
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joanna Głowska-Ciemny | -- | 2693 | 2024-01-23 08:32:29 | | | |
| 2 | Mona Zou | Meta information modification | 2693 | 2024-01-24 09:45:17 | | |
Video Upload Options
The most common association related to alpha-fetoprotein (AFP) is fetal neural tube defect (NTD), and indeed, this is where the international career of this protein began. In times when ultrasonography was not yet technically advanced, the detection of high levels of AFP in maternal serum (MS-AFP) and amniotic fluid was the basis for suspecting neural tube defects. In cases where there was no confirmation of NTD, other causes were sought. It has been established that high titers of MS-AFP could originate in other defects or diseases, such as (1) increased proteinuria in severe fetal kidney diseases; (2) pathological overproduction in liver diseases; (3) penetration through the membranes of gastrointestinal organs exposed to amniotic fluid; (4) passage through the walls of skin vessels; and as a side effect of (5) hepatic hematopoiesis and increased transfer through the edematous placenta in fetal anemia.
1. Central Nervous System
Intraneuronal synthesis of AFP has never been proven, but its presence in the central nervous system has been confirmed by immunohistochemical studies. It enters the cerebrospinal fluid through the process of blood filtration in the choroid plexuses of the ventricular system. In case of open neural tube defects, AFP leaks with cerebrospinal fluid from the defective structures into the amniotic fluid (AF-AFP) and is then absorbed through the fetal membranes into the maternal bloodstream. Maternal-serum alpha-fetoprotein (MS-AFP) determination can detect 65–80% of open neural tube defects (88% of anencephaly, where we typically find values around 6.5 multiple of the median (MoM), and 67% of meningo-spinal hernias, where we typically find values around 3.8 MoM). The highest sensitivity in detecting NTDs occurs when AFP testing is performed between 15–18 weeks of gestation, and declines thereafter, as AFP production and concentration also physiologically decline. The usefulness of AFP determination in closed neural tube defects such as cerebral hernia (encephalocele), cleft occipitalis (iniencephaly), or caudal regression syndrome (CRS) has not been demonstrated, as there is no CSF leakage there. Research is currently underway on other non-invasive NTD markers—glial fibrillary acidic protein (GFAP) and complement system proteins. GFAP is a protein involved in the structure of the glial cytoskeleton and a specific marker of CNS defects. Its sensitivity does not decrease with increasing gestational age and is not present in other defects such as evisceration. It also does not increase in cases of closed NTDs. Complement components, in turn, act as conductors of cells to their target sites in the CNS during embryogenesis. Imbalances in this system are thought to be a cause of NTDs (C3 and C9 levels are significantly reduced in NTDs), schizophrenia, or autism [1][2][3][4][5][6][7].
Meningomyelocele (MMC). It occurs with a frequency of 0.4:1000 births. The underlying cause is a genetic (mutations of dozens of different genes)–environmental interaction (folic acid deficiency, among other things). Due to incomplete closure of the neural tube, there is an exposure of the neuroepithelium to amniotic fluid, resulting in neuronal degeneration. In the perinatal period, MMC manifests as a tumor of the spinal region, most often of the L-S segment of the spine, and Arnold–Chiari syndrome (wedging of the cerebellum, fourth ventricle, and brainstem into the great aperture of the skull and consequent hydrocephalus). After birth, hydrocephalus, fecal and urinary incontinence, and flaccid paresis of the lower extremities are the predominant problems [4][5].
