Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shengjie Wang | -- | 3668 | 2024-01-22 14:04:51 | | | |
| 2 | Lindsay Dong | Meta information modification | 3668 | 2024-01-23 02:16:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tang, C.; Li, X.; Hu, Y.; Du, X.; Wang, S.; Chen, B.; Wang, S. Porphyrin-Based Metal-Organic Framework Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/54198 (accessed on 07 February 2026).
Tang C, Li X, Hu Y, Du X, Wang S, Chen B, et al. Porphyrin-Based Metal-Organic Framework Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/54198. Accessed February 07, 2026.
Tang, Chuanyin, Xiaoyu Li, Yingxu Hu, Xin Du, Shuo Wang, Bo Chen, Shengjie Wang. "Porphyrin-Based Metal-Organic Framework Materials" Encyclopedia, https://encyclopedia.pub/entry/54198 (accessed February 07, 2026).
Tang, C., Li, X., Hu, Y., Du, X., Wang, S., Chen, B., & Wang, S. (2024, January 22). Porphyrin-Based Metal-Organic Framework Materials. In Encyclopedia. https://encyclopedia.pub/entry/54198
Tang, Chuanyin, et al. "Porphyrin-Based Metal-Organic Framework Materials." Encyclopedia. Web. 22 January, 2024.
Copy Citation
Metal-organic frameworks (MOFs) are a novel category of porous crystalline materials with an exceptionally high surface area and adjustable pore structure. They possess a designable composition and can be easily functionalized with different units. Porphyrins with conjugated tetrapyrrole macrocyclic structures can absorb light from ultraviolet to visible light regions, and their structures and properties can be facilely regulated by altering their peripheral groups or central metal ions. Porphyrin-based MOFs constructed from porphyrin ligands and metal nodes combine the unique features of porphyrins and MOFs as well as overcoming their respective limitations.
metal-organic frameworks
porphyrin
photoelectric transmission
1. Introduction
The utilization of fossil energy brings great benefits to human beings. However, over reliance on traditional fossil energy such as coal and petroleum results in serious problems such as energy shortages and environment pollution [1][2]. People search for alternative energy resources to reduce their reliance on traditional fossil fuels [3][4][5]. As is known, solar energy is an important alternative energy source. About 120,000 terawatts (TW) of solar energy reaches the earth’s surface every year, far exceeding the global total energy demand [6]. Moreover, solar energy has emerged as a crucial sustainable energy resource due to its abundance, cleanliness, affordability, and inexhaustibility. However, efficient capturing and utilization of solar energy is still challenging because of its lower energy density and its being affected by the weather, season, and geographical position. Therefore, solar energy is converted to thermal energy, electrical energy, and chemical energy to solve the problem of direct utilization.
Photocatalytic technology is one of the most promising methods to solve both environmental and energy problems due to its simple reaction conditions, low cost, lack of secondary pollution, and environmental protection [7], and it exhibits extensive applications in hydrogen evolution, nitrogen fixation, CO2 reduction, as well as the elimination of organic and inorganic pollutants. The design and development of efficient photocatalysts attract great attention since photocatalysts play a crucial role in photocatalytic reactions [8]. So far, metal oxides [9][10], metal phosphides [11][12], metal sulfides [13][14][15], and carbon-based composites [16][17][18][19] have been developed and used in energy conversion systems. However, these traditional photocatalysts suffered from different challenges such as low electronic conductivity, low apparent quantum efficiency, rapid recombination of electron-hole pairs, poor chemical stability and cycle stability, and so on [20]. Therefore, the construction of photocatalysts with high performance and physical/chemical stability holds great promise in addressing the current energy and environmental issues.
Metal-organic frameworks (MOFs) are crystalline nanoporous hybrid materials composed of metal nodes connected by organic ligands [21]. They can be modularly assembled with suitable inorganic and organic building units, allowing for the design of functional frameworks with specific topologies, adjustable porosity, and desirable properties [22]. Different from other porous compounds such as zeolites, MOFs are easily modifiable. The products can be obtained under relatively simple conditions and thus obtain good specificity and selectivity. The rich coordination chemistry of MOFs enables the adjustment of their photoelectric properties by changing their composition [23]. In this context, MOFs can be widely used in various fields including gas storage and separation [24][25], photocatalysis [22][26][27], sensors [28][29], pollutant degradation [30][31], ion exchange [32], and so on.
2. Photocatalytic Mechanism
2.1. Light Absorption
The light absorption of semiconductors is determined by their band gap. According to the band theory, the energy gap of MOFs depends on the distance between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels, which correspond to VB and CB of inorganic semiconductors [20][33][34], respectively (Figure 1a,b). Photocatalysts absorb light energy and facilitate the transition of electrons from HOMO to LUMO. Charge transfer from ligand to metal cluster or ligand (n→π*, π→π*) constitutes the majority of the charge transition in MOFs under irradiation (Figure 1c) [35].
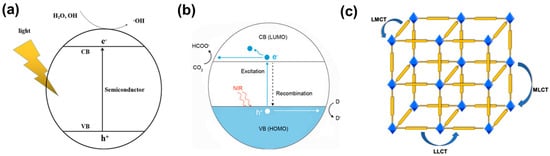
Figure 1. (a) Photocatalytic mechanism of the semiconductor [36]; (b) mechanism of MOF photocatalytic reduction of CO2 [37]; (c) schematic diagram of charge transfer pathways in MOFs, such as LMCT, LLCT, and MLCT.
2.2. Charge Transfer Pathway
The combination of organic ligands and metal clusters in MOFs endows them with semiconductor behavior and a similar principle of photocatalysis [38][39]. Metal clusters and photoactive ligands absorb light energy and result in photo-induced charge separation, that is, electrons with reducing ability e− and holes with oxidizing ability h+. Then the photo-generated charges migrate to the surface of the photocatalyst to participate in the redox reaction. That is to say, metal clusters accept electrons generated by organic ligands for subsequent redox reactions [40]. Transferring e− from the highest occupied molecular orbital (HOMO) of organic ligands to the lowest unoccupied molecular orbital (LUMO) of metal clusters is labeled as the ligand-to-metal charge transfer (LMCT) pathway in MOFs [41].
3. Design and Construction of Porphyrin-Based MOFs
3.1. Design of Porphyrin-Based MOFs
Some drawbacks of MOFs including rapid recombination and deactivation of photo-generated charges as well as poor cycling stability greatly impede their large-scale application in photocatalysis; although, MOFs and their composite materials possess advantages of tunable pores, high porosity, and a large surface area. These limitations hinder the efficient utilization and long-term stability of MOFs in photocatalytic processes. Porphyrins and their derivatives have excellent light absorption capacity and are easily coordinated with metal ions, which facilitates addressing these problems of MOFs. Therefore, the incorporation of porphyrins into MOF materials endows them with the advantages of porphyrin molecules and MOF materials.
