Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | EVANDO ARAÚJO | -- | 2367 | 2024-01-22 10:48:49 | | | |
| 2 | Peter Tang | Meta information modification | 2367 | 2024-01-23 04:38:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cardoso, P.H.N.; Araújo, E.S. Three-Dimensional Printing Techniques Applied to DDS Area. Encyclopedia. Available online: https://encyclopedia.pub/entry/54183 (accessed on 07 March 2026).
Cardoso PHN, Araújo ES. Three-Dimensional Printing Techniques Applied to DDS Area. Encyclopedia. Available at: https://encyclopedia.pub/entry/54183. Accessed March 07, 2026.
Cardoso, Pedro H. N., Evando S. Araújo. "Three-Dimensional Printing Techniques Applied to DDS Area" Encyclopedia, https://encyclopedia.pub/entry/54183 (accessed March 07, 2026).
Cardoso, P.H.N., & Araújo, E.S. (2024, January 22). Three-Dimensional Printing Techniques Applied to DDS Area. In Encyclopedia. https://encyclopedia.pub/entry/54183
Cardoso, Pedro H. N. and Evando S. Araújo. "Three-Dimensional Printing Techniques Applied to DDS Area." Encyclopedia. Web. 22 January, 2024.
Copy Citation
Three-dimensional printing (3DP) technologies are characterized as a set of innovative manufacturing techniques that allow for the creation of complex and/or personalized three-dimensional physical objects on the work surface of a 3D printing machine (based on the computer-aided design (CAD) project designs of these parts). Three-dimensional printing techniques are widely used in various areas of knowledge, such as education, engineering, and biomedicine.
biopolymers
drug delivery systems
3D printing
customized medicines
1. Introduction
Three-dimensional printing (3DP) techniques are technologies for manufacturing three-dimensional objects based on computer-aided design (CAD) and the deposition of successive layers of a molten material of interest on the flat working surface of a three-dimensional printer [1][2].
Currently, 3D printing techniques such as fused deposition modeling (FDM), selective laser sintering (SLS), stereolithography (SLA), and bioprinting are among the 3DP technologies that are available for the development of devices in emerging materials science areas, such as in sensors, supercapacitors, flexible circuits, customized biomedical implants, and personalized drug delivery devices [3][4][5][6]. The choice of one of the 3DP techniques for producing a three-dimensional object of interest involves assessing the required properties for the final product and the mode of deposition that is suitable for the production of the designed part [7]. Polymers are the first choice of all materials used in these 3DP technologies, as they are thermoplastic matrices with good compatibility with other chemical materials. The low melting points of these materials increase the manufacturability of the parts, and flexible materials are needed for various applications [8][9][10].
The term drug delivery systems (DDSs) can be understood as a set of methods for transporting drugs within an organism for a specific therapeutic objective [11]. An increasing number of publications on the application of 3DP techniques in combination with polymers for the production of drug delivery systems (DDSs) of active ingredients were published between 2012 and 2022 (Figure 1). The evolution of this research area followed the temporal progress of publications in the 3DP area but still involved a much smaller number of works (compared to the total number of publications: 40,000 works in the same period). This is an indication that 3DP techniques are widely used in various areas of knowledge, from education to engineering, and that the development of polymeric DDSs by 3DP is currently an active field of research, both in academia and industry (given the potential of this technique for biomedical purposes).

Figure 1. Number of publications on 3DP techniques using polymers for the production of DDSs (from 2012 to 2022). The search considered works containing the terms “3D printing”, “polymer”, and “drug delivery”. Source: scopus.com., (accessed on 11 January 2024)
For example, the first successful commercial product used in medical printing was Spritam (used in epilepsy treatment), which was authorized in 2015 by the Food and Drug Administration (FDA) of the USA. With the emergence of 3DP medicines, pharmacies can now shift from mass production to the specific dosage forms that are available for patients [12].
In the drug delivery system (DDS) area, the different 3DP techniques allow flexibility in the manufacturing of drug dosage forms (tablets, dermal patches, capsules, and suppositories, among others), which would be difficult to achieve using other forms of pharmaceutical production techniques [13]. These technologies have evolved as novel alternatives to provide personalized DDSs to users, with the aim of facilitating the administration of active substances and obtaining the greatest desirable therapeutic effect [12][14].
