Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stoyan Kostov | -- | 5292 | 2024-01-19 07:35:57 | | | |
| 2 | Camila Xu | Meta information modification | 5292 | 2024-01-22 02:39:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kostov, S.; Selçuk, I.; Watrowski, R.; Dineva, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Omental Bursa in Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/54081 (accessed on 07 February 2026).
Kostov S, Selçuk I, Watrowski R, Dineva S, Kornovski Y, Slavchev S, et al. Omental Bursa in Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/54081. Accessed February 07, 2026.
Kostov, Stoyan, Ilker Selçuk, Rafał Watrowski, Svetla Dineva, Yavor Kornovski, Stanislav Slavchev, Yonka Ivanova, Angel Yordanov. "Omental Bursa in Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/54081 (accessed February 07, 2026).
Kostov, S., Selçuk, I., Watrowski, R., Dineva, S., Kornovski, Y., Slavchev, S., Ivanova, Y., & Yordanov, A. (2024, January 19). Omental Bursa in Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/54081
Kostov, Stoyan, et al. "Omental Bursa in Ovarian Cancer." Encyclopedia. Web. 19 January, 2024.
Copy Citation
The omental bursa (OB), also referred to as the lesser peritoneal sac, is a natural space situated between the stomach and the pancreas.
advanced epithelial ovarian cancer
anatomy
neglected anatomical areas
1. Introduction
Ovarian cancer (OC) is a rare disease with specific tumor biology and clinical behavior. Therefore, OC represents one of the major causes of lethality from cancer among women in developed countries [1]. The majority of patients with advanced epithelial ovarian cancer (AEOC) are initially diagnosed at an advanced stage of the disease [1][2]. The main routes of spread include peritoneal and lymphatic dissemination with the upper abdomen being commonly affected in advanced stages, which, in turn, increases the rate of lymph node and peritoneal metastatic involvement and decreases the chance for complete cytoreduction [1]. Therefore, the surgical approach to AEOC has changed in the last few decades [3][4][5]. Optimal cytoreduction with no macroscopic visible disease (RO) remains the most important prognostic factor [3][6][7]. The proficiency and anatomical expertise of surgical teams significantly influence the quality of optimal cytoreduction. Suboptimal cytoreduction often arises from the neglect of potential anatomical sites predisposed to concealing macroscopic tumor residues, often left unexplored during AEOC surgery [8]. Omitting dissection of these particular areas can compromise complete cytoreduction [9]. Anatomical sites that may harbor “neglected” tumor residues include the omental bursa; Morison’s pouch; the base of the round ligament of the liver and hepatic bridge; the splenic hilum; and suprarenal, retrocrural, cardiophrenic and inguinal lymph nodes [3][5][7]. A profound understanding of anatomy is a prerequisite since in most cases the surgeon has to perform steps like liver mobilization, diaphragmatic peritonectomy and splenectomy, as well as dissection of suprarenal, celiac and cardiophrenic lymph nodes [9]. Consequently, oncogynecologists are responsible for the safe, precise and complete dissection of these anatomical areas. Anatomical areas such as the retroperitoneal pelvic and paraaortic lymph nodes, diaphragmatic peritoneum, mesentery of small intestine/colon, gallbladder and omentum are not included in the article, as these areas are always preciously investigated in cases of abdominal exploration during OC surgery.
2. Omental Bursa
2.1. Boundaries
The omental bursa (OB), also referred to as the lesser peritoneal sac, is a natural space situated between the stomach and the pancreas [10]. The boundaries of the OB are defined as follows [5][10][11][12]:
Anterior: The hepatogastric ligament (pars flaccida), the posterior wall of the stomach, the gastrocolic ligament.
Posterior: The parietal peritoneum covering the right crura of the diaphragm, the abdominal aorta, the celiac trunk, the pancreas, the left suprarenal gland and the medial part of the anterior aspect of the left kidney and the duodenum.
Superior: The narrow between the right side of the esophagus and the ligamentum venosum fissure.
Inferior: The fusion line of the layers of the greater omentum and the transverse mesocolon.
Left lateral wall: Lower bound—the gastrosplenic ligament and the splenorenal ligament; left gastroomental fold; upper bound—the gastrophrenic ligament.
Right lateral wall: The epiploic foramen (Winslow’s foramen).
The OB can also be divided into an infragastric and a supragastric part. The infragastric part is located posterior to the greater omentum, caudally and posterior to the stomach. Surgeons may encounter this part of the OB during supracolic (total) omentectomy. The supragastric part is located posterior to the lesser sac and cranial to the pancreas. Accessing this part is more intricate than accessing the infragastric one [13].
2.1.1. Recesses and Vestibule
Within the OB, three peritoneal pouches or recesses can be identified. The superior omental recess is located between the caudal liver lobe and the diaphragm, whereas the inferior omental recess extends between the posterior wall of the stomach, the pancreas and the transverse mesocolon. More caudally, the inferior recess almost vanishes due to the fusion of the layers of the greater omentum. The superior recess communicates with the peritoneal cavity through Winslow’s foramen. The splenic recess is situated between the stomach and the hilum of the spleen [5][10][14].
The vestibule of the OB is located to the left of the epiploic foramen. It is bounded anteriorly by the hepatoduodenal ligament, superiorly by the caudate lobe of the liver and postero-inferiorly by the head of the pancreas [11][15].