The chemical properties and coordination abilities can also be regulated by introducing different functional groups onto porphyrin molecules. For example, the electron affinity and electron conductivity of porphyrins can be changed by the introduction of various substituents or modified peripheral groups [42][43][44]. This modification allows for the attainment of appropriate orbital energy levels and band gaps, and results in the construction of porphyrin-based MOFs with exceptional light-harvesting capabilities and efficient charge separation and transfer efficiencies. 5,10,15,20-tetrakis(4-carboxyphenyl) porphyrin (TCPP) with the capacity to coordinate with Zr, Cu, Al, Cr, La, and other metals, is the most commonly used organic linker in porphyrin-based MOFs.
3.2. Construction of Porphyrin-Based MOFs
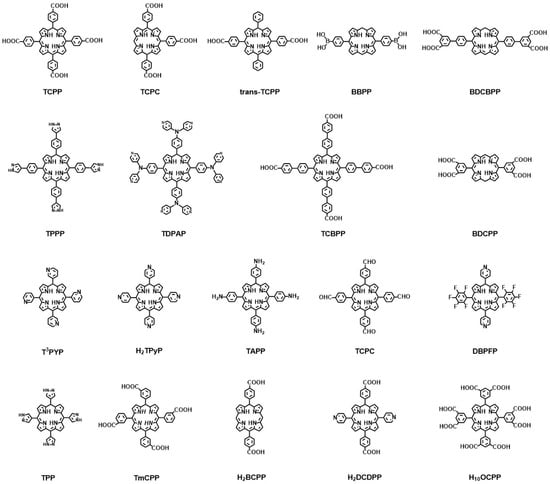
In porphyrin-based MOFs, porphyrin or metal porphyrin molecules act as organic ligands to coordinate with metal ions or metal clusters. The porphyrin linkers in porphyrin-based MOFs mainly include carboxylic acids, pyridines, and polyazole groups [45][46][47]. The following are most of the porphyrin ligands that are commonly used in porphyrin-based MOFs, shown in Figure 2 [48][49][50][51][52][53][54][55].

Figure 2. Representative chemical structures of porphyrin ligands in porphyrin-based MOFs.
3.2.1. Porphyrin-Based MOFs with Carboxylic Acid Linkers
The porphyrin ligand with a carboxylic acid linker is the most widely used in the construction of porphyrin-based MOFs. It is connected to the metal center through a flexible coordination mode, resulting in a controllable and adjustable ligand structure. Such porphyrin-based MOFs usually exhibit high stability and controllability in structures and are suitable for application in gas adsorption, catalysis, and photocatalysis.
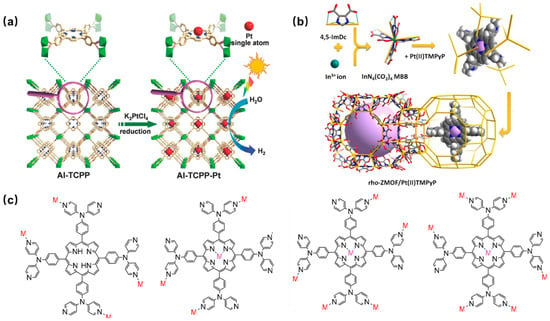
An atomically Pt-dispersed aluminum-porphyrin-based MOF (Al-TCPP-Pt) was synthesized using tetracarboxylic porphyrin (H2TCPP) as the organic ligand to interconnect Al(OH)O4 chains by a hydrothermal reaction and a subsequent Pt(II) metalation [56]. The hybrid Al-TCPP-Pt MOFs showed significantly enhanced photocatalytic activity, utilizing the highly efficient electron transfer channel provided by platinum (Pt) atoms (Figure 3a).
3.2.2. Porphyrin-Based MOFs with Nitrogen-Containing Heterocyclic Linkers
Nitrogen-containing heterocyclic ligands include imidazole, polyazole, pyridine, pyrazole ligands, and so on. The coordination strength between nitrogen atoms and metal ions is greater than that between oxygen atoms and metal ions. Therefore, MOFs composed of nitrogen-containing heterocyclic ligands exhibit comparable chemical and thermal stability to carboxylic acid-based MOFs. At the same time, nitrogen-containing heterocyclic MOFs may exhibit better stability than carboxylic MOFs under extreme pH conditions through appropriate metal-ligand matching.
In 1994, Robson and coauthors [57] first reported a coordinated framework material containing Cu(II) and neutral pyridyl-substituted porphyrin units. Since then, an increasing number of porphyrin-based MOFs have been reported. Pyridine-containing porphyrin-based MOFP was prepared by encapsulating platinum metalized porphyrin (Pt(II)TMPyP) in a rho zeolite-like metal-organic framework (rho-ZMOF) via a post-modification method (Figure 3b). They exhibited excellent sensitivity and selectivity for various anions in both aqueous and methanol media, leading to a significant enhancement in the detection limit of anions [58]. In addition, a novel pyridine-containing porphyrin ligand 5,10,15,20-tetrakis (4,4-dipyridylaminophenylene) porphyrin (TDPAP) was designed and synthesized, and introduced four peripheral 4,4′-bipyridylamine moieties in the porphyrin platform (Figure 3c) [59].

Figure 3. (a) Schematic diagram of the preparation of Al-TCPP-Pt photocatalyst [56]; (b) synthesis method of Pt(II)TMPyP/rho-ZMOF composites [58]; (c) four coordination modes of porphyrin TDPAP [59].
4. Improvement in Photocatalytic Performance of Porphyrin-Based MOFs
4.1. Modification of Porphyrin Ligands
As one of the important components of MOFs, organic ligands play a crucial role in determining their topological structure, pore size, shape, and surface properties. They serve as the main supporting unit of the framework and their size and shape affect the aforementioned characteristics [60][61]. In addition, organic ligands in MOFs act as ‘photosensitive antennas’, determine the light-capturing ability, and are responsible for the charge transfer processes of LMCT or MLCT pathways [62][63]. Therefore, the rational selection and modification of organic ligands can remarkably improve the photocatalytic ability of MOFs. As the most commonly used photosensitive ligand with a large π structure, porphyrin has become an important target for ligand modification and hybridization strategies.
4.1.1. The Introduction of Metal Coordination Center
The modification of porphyrin ligands mainly refers to the insertion of metal ions into the porphyrin core to form metalloporphyrin complexes [64]. After metalation, the coordination bonds are formed between the metal ion and the porphyrin ring which enhances the symmetry of the porphyrin molecule, resulting in stronger absorption in the visible and near-infrared regions and suppressed recombination of photo-generated electron-hole pairs [65][66][67][68][69][70][71]. The optical properties and catalytic activity of metalloporphyrin-based MOFs can be regulated by selecting different metal ions and controlling the structure of the porphyrin ligands.
