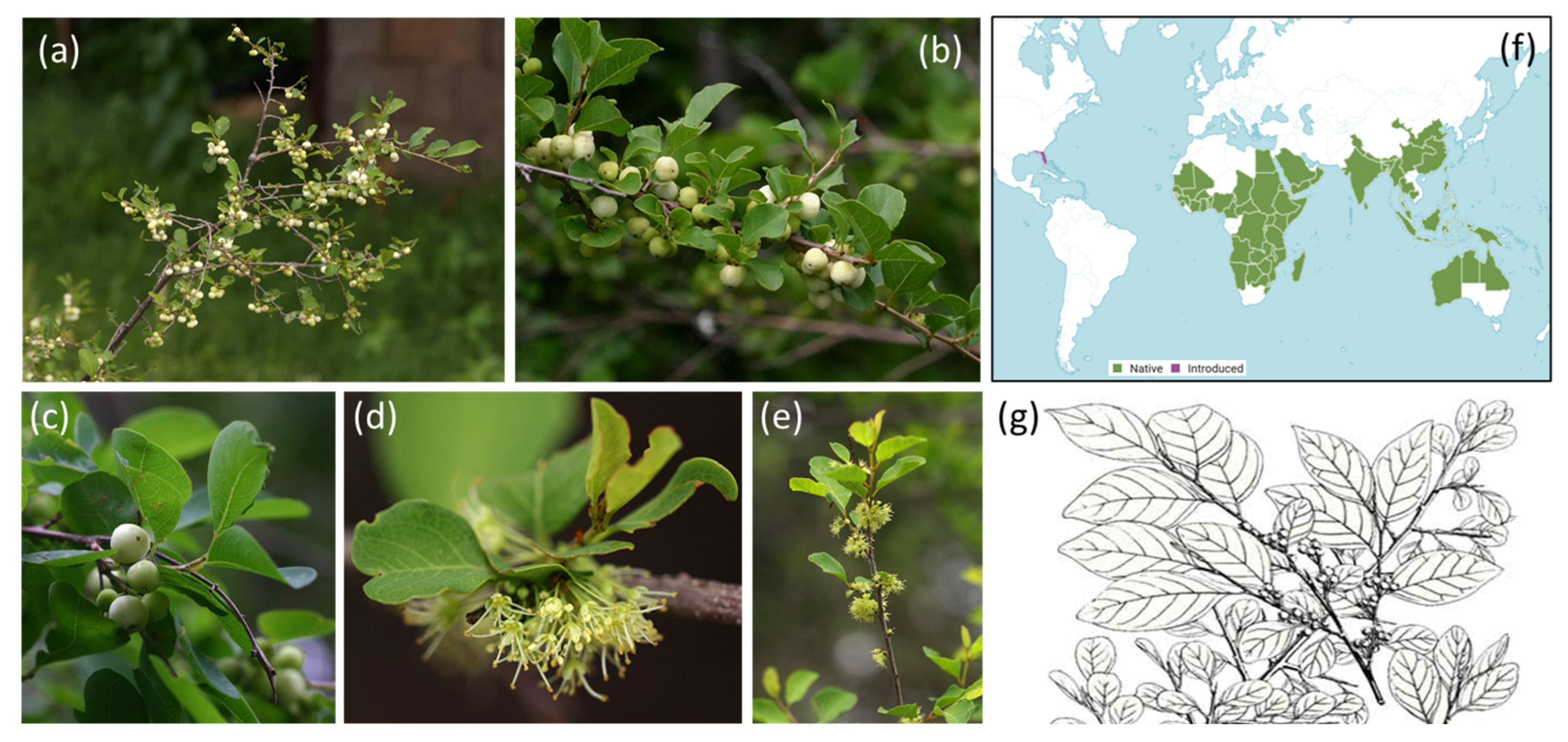
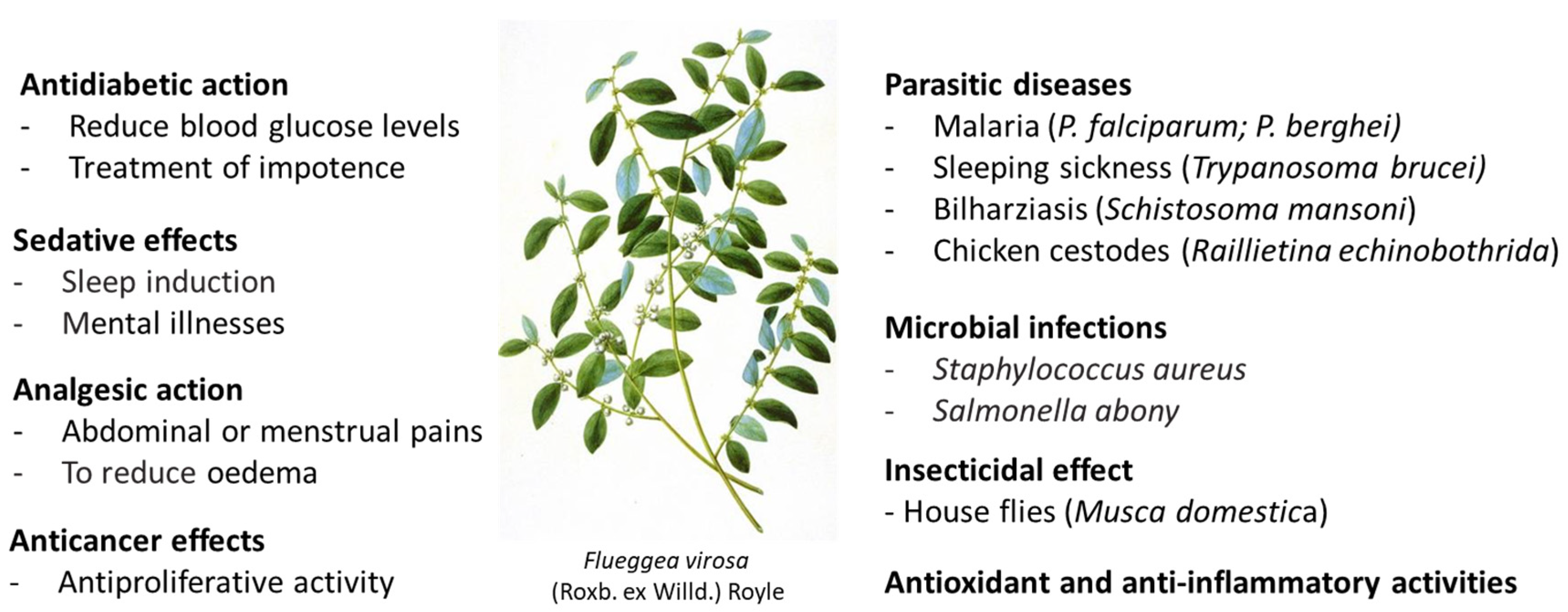
2. Antiparasitic Activities
The plant is used against malaria in Kenyan folk medicine
[9] and in Ghana
[10] and other African countries to treat parasitic diseases. A methanol extract made from fresh leaves of
F. virosa (collected in Comoros) was shown to inhibit the growth of the malaria parasite
Plasmodium falciparum in vitro (IC
50 = 2.28 and 3.64 µg/mL, with strains D6 and W2, respectively), with no significant effects on control non-infected Vero E6 cells (CC
50 = 683 µg/mL). In vivo, the same extract was found to reduce the growth of
Plasmodium berghei in infected mice by more than 70% when administered at 100 mg/kg/day. The effect was modest but noticeable with a mean survival time of 11 days versus 8 days for the vehicle-treated control group. No apparent sign of toxicity was observed
[11][12][13]. Moderate antimalaria activity was confirmed using another chloroquine-resistant strain of
P. falciparum, namely, strain K1 (IC
50 = 7.6 µg/mL)
[14]. A marked antiplasmodial activity was obtained with a methanol/water leaf extract and with a root decoction (IC
50 = 3 µg/mL)
[15]. But the best evidence of activity comes from a study that employed an ethyl acetate fraction of
F. virosa leaves, which revealed dose-dependent activity in
P. berghei-infected mice, with a nearly complete (86%) suppression of the animal parasitemia and a mean survival time of 17.2 days versus 10.8 days for the control animals
[16]. The antimalaria activity was attributed to the presence of the polyphenol bergenin (see below). The plant is not a universal treatment because a similar methanolic extract was found to be inactive against the chloroquine-sensitive strain of
P. falciparum PoW (IC
50 > 50 µg/mL)
[17]. Nevertheless,
F. virosa is recognized among major African medicinal plants to treat malaria
[18].