2. Stereolithography (SLA)
This 3D printing technique involves converting liquid resins into solid parts through the photopolymerization process [15]. The final printed part is produced with successive layers of the material on the flat surface of a stereolithography apparatus [16]. This technique uses an electron beam of UV light to cause a chain reaction in each layer of resin (or also epoxy or acrylic), which is capable of converting the initial material into polymer chains in the solid state. Other processes, such as photocuring, can be used to mechanically improve printed objects [17].
The printing process starts when the equipment work surface is directed to the resin stock compartment with a layer of distance between them. This layer is then cured with UV light, and a new layer of fresh resin is released above the previous layer so that the process is repeated until the last layer of object formation is activated. Generally, the printed part is washed with isopropyl alcohol to remove surface excesses. Finally, the object photocuring process is established in a specific UV chamber. For this process, the choice of resin must take into account the appropriate curing rate and its approval by the FDA for use in pharmaceutical products [16].
SLA technology also stands out for enabling the deposition of extremely thin layers under the work surface. This means that the technique is capable of faithfully reproducing submillimeter details of the designed part, which provides greater quality and precision to the final product [16]. Moreover, objects are printed in a relatively short time compared to other 3D printing techniques. Like with other 3DP technologies, the duration of the printing process is directly determined by the dimensions of the part, the thickness of the deposition layers, and the complexity of the part details [15]. Stereolithography is also superior to other free-form solid fabrication (FFF) techniques in regard to resolution and accuracy (up to 10 μm) [17]. Several types of resins have been developed in recent years with the aim of providing wide variations in final mechanical properties [16]. On the other hand, a limitation of the use of this technique in DDSs is the reduced quantity of resins that are biocompatible and biodegradable for the required applications [17].
Polylactic acid (PLA) and poly(vinyl alcohol) (PVA) are alternative polymers that have been used in SLA technology [18]. Other authors [19] have used poly(methyl methacrylate) (PMMA) and polyvinylpyrrolidone (PVP) for the production of DDSs by stereolithography for applications in dermal wound healing devices and in the area of dentistry. In another study, Healy et al. produced customized controlled-release tablets through stereolithography. The results showed that the incorporation of different drugs (such as aspirin and paracetamol) into the polymeric matrix can directly impact the dimensions of the printed pharmaceutical form [13].
3. Selective Laser Sintering (SLS)
Selective laser sintering (SLS) is a 3D printing technology dating back to the late 1980s. Objects printed by the SLS technique are produced by depositing successive layers of thermoplastic polymer powder, which are sintered by a high-power laser to form structured three-dimensional parts [18].
Currently, SLS technology is widely used in industry due to its cost–benefit relationship and high productivity [19]. The printing process begins with the placement of a layer of powder on the printer work surface following the project design. This layer is preheated with the aim of improving the surface quality of the part that is in contact with the work surface and ensuring that a lower laser power is used for sintering (preventing polymer degradation). When this layer is sintered, a new layer of powder is made available. The layer deposition and sintering processes are repeated until the designed three-dimensional structure is finalized [19][20]. The quality of the final part is also dependent on the layer deposition process. For example, the distance between two subsequent laser scanning paths over the layer and the layer thickness are important parameters for obtaining a part without apparent structural defects [21].
SLS involves high-resolution printing due to the particle size of the material used, which is approximately 50 to 80 µm and allows for the production of objects with more complex geometries [20]. It also has the ability to carry out medicinal printing without the need for a solvent [19]. This printing technique permits the use of polymers or combinations of polymers. It is still possible to recycle the powdered material that is not used during the process to produce new parts [21][22]. In addition, SLS is the least suitable method for oral drug delivery, as the raw surface after printing requires more complex postprocessing finishing. This technique is limited by the use of thermoplastic polymers, such as polylactic acid (PLA) and polycaprolactone (PCL), combined with pharmaceutical molecules that are stable at sintering temperatures [22][23].
Trenfield et al. mentioned that SLS printing has been used recently for manufacturing drug release capsules with more complex geometries [23]. The aim of this research was to achieve significant changes in the drug release profile. Fina et al. also suggested that SLS 3D printing is suitable for the production of solid pharmaceutical solutions for oral administration using polymers with a fast- or slow-release profile [24].