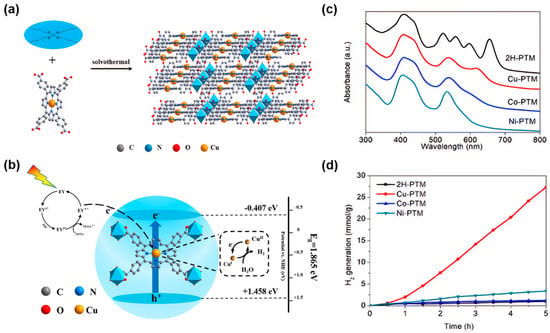
The stability and biocompatibility of the materials can be enhanced to meet the requirements for different applications. For example, a new ligand-to-linker metal charge-transfer (LLMCT) pathway was found by anchoring non-precious metal Cu in the center of the porphyrin in Ti-porphyrin-based MOFs (Figure 4a,c). The photo-generated electrons initially originated from the excited eosin Y (EY*) can transfer from the lowest unoccupied molecular orbital (LUMO) level of the porphyrin to the transient CuI/CuII center via rapid delocalization through the conjugated 18-π electron system of the porphyrin. This can reduce the width of the bandgap and at the same time, improve the charge separation and transfer rate, thereby enhancing the charge transfer efficiency (Figure 4b). Experimental results show that the addition of metal ions in MOFs can achieve a hydrogen evolution rate of 5465.689 μmol g−1 h−1, approximately 27.71 times that of the pristine MOFs (Figure 4d), indicating the Cu in porphyrin core plays a key role in improving the photocatalytic performance [72].

Figure 4. (a) Synthesis of Cu-PTM [72]; (b) photocatalytic mechanism of Cu-PTM for hydrogen evolution under visible light [72]; (c) UV-visible spectra of 2H-PTM, Cu-PTM, Co-PTM, and Ni-PTM [72]; (d) photocatalytic hydrogen evolution diagrams in the presence of 2H-PTM, Cu-PTM, Co-PTM, and Ni-PTM photocatalysts [72].
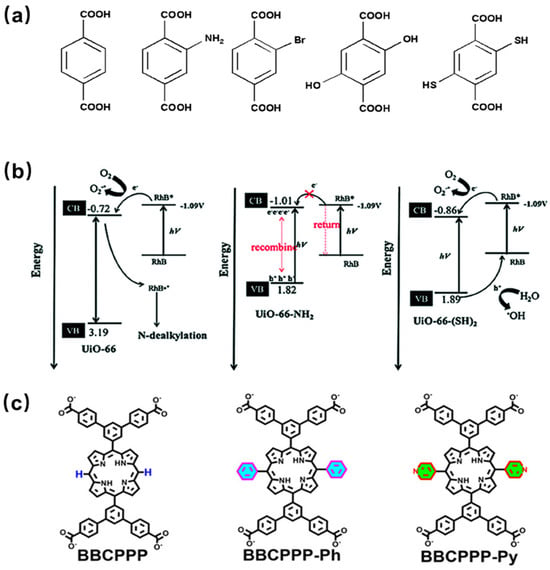
4.1.2. Incorporation of Functional Groups
The structures and properties of MOFs depend on the organic ligands and metal clusters and can be regulated by the incorporation of active ingredients. In addition, by functionalizing organic linkers (ligands) with other groups (such as amino), the light capture properties of MOFs can be regulated [73]. Therefore, the incorporation of functional groups on the ligand can optimize the structure and properties of the materials, such as increasing the porosity and surface area, decreasing the band gap, and thus improving the catalytic performance of MOFs. A series of isomorphic UiO-66-X (X = H, NH2, Br, (OH)2, (SH)2) catalysts have been successfully synthesized by incorporating various functional groups in the ligands (Figure 5a). The ligand modification of UiO-66 was carried out to enhance the photocatalytic activity for the degradation of Rhodamine B under visible light. The results showed that UiO-66-NH2 and UiO-66-(OH)2 have narrow band gaps and good visible light absorption, but they cannot effectively catalyze the degradation of organic dyes, possibly due to their conduction band being close to the redox potential of Rhodamine B (Figure 5b) [74]. However, different results can be obtained when amino groups are incorporated in other MOFs. MIL-125-NH2 modified with amino functional groups can decompose 97% of Congo Red dye under 3 h of irradiation, while the original material MIL-125 can only degrade 60% pollute [75]. Three RE-PMOFs (BUT-224/225/226) with new topological structures were successfully obtained by introducing phenyl/pyridine groups in the middle of the porphyrin core (Figure 5c) and adjusting the symmetry and connectivity of the ligands. In addition, BUT-225 (Co) exhibits stronger CO2 absorption capacity and higher catalytic activity than other MOFs during CO2 reduction [76].

Figure 5. (a) Molecule structure of functionalized terephthalic acid linker, H2BDC-X [74]; (b) band gap diagram, energy transfer between UiO-66 and UiO-66-NH2, and the degradation mechanism of Rhodamine B over UiO-66-(SH)2 [74]; (c) the chemical structures of BBCPPP, BBCPPP-Ph, and BBCPPP-Py [76].
4.2. Construction of Donor-Acceptor System
The design of the internal structure of MOFs is an alternative strategy for high-powered photocatalysts. The donor-acceptor (D-A) system, which involves the interaction between electron-rich and electron-deficient organic components, has garnered significant interest from researchers. This system shows promising potential for optical and electrical materials. The unique electronic properties of the component endow the D-A system with different electron transfer behaviors between the donor and acceptor components [77][78], which lays the foundation for the charge transfer (CT) and energy transfer (ET) of MOFs containing the D-A system. The different connection or packaging of the donor and acceptor components will affect their mode of interaction, thereby determining the properties of the system.
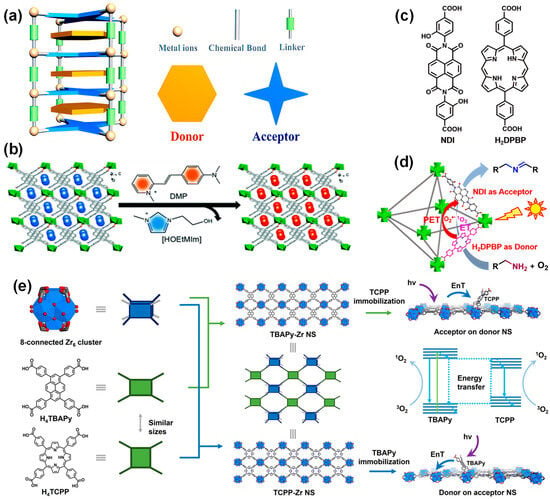
Photo-induced electron transfer (PET) can be achieved by assembling electron donor and acceptor chromophores in a single MOF. Weak interactions, such as electrostatic and hydrogen bonding between the molecular entities of the donor and acceptor, provide a long-range ordered electron transfer channel and help to inhibit the recombination of charges [79]. In addition, MOFs provide an unprecedented level of controlling the D-A system layout through the design of metal nodes and organic linkers, the modularity of the framework topology, and the combination of different subjects (Figure 6a) [80].
4.2.1. Effect of D-A System in Promoting Light Harvesting
A donor-acceptor system containing MOFs with molecular-level heterojunctions was synthesized from a linear trinuclear Mn(II) cluster, a 1,1,3,6-tetraphenyl pyrene-based photoactive linker, and a D-π-A cationic dye cyanine (DMP) under isothermal conditions (Figure 6b) [81]. The interplay between the chromophore and energy acceptor, and the bandgap can be adjusted to a large extent. The resulting MOFs exhibited unique light absorption across the UV/visible to NIR light region, extremely high luminescence polarization anisotropy (0.97), and high light responsivity.