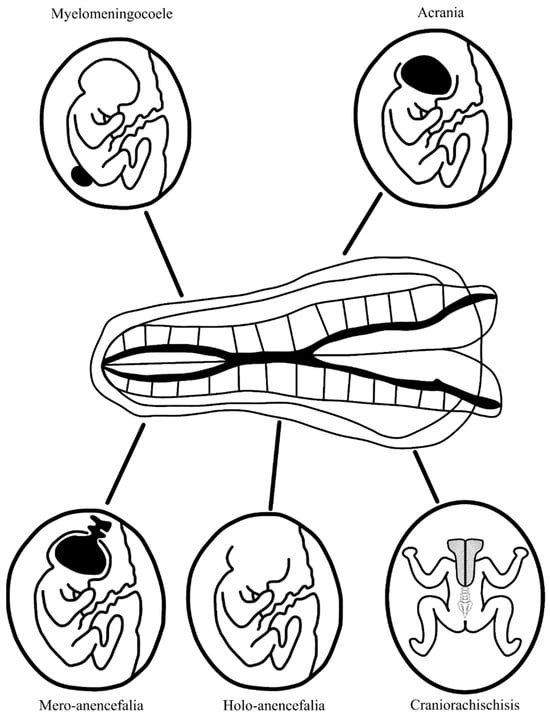
Acrania and anencephaly. They occur with a frequency of 1:10,000–20,000 births. A genetic mutation in the gene encoding hedgehog acyltransferase (HHAT) underlies this condition, which is essential for the production of Hedgehog (Hh) protein, important in the production of kinases that regulate extracellular signals, bone morphogenetic proteins, and fibroblast growth factors [8]. This leads to abnormal bone and cartilage tissue formation. Another cause of acrania can be amniotic band syndrome. This results in the underdevelopment or absence of cranial plates, either partially or entirely. The brain is exposed to amniotic fluid, which eventually leads to the destruction of its structures. There are three forms of anencephaly. Mero-anencephaly involves a small midline gap in the cranial vault with protrusion of immature brain tissue (this is the cerebrovascular area—primitive brain tissue with fields of irregular vascularization) that is not covered by meninges and skin. In holo-anencephaly, the classic form of anencephaly, there is a complete absence of brain tissue except for the brainstem. In craniorachischisis, the most severe form, anencephaly is accompanied by spina bifida, a split in the spinal column to the thoracic level, and the resulting space is filled with the cerebrovascular and medullovascular areas [4][5]. A summary of fetal CNS defects associated with elevated maternal serum AFP levels is shown in Figure 1.
2. Liver
From the 26th day after ovulation, the hepatic diverticulum of the archenteron produces AFP; from the 9th week of pregnancy until birth, the fetal liver is the main source of AFP in the fetal bloodstream [9].
Beckwith–Wiedemann Syndrome (BWS). It occurs with a frequency of 1:136,000 births. It is genetically associated with hypermethylation of the H19 gene on chromosome 11p.15.5. During prenatal development, it is characterized by macrosomia, umbilical hernia, macroglossia, organomegaly, polyhydramnios (swallowing disorder), and enlargement of the placenta. Overall, 5–10% of children have a risk of developing embryonal malignancies in childhood, such as Wilms tumor, hepatoblastoma, adrenocortical carcinoma, gonadoblastoma, neuroblastoma, and rhabdomyosarcoma. Elevated AFP levels result from abnormal liver function and leakage through the enlarged placenta. High AFP also persists after birth and is at higher levels than in healthy children. AFP measurement should be used for oncologic monitoring for hepatoblastoma (96% of children with hepatoblastoma have high AFP), based on special nomograms for children with BWS rather than healthy children (characterized by a different, slower rate of descent of AFP levels in the first year of life) [10][11][12][13].
Hepatic hemangioma. It occurs with a frequency of 5:1,000,000 births. It is the most common benign liver tumor in fetuses and newborns. It undergoes a phase of rapid proliferation during infancy and then regression from 1–5 years of age. It can have a focal, multiple, or diffuse form. Most cases are asymptomatic, but heart failure or signs of hepatitis can rarely occur. It has been suggested that the neoplasm itself does not produce AFP, but the interaction between the primary mesoderm, from which it originates, and the endodermal elements from the yolk sac located in the liver is the source [14][15].
3. Gastrointestinal Tract
AFP is produced in trace amounts by cells of the gastrointestinal tract from the 26th day after ovulation and comes mainly from the endodermal cells of the middle and posterior archenteron. In the gastrointestinal tract, AFP is also absorbed from ingested amniotic fluid. Postnatally, AFP may serve as a marker of gastrointestinal mucosal regeneration following intestinal surgeries for necrotizing enterocolitis (NEC) or other intestinal injuries in children. These possibilities are associated with endodermal stem cells that persist beyond the fetal period of life [9][16].