4. Fused Deposition Modeling (FDM)
The fused deposition modeling (FDM) technology originated in 1988 as an invention by the American Scott Crump [25]. This technology is also known by the term fused filament fabrication (FFF). In an FDM printer, a cylindrical solid filament with a millimeter diameter (generally 1.75 or 3.00 mm) of thermoplastic material (polymer or polymer composite) is heated to a temperature that is greater than its glass transition temperature in the extrusion nozzle with the aim of making the material fluid. By keeping the material flow and extrusion temperature constant, it is possible to form a layer of material on the flat working surface of the printer through the programmed movement of the extruder nozzle in the horizontal plane. With the completion of the first layer, the process is repeated for the other overlapping layers until the three-dimensional part formation project is completed [17][19].
In FDM technology, the thermoplastic characteristic of the filament material is essential for part printing, since each layer of molten material returns to the solid state when the temperature decreases, structuring the formation of the object with dimensional accuracy. Some important parameters for the quality of the final part include the part filling method, diameter of the printer extruder nozzle, thickness of the layers, and extrusion temperature [17]. The extrusion temperature will depend on the polymer/composite material, considering its glass transition and melting temperatures. Generally, these temperatures for pure polymer filaments are indicated by manufacturers, while the use of new composite filaments containing additives of interest on an experimental scale may require additional tests to define the optimized temperature for the process [18].
The main reason for the vast use of FDM technology for 3D printing in drug delivery devices is the low cost of production, including the technology itself, and the wide range of available polymers. The cost-effective characteristics of FDM include preventing material waste and reducing the cost per unit of drug delivery device [26]. Recently, advances in multiple-extrusion FDM printers have demonstrated the potential of this technology for dispersing multiple drugs with different release profiles in the same printed pharmaceutical solid solution [11].
PLA, PCL, and polyvinyl alcohol (PVA) are polymers that stand out in FDM for the production of printed DDSs, as they melt at temperatures that are compatible with the thermal stability range of several drugs, maintaining their bioactivity [19]. Furthermore, when PCL and PVA are used to produce oral formulations, they undergo hydrolysis and are eliminated from the body via excretory routes [26].
Matijašić et al. successfully applied FDM technology to produce 3D-printed capsules. The authors also highlighted the importance of FDM for the area of pharmaceutical technology, given the versatility of the technique for producing customized DDSs in geometry and for different release profiles, in addition to the possibility of administering more than one active ingredient in the same pharmaceutical form [27]. Krause et al. demonstrated the possibility of producing pressure-controlled dosage forms using FDM. This variation in technique was able to print a variety of pharmaceutical solid solutions with different geometries. In addition, using this technique, there was no need to use structural supports during the printing process, which favors the customization of pharmaceutical forms and a reduction in the device printing time [28].
5. Bioprinting
The three-dimensional (3D) bioprinting technology was first developed by Thomas Boland in the early 2000s and continues to grow in popularity in both academia and industry [29]. This technique involves engineering and biological concepts for printing functional biological materials [30].
Bioprinting technology is also an additive manufacturing technology with great potential for developing new personalized biomaterials [31]. This technique uses polymeric fluids/gels containing active cells to precisely manufacture microscale materials layer by layer for tissue engineering applications [32]. The main applications involve the production of tissues containing regenerative cells, similar to natural cells, and drug delivery systems with programmed release [33]. The bioprinting technique involves two well-known procedures, namely, extrusion- and inkjet-based bioprinting.
The technique most commonly used in industry is the extrusion-based bioprinting technique (or pressure-based bioprinting). This technique combines usual bioprinting methods (such as a fluid dispensing system or biological paste) with a robotic system for the material extrusion process. The fluid is inserted into a reservoir coupled to a robotic arm that moves three-dimensionally over the work surface to disperse the material and form the designed object [34]. According to Mobaraki et al., alginate, cellulose, chitosan, gelatin, and hyaluronic acid (HA) have been used for 3D printing of drug delivery devices in hydrogel forms that are usually produced using extrusion-based bioprinting [30]. For example, Kim et al. developed a new biogel for extrusion-based bioprinting. The authors showed that a mixture of alginate and carrageenan in an optimized proportion can be used to develop biomedical devices that satisfactorily inhibit inflammatory processes [32].
On the other hand, using the inkjet-based bioprinting technique, the ejection of droplets of biological fluid onto a flat printing table is established to form the object [35]. With a constant flow of biological gel from the reservoir to the tip of the printing nozzle (a millimetric metallic capillary), an electric field is established between the nozzle and the printing table, with sufficient electrical force to overcome the surface tension of the droplet at the capillary exit. This causes drops of the material to be ejected toward the flat collector to form the first layer of bioink on the work surface as a result of the continuous spraying of fluid drops. This process is repeated to form the second layer, and so on, until the required object is shaped as a junction of these layers [30].