Figure 6. (a) Diagram of donor-acceptor-based MOF structure [80]; (b) schematic diagram of the ion exchange experiment by the incorporation of DMP cations where a, b and c represents the 3D spatial directions [81]; (c) chemical structure of the NDI and H2DPBP ligands [82]; (d) photocatalytic mechanism of amine coupling reaction over the mixed ligands Zr-NDI-H2DPBPMOF based on donor H2DPBP and acceptor NDI [82]; (e) schematic diagrams of the SBUs for the construction of TBAPy-Zr NS and TCPP-Zr NS (left), schematic illustration of the acceptor-on-donor-NS model (right, top), the EnT process (right, middle), and the donor-on-acceptor-NS model (right, bottom) [83].
4.2.2. Effect of D-A System in Facilitating Electron Separation
The porphyrin-based MOF based on the donor-acceptor system also exhibits excellent performance in promoting electron separation. For example, a mixed ligand MOF (Zr-NDI-H2DPBP) photocatalyst was constructed using 5,15-bis (p-benzoic acid) porphyrin (H2DPBP) as the electron donor and naphthalene diimide (NDI) as the electron acceptor (Figure 6c). The prepared MOFs can produce high-efficiency photo-induced electron transfer, optimize charge separation efficiency, and thus improve photocatalytic performance (Figure 6d).
Diverse ligands are used to construct the donor-acceptor system, and the catalytic performance of prepared MOFs can be affected by the functions and properties of the ligands. MOFs with high energy transfer efficiency can be constructed by selecting tetrakis (4-carboxyphenyl) porphyrin (H4TCPP) and 1,3,6,8-tetrakis(p-benzoic acid) pyrene (H4TBAPy) as the acceptor and donor parts, respectively. The results demonstrate that the energy transfer between the TBAPy donor and the TCPP acceptor can be fulfilled. The energy transfer efficiency of the TBAPy-Zr nanosheets with 22% TCPP reached 82% (Figure 6e).
4.3. The Introduction of Co-Catalysts
Photocatalysts are catalysts that can utilize light energy to catalyze reactions. However, the performance of photocatalysts may not be ideal in some applications, which makes it challenging to achieve efficient photocatalytic reactions. The introduction of co-catalysts is a recognized approach to enhance the performance of photocatalysts and amplify their advantageous effectiveness. Unlike optimizing the internal composition and structure design of MOFs, the introduction of co-catalysts can provide additional active sites to enhance the reaction rate. It can also modulate the band structure of the photocatalysts, alter their light absorption and photoelectron transfer properties, accelerate charge transfer, and suppress charge recombination [84][85].
5. Application of Porphyrin-Based MOFs in Photocatalysis
5.1. Photocatalytic Hydrogen Evolution
Hydrogen, a clean and efficient energy resource, produced from solar energy-driven water splitting exhibits great prospects [86][87][88][89]. The visible light absorption of MOFs is effectively broadened when porphyrin ligands are introduced. At the same time, specific active groups such as co-catalysts can be introduced into the larger pore size of MOFs, which endows porphyrin-based MOFs with great potential and advantages in using solar energy to produce hydrogen [90].
The activity in photocatalytic hydrogen evolution of MOFs can be improved by the introduction and assembly of active metals in MOF frameworks. Porphyrin-based MOFs ([FeFe]@ZrPF) have high stability and high photocatalytic hydrogen production efficiency. In this system, the porphyrin unit is used as a photosensitizer, and the iron hydride enzyme is used as a hydrogen evolution catalytic site. The short distance between the two parts and the nature of chemical bonding allows for the fast transfer of photo-generated carriers. The chemical bonding within the materials can provide robust hydrogen elution systems by avoiding the use of electron mediators to transfer electrons from the photosensitizer to the catalyst [89].
5.2. Photocatalytic CO2 Reduction
Fossil fuels including gasoline, oil, coal, and natural gas are used in large quantities every day, and excessive amounts of carbon dioxide are discharged into the air, which causes increasingly serious environmental problems [91][92][93][94]. Therefore, the collection and conversion of carbon dioxide into renewable energy fuels or high-value-added compounds have attracted great interest from scientists and entrepreneurs around the world, and various strategies for converting carbon dioxide into usable substances have been developed [95]. Among them, one of the most promising and economical methods is the photocatalytic reduction of carbon dioxide driven by solar energy [96][97].
Synthesis of MOFs with appropriate organic linkers to promote light absorption is the first step in the photocatalytic reduction of CO2. So far, various organic linkers including porphyrin-based, metal complex-functionalized, and amine-functionalized organic linkers have been used to regulate the band gap of MOF photocatalysts. In 2013, Fu and coauthors [98] demonstrated for the first time that MOF photocatalysts can photocatalytically reduce CO2 to HCOO−.
The stability of MOFs can be enhanced by in situ substitution of porphyrin. A labile MOF (BUT-109(Zr)) was converted to a stable porphyrin-based MOF (BUT-110) by replacing a naphthalene diimide dibenzoate (NDIDB2−) with a size- and geometry-matching porphyrin ligand (DCPP2−). Compared with BUT-109(Zr), BUT-110 MOFs with different porphyrin contents showed various chemical stability enhancements. The obtained BUT-110 material exhibited excellent stability at a wide pH range (1–10) when the porphyrin content exceeded 50%.
5.3. Photocatalytic Synthesis of Organic Compounds
Singlet oxygen is a reactive oxygen species that can be used for many catalytic transformations [99][100][101][102][103]. For the photocatalysts, their ability to effectively generate singlet oxygen and prolong its lifetime is crucial for their use in photooxidation reactions such as sulfide oxidation and amine oxidation.
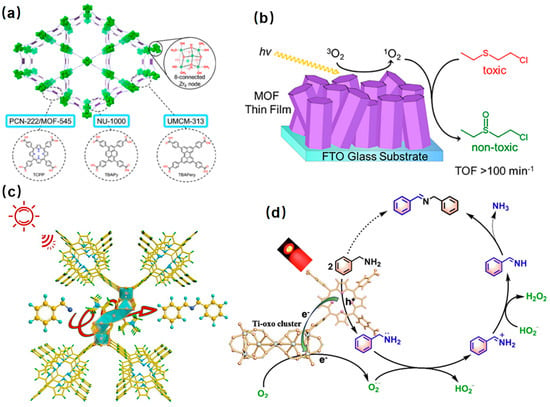
Sulfoxide is an indispensable intermediate in pharmaceutical, agrochemical, and other fine chemical industries [104][105]. The thionyl group in the sulfoxide molecule is chemically unstable and easily overoxidized to sulfone. Strong oxidants such as potassium permanganate or dichromate are usually used for the oxidation of sulfoxide, resulting in serious environmental pollution [106]. Porphyrin-based metal-organic frameworks have remarkable advantages in the photocatalytic oxidation of sulfoxide due to their wide absorption from ultraviolet to the visible light region, high chemical stability, and environmental friendliness. PCN-222/MOF-545, NU-1000, and UMCM-313, MOFs with porphyrin, pyrene, and perylene ligands, respectively, were used as photocatalysts to complete the oxidation of 2-chloroethyl ethyl sulfide (CEES) using the generated singlet oxygen under LED irradiation (Figure 7a,b) [102].