Gastroschisis. It occurs with a frequency of 2–5:10,000 births. It is not associated with genetic mutations but rather with a mother’s young age and propensity to use stimulants and drugs. Typically, a small 2–4 cm opening is found to the right of the umbilicus through which the intestines (and sometimes also the liver or stomach) protrude into the amniotic cavity without being covered by any additional membrane. After birth, the main issue is short bowel syndrome [17][18][19]. A particular form of gastroschisis is Limb–Body Wall Complex (body stalk anomaly), where there is both thoracic and abdominal evisceration, limb abnormalities, and encephalocele [17]. The source of high AFP levels in amniotic fluid and maternal serum is the release of AFP from the exposed intestines.
Umbilical hernia (omphalocele) occurs with a frequency of 1:4000 births. It is associated with chromosomal aberrations, mainly trisomy 13 and 18, as well as various genetic syndromes such as Beckwith–Wiedemann syndrome. The hernia sac protrudes from the umbilical ring and has an umbilical cord attachment to its top. It contains the intestines and/or the liver [19]. If the umbilical hernia coexists with pathologies of the abdominal wall below the umbilicus, it is referred to as OEIS complex (omphalocele, bladder exstrophy, imperforate anus, spinal cleft) [20]. When abdominal and chest wall pathologies coexist, this is referred to as pentalogy of Cantrell (umbilical hernia, diaphragmatic hernia, sternal cleft, cardiac ectopy, cardiac defect) [21][22]. Due to the thick membrane coverage, AFP levels in amniotic fluid and serum may be low, and certainly significantly lower than in gastroschisis.
Epignathus. It occurs with a frequency of 1:20,000–40,000 births, with a predominant incidence in women. The genetic etiology is unclear, but it is associated with trisomy 13, ring chromosome X, and pentasomy of the X chromosome. It is a rare teratoma (germinal cell tumor), with most cases occurring in the sacrococcygeal region during fetal life, and only about 2–9% involving the head and neck area. Epignathus can originate in the mandible, maxilla, palate, fetal throat, and sphenoid or ethmoid sinuses. It contains cells of all three germ layers: ectoderm, endoderm, and mesoderm. It can reach massive sizes, protruding in front of the oral cavity and causing airway obstruction after birth (80–100% risk of perinatal death). The tumor is accompanied by deformation of the craniofacial structures (often cleft palate) and a risk of invasion into the brain structures. Impaired swallowing of amniotic fluid leads to polyhydramnios. High AFP levels result from its production by the huge mass of tumor tissue. Monitoring AFP levels in teratomas is used to detect recurrence or progression toward malignancy [23][24].
4. Kidneys
AFP production has not been found in the kidneys, neither in the prenatal period nor in childhood. However, congenital abnormalities of nephron structure, particularly the filtration membrane, can cause significant proteinuria, leading to high concentrations of AFP in amniotic fluid and maternal serum [9].
Congenital “Finnish type” of nephrotic syndrome. It occurs with a frequency of 1:8000 births in the Finnish population. It is inherited in an autosomal recessive manner due to mutations in the NPHS1 gene encoding nephrin, a protein on the filtration membrane of kidney glomeruli. In fetal life, there are no apparent anatomical changes, although these children already exhibit significant proteinuria. After birth, severe nephrotic syndrome, malnutrition, and susceptibility to infection are diagnosed. The only treatment is kidney transplantation. High AFP levels in amniotic fluid and maternal serum are typical for both homozygotes and heterozygous carriers. However, carriers of the mutation have elevated AFP levels only up to about the 20th week of gestation. This normalizes in the late second trimester due to the completion of glomerular formation and simultaneous withdrawal of proteinuria [25][26].
Steroid-resistant nephritic syndrome associated with CRB2 mutation. It occurs with a frequency of <1:1,000,000 and is inherited in an autosomal recessive manner. It mimics the congenital “Finnish type” of nephrotic syndrome. Histopathological examination reveals obliteration of foot processes of podocytes. Dilatation of the ventricular system of the brain, renal cysts, heterotopia of the gray matter, VSD, Scimitar syndrome, and the ophthalmic disorders retinitis pigmentosa and congenital Leber blindness are diagnosed perinatally [27][28].