The alcoholic plant extract may be used also to combat other parasites. A leaf extract of
F. virosa (collected in Mizoram, India) was found to be active against the intestinal cestode parasite
Raillietina echinobothrida, which infects domestic fowl. The extract significantly reduced the alkaline phosphatase activity and induced protein catabolism in treated parasites
[19]. The anthelmintic activity has been associated with the induction of significant destruction of the parasite tegument with intense vacuolization and swellings of the basal lamina leading to deformities in the cell organelles
[20]. Antitrypanosomal activity was also reported with a petroleum ether extract of
F. virosa, which was found to reduce the growth of
Trypanosoma brucei rhodesiense (IC
50 = 0.5 µg/mL), whereas the maximum tolerated concentration was about 20-fold higher (MTC = 9 µg/mL)
[21]. Here, again, the antitrypanosomal activity of the extracts can be attributed to the presence of bergenin, which was shown to inhibit the growth of the bloodstream form of
Trypanosoma brucei (IC
50 = 1.0 µM)
[22]. This natural product is a model compound for designing semi-synthetic products that are active against
T. brucei [23]. The marked activity observed with the plant extract explains why traditional healers in Uganda use a powder from pounded roots of
F. virosa (one spoonful orally) to treat sleeping sickness
[21].
The root extract of
F. virosa may be used to combat bilharziasis (schistosomiasis). A traditional recipe used in Somalia recommended the use of fresh roots, crushed and boiled with water to prepare a drinkable solution active against the parasite
[24]. But no information about the efficacy of the preparation is available. Similarly, the use of a root decoction to treat urinary schistosomiasis is cited in a review on plants used to treat schistosomiasis in Niono District in Mali, but without further details
[25]. Experimental data to support the antischistosomal activity are needed because an ancient study only revealed a minor effect of a leaf extract of
F. virosa on cercariae and miracidia of
Schistosoma mansoni [26].
3. Antimicrobial Effects
The traditional use of
F. virosa for the treatment of dermal infections and wounds in Ghana has motivated studies aimed at characterizing the potential activity of various polar/apolar extracts of the plant against several organisms. Among the different medicinal plants tested,
F. virosa was the most active, notably the chloroform extract derived for the root bark, which was found to be active against 13 microorganisms tested, but with a variable degree of activity. The CHCl
3 extract was most potent against the pathogenic Gram-negative species
Salmonella abony (MIC = 15.6 µg/mL) and mildly active against
Staphylococcus aureus (MIC = 125 µg/mL). Interestingly, the extract was found to potentiate the activity of the antibiotic norfloxacin against a norfloxacin-resistant strain of
S. aureus possessing the efflux pump NorA. Antimicrobial activities against other pathogens, such as
Bacillus subtilis,
Micrococcus flavus and
Pseudomonas aeruginosa were also observed (17, 19 and 12 mm of growth inhibition when using an agar well diffusion technique)
[27]. A recent study underlined the activity of
F. virosa leaf extracts against
methicillin-resistant Staphylococcus aureus (MRSA)
[28], in agreement with other antimicrobial studies
[29][30].
4. Antiepilepsy and Antipsychotic Activities
The testing of different Malian plants used traditionally to treat epilepsy and convulsions has revealed a marked activity with a leaf extract of
F. virosa (with a plant collected in Bougouni District, Southern Mali). The extract inhibited the spontaneous discharge (SED) in a mouse cortical wedge preparation (IC
50 = 0.2 mg/mL) and was efficient in the [
3H]-flumazenil-binding assay (IC
50 = 0.45 mg/mL), suggesting the presence of GABAergic compounds. The extract showed no effect during anticonvulsive testing in a model of pentylenetetrazol-induced seizure in mice
[31]. A similar methanolic extract, which was also prepared from leaves of
F. virosa, was shown to decrease aporphine-induced stereotypic climbing behavior, reduce swim-induced grooming in mice and increase the mean duration of ketamine-induced sleep in a murine model at a dose of 50 mg/kg
[32][33]. The extract facilitated sleep induction, as was also shown in another study with a model of diazepam-induced sleep. In this case, an extract at 100 mg/kg was shown to prolong the duration of diazepam-induced sleep without affecting the exploratory behavior or the motor coordination of mice
[34]. These observations using experimental models are consistent with the traditional use of plant extract as a sedative in children and for mental illnesses.
Sedative effects were also observed when using root bark extracts of
F. virosa, notably with a butanol extract that contained tannins, saponins, alkaloids, flavonoids and cardiac glycosides, as with the methanol extract. A butanol fraction administered to mice (75 mg/kg) was found to reduce the mean onset of sleep and to double the mean duration of sleep. The sedative effects were clearly established
[35][36]. A bark extract also displayed an antipsychotic activity, reducing swim-induced grooming activity in mice and the mean climbing score
[37]. This latter study was performed using a residual aqueous fraction of methanol root bark extract but it gave results comparable with those obtained with the methanolic leaf extract, suggesting the implication of the same bioactive principles
[33]. One of the active principles was clearly identified: the polyphenolic substance bergenin, which is present in the plant roots and aerial parts and was shown to possess a sleep-inducing property. This compound may, at least in part, be responsible for the sedative potential of the plant extracts
[38]. Bergenin is found in many plants and is known to display antioxidant and antianxiety activities
[39][40].