Figure 7. (a) Structure of NU-1000, PCN-222/MOF-545, and UMCM-313 and their corresponding linkers and common Zr6-node [102]. (b) Mechanistic diagram of photocatalytic oxidation of 2-chloroethyl ethyl sulfide over the MOF thin film [102]; (c) selective photocatalytic oxidation of amines on Ti-PMOF-DMA under red LED irradiation [107]; (d) mechanism of selective photocatalytic aerobic oxidation of benzylamine over Ti-PMOF-DMA [107].
Imines are important biomedical intermediates with excellent pharmacological and biological activities. The Ti-porphyrin-based metal-organic framework (Ti-PMOF-DMA) was synthesized by solvent-controlled synthesis, showed excellent catalytic activity, and was selective in the photocatalytic aerobic oxidation of benzylamine. The results showed that benzylamine could be oxidized to N-benzylbenzaldehyde diamine in 40 min, achieving 94% conversion and 88% selectivity (Figure 7c,d).
5.4. Photocatalytic Removal of Pollutants
Environmental issues related to clean energy and pollution have become increasingly prominent. In the past few decades, a large quantity of pollutants has been discharged into the environment due to urbanization and population growth [108][109][110][111]. Organic dyes pose a serious threat to the environment and are difficult to remove from the environment due to their varying resistance to photodecomposition and oxidation [112]. So far, many technologies have been developed and used to remove harmful pollutants in the environment, such as adsorption [113], biological oxidation [114], chemical treatment, and photo-degradation [115][116].
5.5. Photocatalytic Nitrogen Fixation
Nitrogen fixation, as it can convert atmospheric nitrogen into nitrogen-containing molecules that can be used by all living organisms including humans, has been regarded as one of the most important natural processes in the Earth’s nitrogen cycle. For example, ammonia (NH3) is the most common industrial chemical and carbon-free energy storage molecule [117]. However, the industrial preparation of NH3 is a highly energy-intensive and environmentally unfriendly process. Therefore, an alternative sustainable method for efficient generation of NH3 from N2 under mild conditions is sorely needed to alleviate the increasing energy and environmental issues. Porphyrin-based MOFs are highly porous crystalline materials with large surface areas and highly ordered pores, which can effectively enhance N2 adsorption. Porphyrin molecules, as a strong photosensitive linker, can significantly broaden the light response of MOFs. Furthermore, the photocatalytic nitrogen fixation efficiency can be significantly improved by rationally designed porphyrin-based MOFs to enhance the separation of photo-generated charges [118].
References
- McGlade, C.; Ekins, P. The geographical distribution of fossil fuels unused when limiting global warming to 2 degrees C. Nature 2015, 517, 187–190.
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034.
- Liu, X.; Liu, Y.; Zhang, W.; Zhong, Q.; Ma, X. In situ self-assembly of 3D hierarchical 2D/2D CdS/g-C3N4 hereojunction with excellent photocatalytic performance. Mater. Sci. Semicond. Proc. 2020, 105, 104734.
- Wang, S.; Li, M.; Patil, A.J.; Sun, S.; Tian, L.; Zhang, D.; Cao, M.; Mann, S. Design and construction of artificial photoresponsive protocells capable of converting day light to chemical energy. J. Mater. Chem. A 2017, 5, 24612–24616.
- Liu, F.; Cao, H.; Xu, L.; Fu, H.; Sun, S.; Xiao, Z.; Sun, C.; Long, X.; Xia, Y.; Wang, S. Design and preparation of highly active TiO2 photocatalysts by modulating their band structure. J. Colloid Interface Sci. 2023, 629, 336–344.
- Zhang, Y.; Ren, K.; Wang, L.; Wang, L.; Fan, Z. Porphyrin-based heterogeneous photocatalysts for solar energy conversion. Chin. Chem. Lett. 2022, 33, 33–60.
- Ni, Y.; Yan, K.; Xu, F.; Zhong, W.; Zhao, Q.; Liu, K.; Yan, K.; Wang, D. Synergistic effect on TiO2 doped poly (vinyl alcohol-co-ethylene) nanofibrous film for filtration and photocatalytic degradation of methylene blue. Compos. Commun. 2019, 12, 112–116.
- Qian, Y.T.; Zhang, F.F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231.
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO(2) photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661.
- Nguyen, C.T.K.; Quang Tran, N.; Seo, S.; Hwang, H.; Oh, S.; Yu, J.; Lee, J.; Anh Le, T.; Hwang, J.; Kim, M.; et al. Highly efficient nanostructured metal-decorated hybrid semiconductors for solar conversion of CO2 with almost complete CO selectivity. Mater. Today 2020, 35, 25–33.
- Cao, S.; Chen, Y.; Wang, H.; Chen, J.; Shi, X.; Li, H.; Cheng, P.; Liu, X.; Liu, M.; Piao, L. Ultrasmall CoP Nanoparticles as Efficient Cocatalysts for Photocatalytic Formic Acid Dehydrogenation. Joule 2018, 2, 549–557.
- Dong, H.; Xiao, M.; Yu, S.; Wu, H.; Wang, Y.; Sun, J.; Chen, G.; Li, C. Insight into the Activity and Stability of RhxP Nano-Species Supported on g-C3N4 for Photocatalytic H2 Production. ACS Catal. 2019, 10, 458–462.
- Yu, J.; Seo, S.; Luo, Y.; Sun, Y.; Oh, S.; Nguyen, C.T.K.; Seo, C.; Kim, J.H.; Kim, J.; Lee, H. Efficient and Stable Solar Hydrogen Generation of Hydrophilic Rhenium-Disulfide-Based Photocatalysts via Chemically Controlled Charge Transfer Paths. ACS Nano 2020, 14, 1715–1726.
- Sun, Q.; Wang, N.; Yu, J.; Yu, J.C. A Hollow Porous CdS Photocatalyst. Adv. Mater. 2018, 30, e1804368.
- Meng, X.-B.; Sheng, J.-L.; Tang, H.-L.; Sun, X.-J.; Dong, H.; Zhang, F.-M. Metal-organic framework as nanoreactors to co-incorporate carbon nanodots and CdS quantum dots into the pores for improved H2 evolution without noble-metal cocatalyst. Appl. Catal. B Environ. 2019, 244, 340–346.
- Velumani, A.; Sengodan, P.; Arumugam, P.; Rajendran, R.; Santhanam, S.; Palanisamy, M. Carbon quantum dots supported ZnO sphere based photocatalyst for dye degradation application. Curr. Appl. Phys. 2020, 20, 1176–1184.
- Syed, N.; Huang, J.; Feng, Y.; Wang, X.; Cao, L. Carbon-Based Nanomaterials via Heterojunction Serving as Photocatalyst. Front. Chem. 2019, 7, 713.
- Kundu, S.; Bramhaiah, K.; Bhattacharyya, S. Carbon-based nanomaterials: In the quest of alternative metal-free photocatalysts for solar water splitting. Nanoscale Adv. 2020, 2, 5130–5151.