Multiple Acyl-CoA Dehydrogenase Deficiency (MADD) = MAD deficiency = Glutaric Acidosis Type 2 = Glutaric Aciduria Type 2. It occurs with a frequency of 1–9:1,000,000 births and is inherited in an autosomal recessive manner. It is caused by mutations in the Electron Transfer Flavoprotein Subunit Alpha—ETFA (15q23-q25), Electron Transfer Flavoprotein Subunit Beta—ETFB (19q13.3-q13.4), and Electron Transfer Flavoprotein Dehydrogenase—ETFDH (4q32-q35) genes, which encode the alpha and beta subunits of ETF-Q oxidoreductase, a component of the mitochondrial respiratory chain. The prenatal period is characterized by fetal growth retardation, oligohydramnios, and polycystic kidneys.
There are two known clinical forms: severe MADD (onset in the neonatal period, with or without congenital malformations) and mild MADD (late onset). The severe form is characterized by prematurity and clinical symptoms appearing within the first day of life, including hypoglycemia, hypotonia, hepatomegaly, metabolic acidosis, and death in the first week of life. Congenital malformations such as polycystic kidneys, facial dysmorphia, genital abnormalities, and rocker-bottom foot may also occur. The late onset form manifests in the first months of life with episodes of vomiting, metabolic acidosis, and hypoglycemia, or only in adolescence in the form of a syndrome resembling Reye’s syndrome with ketoacidosis and lipid storage myopathy. High AFP in maternal serum and amniotic fluid is associated with progressive fetal kidney failure, negative acetylcholinesterase, and an abnormal acylcarnitine profile in amniotic fluid [29][30].
5. Skin
Epidermolysis Bullosa Simplex. It occurs with a frequency of 2:100,000 births. It is caused by mutations in 16 different genes. Most cases of EB simplex are inherited in an autosomal dominant manner, with mutations affecting the KRT5 and KRT14 genes. However, in about 5% of simplex-type EB cases, the disease is inherited in an autosomal recessive manner and caused by mutations in the KRT14 gene. The remaining forms of EB of the simplex type caused by mutations in other genes (ITGA6, ITGB4, DSP1, PKP1) are inherited in an autosomal recessive manner. An exception is the PLEC1 gene, mutations of which can occur in both the autosomal dominant and recessive inherited subtype of EB simplex. The underlying histopathology is an abnormal junction of the epidermis and dermis, resulting in the formation of blisters due to mechanical trauma, which then rupture, leaving erosions and scars. The formation of blisters on the hands and feet, along with their scarring, can lead to contractures. Narrowing of the gastrointestinal tract, urinary tract, and lungs can also occur. High levels of AFP result from its release into the amniotic fluid from exposed blood vessels that would normally be covered by the skin [31][32][33].
Aplasia cutis congenita. It occurs with a frequency of 1:10,000 births. The etiology is heterogeneous and can include occasional mutations in the BMS1 (10q11.21) and DLL4 (15q15.1) genes, as well as the influence of drugs, narcotics, and herpes virus infections. The syndrome is characterized by the absence of skin, typically from the scalp, although other areas may be affected as well. The high AFP levels are due to its release into the amniotic fluid from exposed vessels that would normally be covered by the skin [34][35].
6. Hematopoietic System
The main source of AFP is multipotent progenitor cells in the bone marrow. Two subtypes of these cells are described: (1) fetal hepatic stem/progenitor cells (FHSCs) and (2) intrinsic hematopoietic stem/progenitor cells (HSPCs). HSPCs can also migrate to the liver and act as precursors of hepatic stem cells. Another source is bone marrow mesenchymal cells with the ability to differentiate into hepatic stem cells and migrate to the liver in case of damage [9][36].