5. Antidiabetic Effects
One of the traditional usages of
F. virosa is the treatment of diabetes-related impotence
[41]. The chronic hyperglycemia that characterizes type 2 diabetes can lead to alteration of the vascular endothelium and associated tissue damage, notably a marked erectile dysfunction, which is considered the most important sexual dysfunction in men with diabetes mellitus
[42]. Traditional healers in the Tanga region of Tanzania (northeastern part) use aqueous extracts of
F. virosa to treat impotence and as an aphrodisiac
[43]. Experimental data show that the oral administration of an aqueous extract prepared from dried roots of
F. virosa reduces the blood glucose level in rabbit, but only during hyperglycemia. The extract has no effect once blood glucose has reached fasting levels. A dose-dependent (0.1–1.0 g/kg body weight) reduction in the area under the oral glucose tolerance curve was observed and no major toxicity was noted
[44]. Antidiabetic effects have also been reported with a leaf extract of
F. virosa administered intraperitoneally to diabetic rats. In this case, the extract reduced blood glucose levels after 4–24 h of administration, with an efficacy relatively close to that of the reference product insulin (at 24 h, the glucose levels were 328.2, 165.4 and 137.0 mg/dL in the groups treated with saline buffer (control), 100 mg/kg
F. virosa leaf extract and 6 i.u./kg insulin, respectively)
[45]. Thus, the extract clearly presented an insulin-like effect. Recently, a similar observation was made with a hydro-ethanolic plant extract (200 mg/kg) administered to streptozotocin-induced diabetic rats. The plant treatment reduced hyperglycemia and the progression of diabetic nephropathy
[46].
F. virosa is regularly cited as a plant used traditionally in Africa to treat diabetes mellitus
[43][47][48], but the natural products at the origin of the antidiabetic effects have not been identified at all. This aspect of the plant warrants further investigation. Among the antidiabetic compounds, it is worth mentioning the trimeric alkaloid fluevirosine A (see below). Its use in the preparation of blood-sugar-reducing medicine was patented in China
[49].
6. Antidiabetic Effects
In relation to diabetes, we can also refer to the use of
F. virosa extracts for the treatment of diabetes-associated pain. In Mali, decoctions made from the root, root bark or leaves are taken orally to relieve painful conditions, including stomach ache, menstrual pain and pain due to diabetes
[50]. A methanol root bark extract of
F. virosa was shown to display analgesic effects in animal models. The extract inhibited acetic acid-induced abdominal constrictions and attenuated formalin-induced neurogenic pain. The extract efficiently reduced abdominal writhing in mice in a dose-dependent manner. Remarkably, the inhibition of abdominal constriction with the plant extract at 25 mg/kg was greater than that of the standard non-steroidal analgesic piroxicam. Moreover, in a model of carrageenan-induced paw edema in rats, the methanol root bark extract was shown to efficiently reduce the edema diameter, with an efficacy close to that of the reference product ketoprofen
[51]. In these in vivo tests, the most active doses were 25–100 mg/kg, which was largely inferior to the intraperitoneal median lethal dose (LD
50 = 1.26 g/kg), suggesting that the extract is relatively safe at the analgesic doses
[52]. Analgesic effects were also observed with an aqueous root extract (100–400 mg/kg) in a model of thermally induced pain in rats
[53]. The aqueous root extract was apparently less efficient than the methanol root extract. Experiments were also performed with aqueous extracts prepared from the leaves and stems of
F. virosa using a model of acetic acid-induced pain in mice. Interestingly, the stem extract displayed a marked analgesic effect (65% pain inhibition) coupled with a significant anti-inflammatory activity (59% inhibition of carrageenan-induced inflammation in mice)
[54]. Collectively, these different studies support the traditional use of
F. virosa to manage pain associated with different diseases and conditions, such as diabetes, but also benign prostatic hyperplasia (BPH).