- Srisasiwimon, N.; Chuangchote, S.; Laosiripojana, N.; Sagawa, T. TiO2/Lignin-Based Carbon Composited Photocatalysts for Enhanced Photocatalytic Conversion of Lignin to High Value Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 13968–13976.
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Chen, Y.; Ma, T.; Sun, X. Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Mater. 2023, 13, 2300086.
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Accounts. Chem. Res. 2001, 34, 319–330.
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020, 2, 555–568.
- Qin, Y.; Hao, M.; Li, Z. Metal-organic frameworks for photocatalysis. In Surface Science of Photocatalysis; Elsevier: London, UK, 2020; Volume 31, pp. 541–579.
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gomez-Gualdron, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303.
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472.
- Xin, X.; Zhao, Z.; Chen, Y.; Tan, J.; Shi, Y.; Ren, H.; Yang, D.; Jiang, Z. Dual-Ligand Ti-MOFs with Push-Pull Effect for Photocatalytic H(2) Production. ACS Appl. Mater. Interfaces 2023, 15, 1053–1062.
- Hussain, M.B.; Kang, B.; Cheng, X.; Ma, C.; Wang, X.; Mehmood, R.; Iqbal, S. Oxygen vacancy induced Pt-decorated MOF photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2023, 48, 13780–13790.
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing Conductive Metal-Organic Frameworks for Voltammetric Detection of Neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733.
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125.
- Rojas, S.; Garcia-Gonzalez, J.; Salcedo-Abraira, P.; Rincon, I.; Castells-Gil, J.; Padial, N.M.; Marti-Gastaldo, C.; Horcajada, P. Ti-based robust MOFs in the combined photocatalytic degradation of emerging organic contaminants. Sci. Rep. 2022, 12, 14513.
- Xie, W.; Yuan, Y.; Jiang, W.; Zhang, S.-R.; Xu, G.-J.; Xu, Y.-H.; Su, Z.-M. Heterogeneous activation of peroxymonosulfate by stable Co-MOF for the efficient degradation of organic dye pollutants. CrystEngComm 2022, 24, 6786–6792.
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal-organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615.
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339.
- Li, X.; Tang, C.; Zhang, L.; Song, M.; Zhang, Y.; Wang, S. Porphyrin-Based Covalent Organic Frameworks: Design, Synthesis, Photoelectric Conversion Mechanism, and Applications. Biomimetics 2023, 8, 171.
- Xu, C.; Pan, Y.; Wan, G.; Liu, H.; Wang, L.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. Turning on Visible-Light Photocatalytic C-H Oxidation over Metal-Organic Frameworks by Introducing Metal-to-Cluster Charge Transfer. J. Am. Chem. Soc. 2019, 141, 19110–19117.
- Li, S.; Luo, P.; Wu, H.; Wei, C.; Hu, Y.; Qiu, G. Strategies for Improving the Performance and Application of MOFs Photocatalysts. ChemCatChem 2019, 11, 2978–2993.
- Zeng, J.Y.; Wang, X.S.; Xie, B.R.; Li, Q.R.; Zhang, X.Z. Large pi-Conjugated Metal-Organic Frameworks for Infrared-Light-Driven CO(2) Reduction. J. Am. Chem. Soc. 2022, 144, 1218–1231.
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Account. Chem. Res. 2019, 52, 356–366.
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342.
- Alvaro, M.; Carbonell, E.; Ferrer, B.; LlabrésiXamena, F.X.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem.-Eur. J. 2007, 13, 5106–5112.
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097.
- Li, L.-L.; Yang, C.-J.; Chen, W.-H.; Lin, K.-J. Towards the Development of Electrical Conduction and Lithium-Ion Transport in a Tetragonal Porphyrin Wire. Angew. Chem. 2003, 115, 1543–1546.
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277.
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713.
- Tian, Y.-Q.; Zhao, Y.-M.; Chen, Z.-X.; Zhang, G.-N.; Weng, L.-H.; Zhao, D.-Y. Design and generation of extended zeolitic metal-organic frameworks (ZMOFs): Synthesis and crystal structures of zinc(II) imidazolate polymers with zeolitic topologies. Chem.-Eur. J. 2007, 13, 4146–4154.
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495.
- Dybtsev, D.N.; Chun, H.; Kim, K. Three-dimensional metal-organic framework with (3,4)-connected net, synthesized from an ionic liquid medium. Chem. Commun. 2004, 14, 1594–1595.
- Wang, Z.; Sun, Q.; Liu, B.; Kuang, Y.; Gulzar, A.; He, F.; Gai, S.; Yang, P.; Lin, J. Recent advances in porphyrin-based MOFs for cancer therapy and diagnosis therapy. Coord. Chem. Rev. 2021, 439, 213945.
- Kubovics, M.; Careta, O.; Vallcorba, O.; Romo-Islas, G.; Rodríguez, L.; Ayllón, J.A.; Domingo, C.; Nogués, C.; López-Periago, A.M. Supercritical CO2 Synthesis of Porous Metalloporphyrin Frameworks: Application in Photodynamic Therapy. Chem. Mater. 2023, 35, 1080–1093.
- Zhao, Y.; Qi, S.; Niu, Z.; Peng, Y.; Shan, C.; Verma, G.; Wojtas, L.; Zhang, Z.; Zhang, B.; Feng, Y.; et al. Robust Corrole-Based Metal-Organic Frameworks with Rare 9-Connected Zr/Hf-Oxo Clusters. J. Am. Chem. Soc. 2019, 141, 14443–14450.
- Wu, H.; Yang, F.; Lv, X.-L.; Wang, B.; Zhang, Y.-Z.; Zhao, M.-J.; Li, J.-R. A stable porphyrinic metal-organic framework pore-functionalized by high-density carboxylic groups for proton conduction. J. Mater. Chem. A 2017, 5, 14525–14529.
- Yang, X.-L.; Xie, M.-H.; Zou, C.; He, Y.; Chen, B.; O’Keeffe, M.; Wu, C.-D. Porous Metalloporphyrinic Frameworks Constructed from Metal 5,10,15,20-Tetrakis(3,5-biscarboxylphenyl)porphyrin for Highly Efficient and Selective Catalytic Oxidation of Alkylbenzenes. J. Am. Chem. Soc. 2012, 134, 10638–10645.
- Johnson, J.A.; Zhang, X.; Reeson, T.C.; Chen, Y.-S.; Zhang, J. Facile Control of the Charge Density and Photocatalytic Activity of an Anionic Indium Porphyrin Framework via in Situ Metalation. J. Am. Chem. Soc. 2014, 136, 15881–15884.
- Shultz, A.M.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A Catalytically Active, Permanently Microporous MOF with Metalloporphyrin Struts. J. Am. Chem. Soc. 2009, 131, 4204–4205.
- Liu, D.; Liu, T.-F.; Chen, Y.-P.; Zou, L.; Feng, D.; Wang, K.; Zhang, Q.; Yuan, S.; Zhong, C.; Zhou, H.-C. A Reversible Crystallinity-Preserving Phase Transition in Metal-Organic Frameworks: Discovery, Mechanistic Studies, and Potential Applications. J. Am. Chem. Soc. 2015, 137, 7740–7746.