Inkjet printing has been used to create DDSs that improve the bioavailability of poorly soluble molecules for the administration of low concentrations of drugs and on-demand medications [30]. Cellulose, chitosan, and hyaluronic acid are the most popular polymers for the production of drug delivery systems by the abovementioned technique [30][36]. The working principles, advantages, and applications of these 3D printing techniques for developing DDSs are summarized in Figure 2.
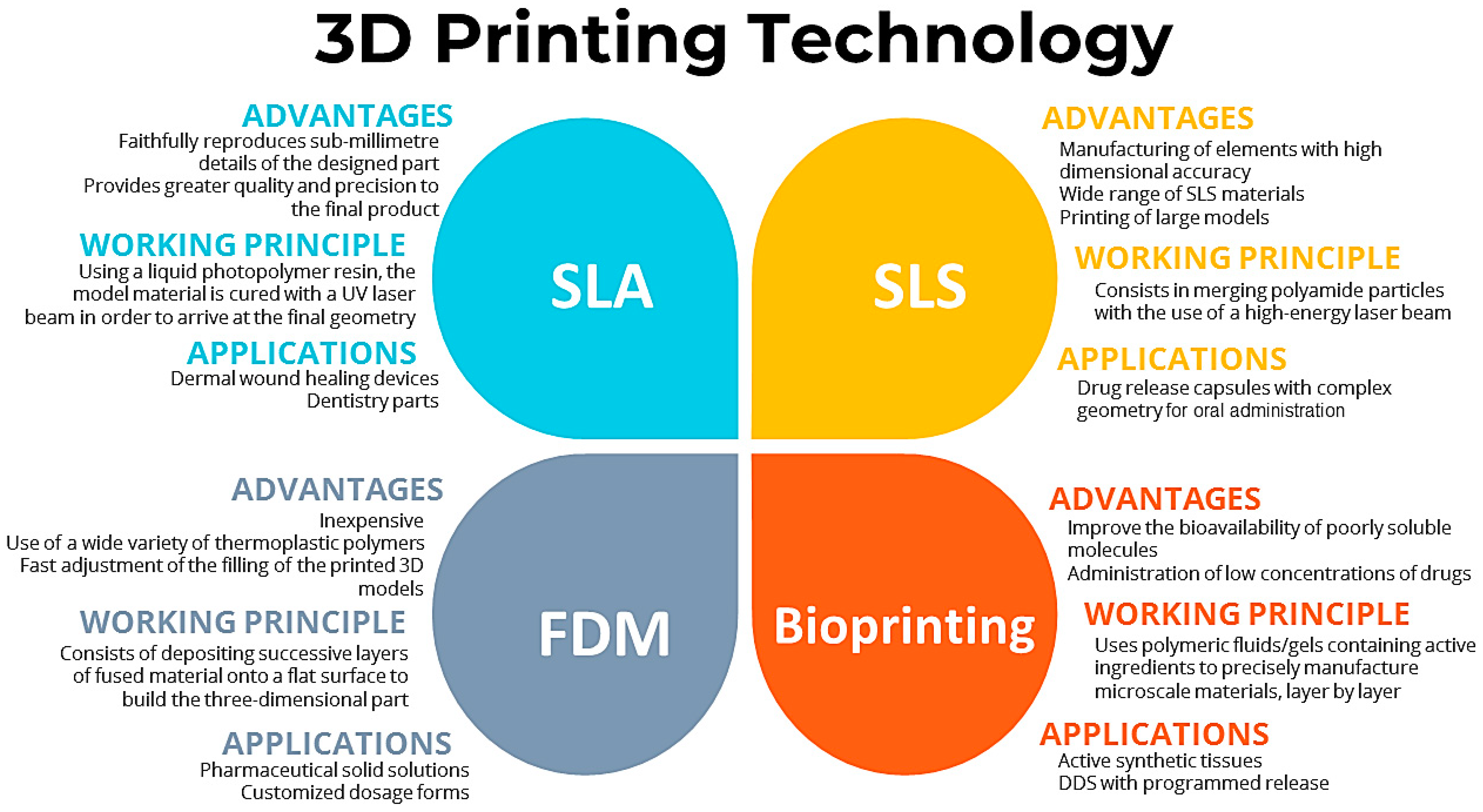
Figure 2. Working principles, advantages, and applications of 3D printing techniques for developing DDSs.
