseline Values With Telemonitoring Before Major Abdominal Surgery: Pilot Cohort Study. Jmir Perioper. Med. 2022, 5, e40815.
- Lin, S.; Wu, X.; Martinez, G.; Chawla, N.V. Filling missing values on wearable-sensory time series data. In Proceedings of the 2020 SIAM International Conference on Data Mining, Cincinnati, OH, USA, 7–9 May 2020; pp. 46–54.
- Lin, J.Y.; Lu, Y.; Tu, X. How to avoid missing data and the problems they pose: Design considerations. Shanghai Arch. Psychiatry 2012, 24, 181.
- Hallgrímsson, H.T.; Jankovic, F.; Althoff, T.; Allen, P.G.; Foschini, L. Learning Individualized Cardiovascular Responses from Large-scale Wearable Sensors Data. arXiv 2018, arXiv:1812.01696.
- Angelucci, A.; Greco, M.; Canali, S.; Marelli, G.; Avidano, G.; Goretti, G.; Cecconi, M.; Aliverti, A. Fitbit Data to Assess Functional Capacity in Patients Before Elective Surgery: Pilot Prospective Observational Study. J. Med. Internet Res. 2023, 25, e42815.
- Meesad, P.; Hengpraprohm, K. Combination of KNN-based feature selection and KNN-based missing-value imputation of microarray data. In Proceedings of the 3rd International Conference on Innovative Computing Information and Control, ICICIC’08, Dalian, China, 18–20 June 2008.
- Commons, C. CC BY 4.0 Deed. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 24 November 2023).
- Chakrabarti, S.; Biswas, N.; Karnani, K.; Padul, V.; Jones, L.D.; Kesari, S.; Ashili, S. Binned Data Provide Better Imputation of Missing Time Series Data from Wearables. Sensors 2023, 23, 1454.
- Bashar, S.K.; Ding, E.; Walkey, A.J.; McManus, D.D.; Chon, K.H. Noise Detection in Electrocardiogram Signals for Intensive Care Unit Patients. IEEE Access Pract. Innov. Open Solut. 2019, 7, 88357.
- Kang, S.; Paul, A.; Jeon, G. Reduction of mixed noise from wearable sensors in human-motion estimation. Comput. Electr. Eng. 2017, 61, 287–296.
- Stegle, O.; Fallert, S.V.; MacKay, D.J.; Brage, S. Gaussian process robust regression for noisy heart rate data. IEEE Trans. Bio-Med. Eng. 2008, 55, 2143–2151.
- Wu, Y.; Spathis, D.; Jia, H.; Perez-Pozuelo, I.; Gonzales, T.I.; Brage, S.; Wareham, N.; Mascolo, C. Turning Silver into Gold: Domain Adaptation with Noisy Labels for Wearable Cardio-Respiratory Fitness Prediction. arXiv 2022, arXiv:2211.10475.
- Altini, M.; Casale, P.; Penders, J.; Amft, O. Cardiorespiratory fitness estimation in free-living using wearable sensors. Artif. Intell. Med. 2016, 68, 37–46.
- Beltrame, T.; Amelard, R.; Wong, A.; Hughson, R.L. Prediction of oxygen uptake dynamics by machine learning analysis of wearable sensors during activities of daily living. Sci. Rep. 2017, 7, 45738.
- Cui, H.W.; Kirby, G.S.; Surmacz, K.; Hargrove, C.; Griffiths, J.; Turney, B.W. The association of pre-operative home accelerometry with cardiopulmonary exercise variables. Anaesthesia 2018, 73, 738–745.
- Dunn, J.; Kidzinski, L.; Runge, R.; Witt, D.; Hicks, J.L.; Rose, S.M.S.F.; Li, X.; Bahmani, A.; Delp, S.L.; Hastie, T.; et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med. 2021, 27, 1105–1112.
- Spathis, D.; Perez-Pozuelo, I.; Gonzales, T.I.; Wu, Y.; Brage, S.; Wareham, N.; Mascolo, C. Longitudinal cardio-respiratory fitness prediction through wearables in free-living environments. npj Digit. Med. 2022, 5, 176.