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H.L. Single Pt Atoms Confined into a Metal-Organic Framework for Efficient Photocatalysis. Adv. Mater. 2018, 30, 1705112.
- Abrahams, B.F.; Hoskins, B.F.; Michail, D.M.; Robson, R. Assembly of porphyrin building blocks into network structures with large channels. Nature 1994, 369, 727–729.
- Masih, D.; Chernikova, V.; Shekhah, O.; Eddaoudi, M.; Mohammed, O.F. Zeolite-like Metal-Organic Framework(MOF) Encaged Pt(II)-Porphyrin for Anion-Selective Sensing. ACS Appl. Mater. Interfaces 2018, 10, 11399–11405.
- Zha, Q.; Ding, C.; Rui, X.; Xie, Y. A Novel Porphyrin-Based Ligand Containing Four 4,4′-Dipyridylamine Moieties: Syntheses, Structures, and Luminescent Properties of Mn(II), Cu(II), Zn(II), and Cd(II) Coordination Polymers. Cryst. Growth Des. 2013, 13, 4583–4590.
- Feng, L.; Day, G.S.; Wang, K.-Y.; Yuan, S.; Zhou, H.-C. Strategies for Pore Engineering in Zirconium Metal-Organic Frameworks. Chem 2020, 6, 2902–2923.
- Zhang, X.; Frey, B.L.; Chen, Y.S.; Zhang, J. Topology-Guided Stepwise Insertion of Three Secondary Linkers in Zirconium Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 7710–7715.
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945.
- Pan, S.; Kong, X.; Zhang, Q.; Xu, Q.; Wang, M.; Wei, C.; Zhao, Y.; Zhang, X. Rational modulating electronegativity of substituents in amorphous metal-organic frameworks for water oxidation catalysis. Int. J. Hydrogen Energy 2020, 45, 9723–9732.
- De, S.; Devic, T.; Fateeva, A. Porphyrin and phthalocyanine-based metal-organic frameworks beyond metal-carboxylates. Dalton Trans. 2021, 50, 1166–1188.
- Shi, L.; Yang, L.; Zhang, H.; Chang, K.; Zhao, G.; Kako, T.; Ye, J. Implantation of Iron(III) in porphyrinic metal-organic frameworks for highly improved photocatalytic performance. Appl. Catal. B Environ. 2018, 224, 60–68.
- Wu, Y.; Luo, H.; Wang, H. Synthesis of iron(iii)-based metal-organic framework/graphene oxide composites with increased photocatalytic performance for dye degradation. RSC Adv. 2014, 4, 40435–40438.
- Wang, M.; Ma, Y.; Lv, B.; Hua, F.; Meng, S.; Lei, X.; Wang, Q.; Su, B.; Lei, Z.; Yang, Z. Fe Doped MIL-101/Graphene Nanohybrid for Photocatalytic Oxidation of Alcohols Under Visible-Light Irradiation. Catal. Lett. 2021, 151, 2384–2395.
- Choi, S.; Jung, W.-J.; Park, K.; Kim, S.-Y.; Baeg, J.-O.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Rapid Exciton Migration and Amplified Funneling Effects of Multi-Porphyrin Arrays in a Re(I)/Porphyrinic MOF Hybrid for Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2021, 13, 2710–2722.
- Kong, X.J.; He, T.; Zhou, J.; Zhao, C.; Li, T.C.; Wu, X.Q.; Wang, K.; Li, J.R. In Situ Porphyrin Substitution in a Zr(IV)-MOF for Stability Enhancement and Photocatalytic CO2 Reduction. Small 2021, 17, 2005357.
- Fang, Z.-B.; Liu, T.-T.; Liu, J.; Jin, S.; Wu, X.-P.; Gong, X.-Q.; Wang, K.; Yin, Q.; Liu, T.-F.; Cao, R.; et al. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523.
- Qin, J.-H.; Xu, P.; Huang, Y.-D.; Xiao, L.-Y.; Lu, W.; Yang, X.-G.; Ma, L.-F.; Zang, S.-Q. High loading of Mn(ii)-metallated porphyrin in a MOF for photocatalytic CO2 reduction in gas-solid conditions. Chem. Commun. 2021, 57, 8468–8471.
- Chen, M.; Zhang, C.; Tang, Y.; Cai, Q.; Yu, J.; Chen, Y.; He, Y.; Bai, J.; Fu, M.; Chen, S.; et al. Cu ions anchored in the porphyrin center act as transient metal centers of 2D-MOFs to enhance photocatalytic hydrogen production. Catal. Sci. Technol. 2023, 13, 581–586.
- Pan, Y.; Abazari, R.; Tahir, B.; Sanati, S.; Zheng, Y.; Tahir, M.; Gao, J. Iron-based metal-organic frameworks and their derived materials for photocatalytic and photoelectrocatalytic reactions. Coord. Chem. Rev. 2024, 499, 215538.
- Mu, X.; Jiang, J.; Chao, F.; Lou, Y.; Chen, J. Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation. Dalton Trans. 2018, 47, 1895–1902.
- Castellanos, N.J.; Martinez Rojas, Z.; Camargo, H.A.; Biswas, S.; Granados-Oliveros, G. Congo red decomposition by photocatalytic formation of hydroxyl radicals (·OH) using titanium metal-organic frameworks. Transit. Metal Chem. 2018, 44, 77–87.
- Wu, W.; Xie, Y.; Lv, X.-L.; Xie, L.-H.; Zhang, X.; He, T.; Si, G.-R.; Wang, K.; Li, J.-R. Expanding the Structural Topologies of Rare-Earth Porphyrinic Metal-Organic Frameworks through Ligand Modulation. ACS Appl. Mater. Interfaces 2023, 15, 5357–5364.
- Jin, S.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D. Creation of Superheterojunction Polymers via Direct Polycondensation: Segregated and Bicontinuous Donor-Acceptor pi-Columnar Arrays in Covalent Organic Frameworks for Long-Lived Charge Separation. J. Am. Chem. Soc. 2015, 137, 7817–7827.
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511.
- Zeng, Y.; Fu, Z.; Chen, H.; Liu, C.; Liao, S.; Dai, J. Photo- and thermally induced coloration of a crystalline MOF accompanying electron transfer and long-lived charge separation in a stable host-guest system. Chem. Commun. 2012, 48, 8114–8116.
- Zhang, B.; Qian, B.-B.; Li, C.-T.; Li, X.-W.; Nie, H.-X.; Yu, M.-H.; Chang, Z. Donor-acceptor systems in metal-organic frameworks: Design, construction, and properties. CrystEngComm 2022, 24, 5538–5551.
- Yang, X.-G.; Qin, J.-H.; Huang, Y.-D.; Zhai, Z.-M.; Ma, L.-F.; Yan, D. Highly enhanced UV-vis-NIR light harvesting and photoelectric conversion of a pyrene MOF by encapsulation of the D-π-A cyanine dye. J. Mater. Chem. C 2020, 8, 17169–17175.