Hemoglobin Barts (Hb Barts). It occurs with a frequency of 1:200–1:2000 births and is the most common cause of fetal anemia in Southeast Asia. It is the most severe form of alpha-thalassemia. It is inherited in an autosomal recessive manner and results from a deletion in the HBA1 and HBA2 genes (16p13.3). The mutation leads to an abnormal structure of hemoglobin, which consists of four gamma-globin chains (Hb Barts) instead of alpha-globin in fetal life and four beta-globin chains after birth (HbH). Hb Barts and HbH have a high affinity for oxygen but do not release it to the tissues, resulting in anemia and tissue hypoxia, hepatosplenomegaly, placentomegaly, generalized edema appearing before 10 weeks of gestation, and intrauterine or perinatal death. Most children rescued with the help of intrauterine transfusions require lifelong intensive blood transfusions. Elevated maternal serum AFP results from increased fetal–maternal transfer through the edematous placenta and/or from increased extramedullary hematopoiesis in the liver. The increase in MS-AFP levels precedes the increase in MCA-PSV by approximately 2.7 weeks [37][38].
7. Placenta
It has been demonstrated that the human cytotrophoblast already produces AFP in the early stages of pregnancy (the transient presence of the AFP gene has been confirmed) before its production begins in the yolk sac at 6 weeks gestation. At the time of delivery, the concentration of AFP in the intervillous space of the placenta is higher than in maternal serum and similar to the concentration in the umbilical cord. During this period of pregnancy, this results from the transfer from the fetal blood through the villi of the placenta. In the case of a normally developed fetus, specific situations such as damage to the syncytiotrophoblast of the placental villi or vessels passing through the basal plate (larger placental volume, abnormal basal plate structure, chronic inflammation of the villi, and placental infractions) can lead to increased leakage of AFP from the fetus to maternal serum [39][40].
References
- Flick, A.; Krakow, D.; Martirosian, A.; Silverman, N.; Platt, L.D. Routine measurement of amniotic fluid alpha-fetoprotein and acetylcholinesterase: The need for a reevaluation. Am. J. Obstet. Gynecol. 2014, 211, 139.e1–139.e6.
- Lopez, J.; Mikaelian, I.; Gonzalo, P. Amniotic fluid glial fibrillary acidic protein (AF-GFAP), a biomarker of open neural tube defects. Prenat. Diagn. 2013, 33, 990–995.
- Dong, N.; Gu, H.; Liu, D.; Wei, X.; Ma, W.; Ma, L.; Liu, Y.; Wang, Y.; Jia, S.; Huang, J.; et al. Complement factors and alpha-fetoprotein as biomarkers for noninvasive prenatal diagnosis of neural tube defects. Ann. N. Y. Acad. Sci. 2020, 1478, 75–91.
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.P.; Finnell, R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019, 111, 1455–1467.
- Copp, A.J.; Stanier, P.; Greene, N.D. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 12, 799–810.
- Kole, M.J.; Fridley, J.S.; Jea, A.; Bollo, R.J. Currarino syndrome and spinal dysraphism. J. Neurosurg. Pediatr. 2014, 13, 685–689.
- Holmes, L.B.; Toufaily, M.H.; Westgate, M.N. Iniencephaly. Birth Defects Res. 2018, 110, 128–133.
- Dennis, J.F.; Kurosaka, H.; Iulianella, A.; Pace, J.; Thomas, N.; Beckham, S.; Williams, T.; Trainor, P.A. Mutations in Hedgehog acyltransferase (Hhat) perturb Hedgehog signaling, resulting in severe acrania-holoprosencephaly-agnathia craniofacial defects. PLoS Genet. 2012, 8, e1002927.
- Mizejewski, G.J. Levels of alpha-fetoprotein during pregnancy and early infancy in normal and disease states. Obstet. Gynecol. Surv. 2003, 58, 804–826.
- Everman, D.B.; Shuman, C.; Dzolganovski, B.; O’riordan, M.A.; Weksberg, R.; Robin, N.H. Serum α-fetoprotein levels in Beckwith-Wiedemann syndrome. J. Pediatr. 2000, 137, 123–127.
- Clericuzio, C.L.; Chen, E.; McNeil, D.E.; O’Connor, T.; Zackai, E.H.; Medne, L.; Tomlinson, G.; DeBaun, M. Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J. Pediatr. 2003, 143, 270–272.