7. Anticancer Effects
Decoctions from the roots of
F. virosa are traditionally used to treat cancer patients. Notably, the Embu and Mbeere peoples in Kenya use root decoctions to treat prostate and breast cancers and kidney problems
[55][56][57]. An antiproliferative action was also observed with a methanol extract and the human cancer cell lines RD (rhabdomysarcoma) and Hep-2C (laryngeal carcinoma) (IC
50 = 11.3 and 7.2 μg/mL, respectively)
[58]. The plant contains numerous alkaloids that can inhibit the proliferation of cancer cells. These different products are discussed below, notably a series of antiproliferative indolizidine alkaloids. There are also anticancer studies with the plant extracts, notably a study revealing the capacity of a methanolic leaf extract to block the proliferation of MCF7 breast cancer cells and NCI-H460 lung cancer cells (GI
50 = 42.2 and 78.3 µg/mL, respectively). The root bark extract was a little less active than the leaf extract, and the chloroform fraction was more active than the aqueous fraction (essentially inactive)
[59]. Interesting data have also been reported for a root bark extract, which was found to inhibit the proliferation of U-1242 glioblastoma multiforme tumor cells. Here, again, the chloroform fraction was the most active, and the crude methanol root bark extract was shown to modulate the epidermal growth factor receptor (EGFR) pathway
[60].
8. Antioxidant Effects
Unsurprisingly, several studies referred to the antioxidant activity of
F. virosa extracts, which is a property commonly observed with plant extracts containing flavonoids and phenolic compounds
[61]. The best activity was obtained with ethanolic leaf extracts, whereas hexane extracts were much less active
[62]. The leaf methanol extract presented higher antioxidant effects than the stem bark methanol extract
[63][64]. The leaf extract was found to be as efficient as the reference product ascorbic acid (IC
50 = 25 μg/mL in a DPPH assay)
[39]. An extract made from the aerial parts of
F. virosa revealed an antioxidant action that was a little inferior to that of ascorbic acid (IC
50 = 0.01 versus 0.008 mg/mL, respectively), coupled with antifungal and antiproliferation effects
[58]. Diverse antioxidant products were isolated from the leaves, such as bergenin, but also kaempferol 3-
O-(4-galloyl)-β-D-glucopyranoside, 11-
O-caffeoylbergenin and glucogallin acting as hydroxyl radical scavengers
[27][65]. Root extracts also displayed a marked antioxidant profile
[29]. The main component bergenin contributes significantly to the antioxidant effects. This phenolic compound alleviates hydrogen peroxide-induced oxidative stress in cells
[66][67]. The antioxidant properties of
F. virosa have been amply documented. Among 47 plant species (27 families) used in traditional medicine in Burkina Faso, a total extract of
F. virosa revealed the most important antioxidant capacity
[68].
9. Other Activities
Occasionally, other properties were observed when using extracts of
F. virosa, such as antiarrhythmic effects attributed to bergenin
[69]. The capacity of bergenin to protect from myocardial ischemia–reperfusion injury is well documented
[70]. Recently, an ethanolic extract made from the aerial parts of
F. virosa has demonstrated antisickle cell activity via a capacity to normalize the shape of the circulating abnormal erythrocytes, which is the so-called antifalcemic or antisickling activity
[71]. The same type of activity was demonstrated previously with an aqueous methanolic leave extract, which inhibited sodium metabisulphite-induced sickling of hemoglobin sickle-shaped (Hbss) red blood cells in a concentration-dependent manner
[72]. The antisickling is attributed to the presence of phenolic compounds capable of modulating the inflammatory response and reducing the vasocclusive crisis, but also to amino acids and flavonoids targeting Hbss polymerization and reducing endothelial dysfunctions
[71].
There are not many studies that investigated the activity of
F. virosa extracts against pathogenic fungi. Minor activities against
Candida albicans [9] and the dermatophyte
Trichophyton interdigitale (MIC = 125 µg/mL)
[27] were noted. Similarly, modest activity was observed with a root bark extract tested against
Candida albicans,
C. glabrata and
C. tropicalis [73]. The plant extracts are not very active against fungi and different alkaloids from the plant tested, as antifungal agents revealed little or no activity. For example, the alkaloid virosecurinine was found to be inactive against various fungi, such as
Aspergillus niger,
Penicillium viridicatum and
Fusarium monoliforme [74].
Other pharmacological activities have occasionally been reported, such as an antidiarrheal activity with
F. virosa methanolic extract
[75][76] or the treatment of skin rashes and HIV infection
[77]. The plant contains monomeric and dimeric alkaloids acting as inhibitors of HIV replication, such as virosinine A and flueggenine D (see below)
[6][78][79]. A leaf powder of
F. virosa can also be used as an insecticidal agent, for example, to control the development of the house fly
Musca domestica and the transmission of diseases to humans
[80].