References
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T. Three-Dimensional Printing and 3D Scanning: Emerging Technologies Exhibiting High Potential in the Field of Cultural Heritage. Appl. Sci. 2023, 13, 4777.
- Shishatskaya, E.I.; Demidenko, A.V.; Sukovatyi, A.G.; Dudaev, A.E.; Mylnikov, A.V.; Kisterskij, K.A.; Volova, T.G. Three-Dimensional Printing of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation. Int. J. Mol. Sci. 2023, 24, 12969.
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159.
- Surini, S.; Bimawanti, Y.; Kurniawan, A. The Application of Polymers in Fabricating 3D Printing Tablets by Fused Deposition Modeling (FDM) and The Impact on Drug Release Profile. Pharm. Sci. 2023, 29, 156–164.
- Karanwad, T.; Lekurwale, S.; Banerjee, S. Chapter 4—Selective Laser Sintering (SLS) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals, 1st ed.; Banerjee, S., Ed.; Springer Nature: Singapore, 2023; pp. 125–169.
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D bioprinting and nanotechnology in tissue engineering scaffolds. Biomimetics 2023, 8, 94.
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single-and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155.
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. J. 2016, 32, 54–64.
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290.
- Puza, F.; Lienkamp, K. 3D printing of polymer hydrogels—From basic techniques to programmable actuation. Adv. Funct. Mater. 2022, 32, 2205345.
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245.
- Ramola, M.; Yadav, V.; Jain, R. On the adoption of additive manufacturing in healthcare: A literature review. J. Manuf. Technol. Manag. 2018, 30, 48–69.
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive manufacturing of personalized pharmaceutical dosage forms via stereolithography. Pharmaceutics 2019, 11, 645.
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics 2020, 12, 266.
- Małek, E.; Miedzińska, D.; Popławski, A.; Szymczyk, W. Application of 3D printing technology for mechanical properties study of the photopolymer resin used to print porous structures. Tech. Sci. 2019, 22, 183–194.
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479.
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B. Eng. 2018, 143, 172–196.
- Caballero-Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Chapter 6—Three-dimensional printed drug delivery systems. In Engineering Drug Delivery Systems, 1st ed.; Seyfoddin, A., Dezfooli, S.M., Greene, C.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 147–162.
- Wulf, K.; Riess, C.; Rekowska, N.D.; Eickner, T.; Seitz, H.; Grabow, N.; Teske, M. PMMA and PVP based polymers for stereolitho-graphic manufacture of tailored drug release. Trans. Addit. Manuf. Meets Med. 2019, 1, 1–2.
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116.
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D printed sensors for biomedical applications: A review. Sensors 2019, 19, 1706.
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67.
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094.
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 519, 285–293.
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596.
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17.
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D printing of multi-compartmental PVA capsules for drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686.
- Krause, J.; Bogdahn, M.; Schneider, F.; Koziolek, M.; Weitschies, W. Design and characterization of a novel 3D printed pressure-controlled drug delivery system. Eur. J. Pharm. Sci. 2019, 140, 105060.
- Thayer, P.; Martinez, H.; Gatenholm, E. Chapter 1—History and trends of 3D bioprinting. In 3D Bioprinting: Principles and Protocols, 1st ed.; Crook, J.M., Ed.; Humana Press: New York, NY, USA, 2020; Volume 1, pp. 3–18.
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080.
- Chircov, C.; Grumezescu, A.M. Chapter 2—Three-dimensional bioprinting in drug delivery. In Materials for Biomedical Engineering: Nanomaterials-Based Drug Delivery, 1st ed.; Holban, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 19–40.
- Huang, Y.; Zhang, X.-F.; Gao, G.; Yonezawa, T.; Cui, X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12, 1600734.
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063.
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D printing processes for biomedical field: Opportunities and challenges. J. Polym. Environ. 2020, 28, 1345–1367.
- Saygili, E.; Dogan-Gurbuz, A.A.; Yesil-Celiktas, O.; Draz, M.S. A powerful tool to leverage tissue engineering and microbial systems. Bioprinting 2020, 18, e00071.
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
467
Revisions:
2 times
(View History)
Update Date:
23 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