- Aagaard-Tillery, K.M.; Buchbinder, A.; Boente, M.P.; Ramin, K.D. Beckwith-Wiedemann syndrome presenting with an elevated triple screen in the second trimester of pregnancy. Fetal Diagn. Ther. 2007, 22, 18–22.
- Guanciali-Franchi, P.; Di Luzio, L.; Iezzi, I.; Celentano, C.; Matarrelli, B.; Liberati, M.; Palka, G. Elevated maternal serum α-fetoprotein level in a fetus with Beckwith-Wiedemann syndrome in the second trimester of pregnancy. J. Prenat. Med. 2012, 6, 7–9.
- Itinteang, T.; Chibnall, A.M.; Marsh, R.; Dunne, J.C.; de Jong, S.; Davis, P.F.; Leadbitter, P.; Tan, S.T. Elevated Serum Levels of Alpha-Fetoprotein in Patients with Infantile Hemangioma Are Not Derived from within the Tumor. Front. Surg. 2016, 3, 5.
- Gnarra, M.; Behr, G.; Kitajewski, A.; Wu, J.K.; Anupindi, S.A.; Shawber, C.J.; Zavras, N.; Schizas, D.; Salakos, C.; Economopoulos, K.P. History of the infantile hepatic hemangioma: From imaging to generating a differential diagnosis. World J. Clin. Pediatr. 2016, 5, 273–280.
- Wojtulewicz, J.P.; Coakley, J.C. Alpha-fetoprotein—A potential biomarker of intestinal regeneration in the infant. Med. Hypotheses 2013, 81, 335–337.
- Gulczyński, J.; Świątkowska-Freund, M.; Paluchowski, P.; Hermann-Okoniewska, B.; Iżycka-Świeszewska, E. Limb body wall complex–the history of the entity and presentation of our series of cases. Pol. J. Pathol. 2019, 70, 33–41.
- Goldfine, C.; Haddow, J.E.; Knight, G.J.; Palomaki, G.E. Amniotic fluid alpha-fetoprotein and acetylcholinesterase measurements in pregnancies associated with gastroschisis. Prenat. Diagn. 1989, 9, 697–700.
- Palomaki, G.E.; Hill, L.E.; Knight, G.J.; Haddow, J.E.; Carpenter, M. Second-trimester maternal serum alpha-fetoprotein levels in pregnancies associated with gastroschisis and omphalocele. Obstet. Gynecol. 1988, 71, 906–909.
- Kutzner, D.K.; Wilson, W.G.; Hogge, W.A. OEIS complex (cloacal exstrophy): Prenatal diagnosis in the second trimester. Prenat. Diagn. 1988, 8, 247–253.
- Sana, M.K.; Rentea, R.M. Pentalogy of Cantrell. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Kylat, R.I. Complete and Incomplete Pentalogy of Cantrell. Children 2019, 6, 109.
- Clement, K.; Chamberlain, P.; Boyd, P.; Molyneux, A. Prenatal diagnosis of an epignathus: A case report and review of the literature. Ultrasound Obstet. Gynecol. 2001, 18, 178–181.
- Tonni, G.; Centini, G.; Inaudi, P.; Rosignoli, L.; Ginanneschi, C.; De Felice, C. Prenatal diagnosis of severe epignathus in a twin: Case report and review of the literature. Cleft Palate-Craniofacial J. 2010, 47, 421–425.
- Brady, T.B.; Mitra, A.G.; Hooks, J. Maternal serum alpha-fetoprotein levels peak at 19–21 weeks’ gestation and subsequently decline in an NPHS1 sequence variant heterozygote; implications for prenatal diagnosis of congenital nephrosis of the Finnish type. Prenat. Diagn. 2014, 34, 812–814.
- Patrakka, J.; Martin, P.; Salonen, R.; Kestilä, M.; Ruotsalainen, V.; Männikkö, M.; Ryynänen, M.; Rapola, J.; Holmberg, C.; Tryggvason, K.; et al. Proteinuria and prenatal diagnosis of congenital nephrosis in fetal carriers of nephrin gene mutations. Lancet 2002, 359, 1575–1577.