- Xu, W.-J.; Huang, B.-X.; Li, G.; Yang, F.; Lin, W.; Gu, J.-X.; Deng, H.-G.; Gu, Z.-G.; Jin, H.-G. Donor-Acceptor Mixed-Naphthalene Diimide-Porphyrin MOF for Boosting Photocatalytic Oxidative Coupling of Amines. ACS Catal. 2023, 13, 5723–5732.
- Cai, P.; Xu, M.; Meng, S.S.; Lin, Z.; Yan, T.; Drake, H.F.; Zhang, P.; Pang, J.; Gu, Z.Y.; Zhou, H.C. Precise Spatial-Designed Metal-Organic-Framework Nanosheets for Efficient Energy Transfer and Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 27258–27263.
- Cui, G.W.; Wang, W.L.; Ma, M.Y.; Zhang, M.; Xia, X.Y.; Han, F.Y.; Shi, X.F.; Zhao, Y.Q.; Dong, Y.B.; Tang, B. Rational design of carbon and TiO2 assembly materials: Covered or strewn, which is better for photocatalysis? Chem. Commun. 2013, 49, 6415–6417.
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218.
- Wang, Q.; Xu, R.; Wang, X.-S.; Liu, S.-D.; Huang, Y.-B.; Cao, R. Platinum Nanoparticle-Decorated Porous Porphyrin-Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Chin. J. Inorg. Chem. 2017, 33, 2038–2044.
- Zuo, Q.; Liu, T.; Chen, C.; Ji, Y.; Gong, X.; Mai, Y.; Zhou, Y. Ultrathin Metal-Organic Framework Nanosheets with Ultrahigh Loading of Single Pt Atoms for Efficient Visible-Light-Driven Photocatalytic H(2) Evolution. Angew. Chem. Int. Ed. 2019, 58, 10198–10203.
- He, T.; Chen, S.; Ni, B.; Gong, Y.; Wu, Z.; Song, L.; Gu, L.; Hu, W.; Wang, X. Zirconium-Porphyrin-Based Metal-Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms. Angew. Chem. Int. Ed. 2018, 57, 3493–3498.
- Sasan, K.; Lin, Q.; Mao, C.; Feng, P. Incorporation of iron hydrogenase active sites into a highly stable metal-organic framework for photocatalytic hydrogen generation. Chem. Commun. 2014, 50, 10390–10393.
- Liang, Z.; Guo, H.; Lei, H.; Cao, R. Co porphyrin-based metal-organic framework for hydrogen evolution reaction and oxygen reduction reaction. Chin. Chem. Lett. 2022, 33, 3999–4002.
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126.
- Afroz, R.; Hassan, M.N.; Ibrahim, N.A. Review of air pollution and health impacts in Malaysia. Environ. Res. 2003, 92, 71–77.
- Choe, J.H.; Kim, H.; Hong, C.S. MOF-74 type variants for CO2 capture. Mater. Chem. Front. 2021, 5, 5172–5185.
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814.
- Xu, L.; Xiu, Y.; Liu, F.; Liang, Y.; Wang, S. Research Progress in Conversion of CO2 to Valuable Fuels. Molecules 2020, 25, 3653.
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051.
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561.
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO(2) reduction over NH2-Uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks. Chemistry 2013, 19, 14279–14285.
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209.
- Jiang, Z.W.; Zou, Y.C.; Zhao, T.T.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Controllable Synthesis of Porphyrin-Based 2D Lanthanide Metal-Organic Frameworks with Thickness- and Metal-Node-Dependent Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 3300–3306.
- Liu, Y.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal-Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 9001–9005.
- Buru, C.T.; Majewski, M.B.; Howarth, A.J.; Lavroff, R.H.; Kung, C.W.; Peters, A.W.; Goswami, S.; Farha, O.K. Improving the Efficiency of Mustard Gas Simulant Detoxification by Tuning the Singlet Oxygen Quantum Yield in Metal-Organic Frameworks and Their Corresponding Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 23802–23806.
- Li, L.; Yu, X.; Xu, L.; Zhao, Y. Fabrication of a novel type visible-light-driven heterojunction photocatalyst: Metal-porphyrinic metal-organic framework coupled with PW12/TiO2. Chem. Eng. J. 2020, 386, 123955.
- Padwa, A.; Bullock, W.H.; Dyszlewski, A.D. Studies dealing with the alkylation--rearrangement reaction of some phenylthio-substituted allylic sulfones. J. Org. Chem. 2002, 55, 955–964.
- Fernandez, I.; Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 2003, 103, 3651–3705.
- Ren, C.; Fang, R.; Yu, X.; Wang, S. A highly efficient reusable homogeneous copper catalyst for the selective aerobic oxygenation sulfides to sulfoxides. Tetrahedron Lett. 2018, 59, 982–986.
- Sheng, W.; Huang, F.; Dong, X.; Lang, X. Solvent-controlled synthesis of Ti-based porphyrinic metal-organic frameworks for the selective photocatalytic oxidation of amines. J. Colloid. Interface Sci. 2022, 628 Pt A, 784–793.
- Wei, F.; Zhang, H.; Ren, Q.; Chen, H.; Yang, L.; Ding, B.; Yu, M.; Liang, Z. Removal of organic contaminants from wastewater with GO/MOFs composites. PLoS ONE 2021, 16, e0253500.
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2-x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055.
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO(2) composites: A critical review of recent literature. Water Res. 2018, 142, 26–45.
- Fang, H.; Zhang, H.; Han, L.; Mei, J.; Ge, Q.; Long, Z.; Yu, Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ. Pollut. 2018, 243 Pt B, 1206–1216.
- Ramasubbu, V.; Kumar, P.R.; Chellapandi, T.; Madhumitha, G.; Mothi, E.M.; Shajan, X.S. Zn(II) porphyrin sensitized (TiO2@Cd-MOF) nanocomposite aerogel as novel photocatalyst for the effective degradation of methyl orange (MO) dye. Opt. Mater. 2022, 132, 112558.
- Akpinar, I.; Yazaydin, A.O. Rapid and Efficient Removal of Carbamazepine from Water by UiO-67. Ind. Eng. Chem. Res. 2017, 56, 15122–15130.
- Trapido, M.; Tenno, T.; Goi, A.; Dulova, N.; Kattel, E.; Klauson, D.; Klein, K.; Tenno, T.; Viisimaa, M. Bio-recalcitrant pollutants removal from wastewater with combination of the Fenton treatment and biological oxidation. J. Water Process Eng. 2017, 16, 277–282.
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 14642.
- Cao, H.; Liu, F.; Tai, Y.; Wang, W.; Li, X.; Li, P.; Zhao, H.; Xia, Y.; Wang, S. Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem. Eng. J. 2023, 466, 143219.
- Li, L.; Lv, X.; Jin, L.; Du, K.; Jiang, J.; Zhao, X.; Liang, H.; Guo, Y.; Wang, X. Facile synthesis of Sn-doped MOF-5 catalysts for efficient photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2024, 344, 123586.
- Fan, L.; Yu, Q.; Chen, J.; Khan, U.; Wang, X.; Gao, J. Achievements and Perspectives in Metal-Organic Framework-Based Materials for Photocatalytic Nitrogen Reduction. Catalysts 2022, 12, 1005.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
23 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