- Slavotinek, A.; Kaylor, J.; Pierce, H.; Cahr, M.; DeWard, S.J.; Schneidman-Duhovny, D.; Alsadah, A.; Salem, F.; Schmajuk, G.; Mehta, L. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am. J. Hum. Genet. 2015, 96, 162–169.
- Lamont, R.E.; Tan, W.H.; Innes, A.M.; Parboosingh, J.S.; Schneidman-Duhovny, D.; Rajkovic, A.; Pappas, J.; Altschwager, P.; DeWard, S.; Fulton, A.; et al. Expansion of phenotype and genotypic data in CRB2-related syndrome. Eur. J. Hum. Genet. 2016, 24, 1436–1444.
- Prasun, P. Multiple Acyl-CoA Dehydrogenase Deficiency. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2020.
- Chisholm, C.A.; Vavelidis, F.; Lovell, M.A.; Sweetman, L.; Roe, C.R.; Roe, D.S.; Frerman, F.E.; Wilson, W.G. Prenatal diagnosis of multiple acyl-CoA dehydrogenase deficiency: Association with elevated ?-fetoprotein and cystic renal changes. Prenat. Diagn. 2001, 21, 856–859.
- Nesin, M.; Seymour, C.; Kim, Y. Role of elevated alpha-fetoprotein in prenatal diagnosis of junctional epidermolysis bullosa and pyloric atresia. Am. J. Perinatol. 1994, 11, 286–287.
- Drugan, A.; Vadas, A.; Sujov, P.; Gershoni-Baruch, R. Markedly elevated alpha-fetoprotein and positive acetylcholinesterase in amniotic fluid from a pregnancy affected with dystrophic epidermolysis bullosa. Fetal Diagn. Ther. 1995, 10, 37–40.
- Leschot, N.J.; Treffers, P.E.; Becker-Bloemkolk, M.; van Zanten, S.; de Groot, W.; Verjaal, M. Severe congenital skin defects in a newborn. Case report and relevance of several obstetrical parameters. Eur. J. Obstet. Gynecol. Reprod. Biol. 1980, 10, 381–388.
- Mazza, J.M.; Klein, J.F.; Christopher, K.; Silverberg, N.B. Aplasia cutis congenita in a setting of fetus papyraceus associated with small fetal abdominal circumference and high alpha-fetoprotein and amniotic acetylcholinesterase. Pediatr. Dermatol. 2013, 32, 138–140.
- Gerber, M.; de Veciana, M.; Towers, C.V.; Devore, G.R. Aplasia cutis congenita: A rare cause of elevated α-fetoprotein levels. Am. J. Obstet. Gynecol. 1995, 172, 1040–1041.
- Lakhi, N.A.; Mizejewski, G.J. Alpha-fetoprotein and Fanconi Anemia: Relevance to DNA Repair and Breast Cancer Susceptibility. Fetal Pediatr. Pathol. 2017, 36, 49–61.
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Second trimester maternal serum alpha-fetoprotein (MSAFP) as predictor of fetal hemoglobin Bart’s disease. Prenat. Diagn. 2014, 34, 1277–1282.
- Wanapirak, C.; Piyamomgkol, W.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Jatavan, P.; Tongsong, T. Second-trimester maternal serum screening for fetal Down syndrome: As a screening test for hemoglobin Bart’s disease: A prospective population-based study. Prenat. Diagn. 2018, 38, 700–705.
- Brownbill, P.; Edwards, D.; Jones, C.; Mahendran, D.; Owen, D.; Sibley, C.; Johnson, R.; Swanson, P.; Nelson, D.M. Mechanisms of alphafetoprotein transfer in the perfused human placental cotyledon from uncomplicated pregnancy. J. Clin. Investig. 1995, 96, 2220–2226.
- Lafuste, P.; Robert, B.; Mondon, F.; Danan, J.L.; Rossi, B.; Duc-Goiran, P.; Mignot, T.M.; Nunez, E.; Benassayag, C.; Ferré, F. Alpha-fetoprotein gene expression in early and full-term human trophoblast. Placenta 2002, 23, 600–612.