2.1.2. Hepatoduodenal Ligament and Foramen of Winslow
The greater omentum consists of the gastrosplenic, splenorenal, gastrocolic and gastrophrenic ligaments, whereas the lesser omentum is composed of the hepatogastric ligament and the hepatoduodenal ligament (HDL) [11]. The latter is of great interest, as the portal triad (common bile duct, proper hepatic artery, portal vein) is located beneath the two peritoneal leaves of the lesser omentum (visceral and parietal peritoneum). The HDL forms a thick right-sided margin of the lesser omentum, connecting the porta hepatis of the liver and the superior duodenal flexure [11][15][16]. Between the two leaves of the HDL, the common bile duct runs right to the portal vein. The proper hepatic artery runs left to the portal vein [16]. The common bile duct and the proper hepatic artery are located anterior to the portal vein. The HDL also contains nerves, lymphatics, and fatty and connective tissue. The anterior vagal trunk of the vagus nerve is also a part of this complex ligamentous structure, and the lesser curvature of the stomach lies at the left part of the HDL, in close proximity with the anterior vagal nerve [11][15].
The foramen of Winslow (also referred to as the omental or epiploic foramen) is located posterior to the HDL. As mentioned above, this foramen is the only natural connection between the OB and the greater sac. The foramen has the following boundaries: anterior—the HDL; posterior—the parietal peritoneum covering the inferior vena cava, right crus of the diaphragm; inferior—the superior part of the duodenum; superior—the caudate lobe of the liver [11][12].
The anatomy of the supragastric OB is shown in Figure 1.
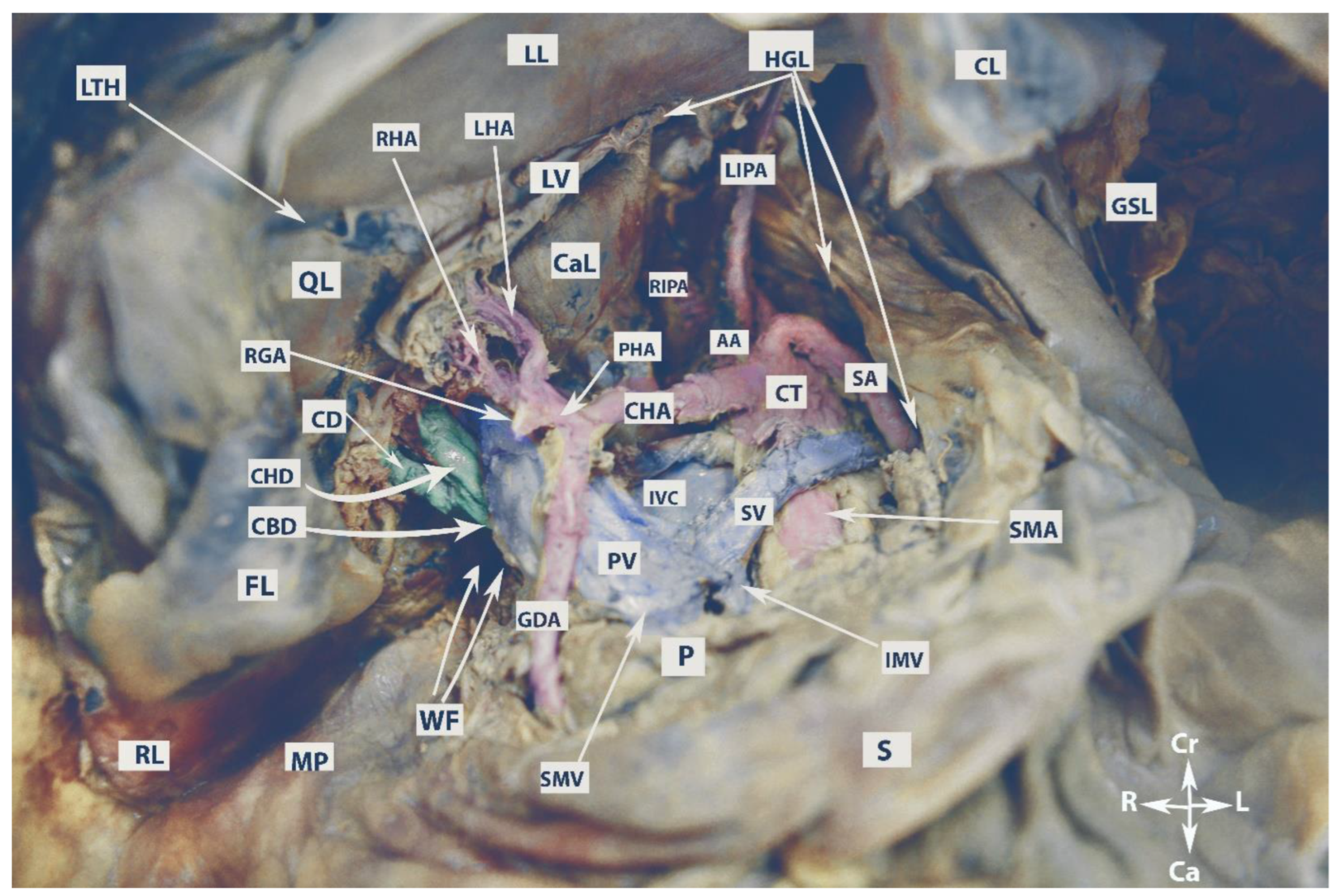
Figure 1. Anatomy of the supragastric omental bursa (embalmed cadaver, authors’ own material). LL—left lobe of the liver; RL—right lobe of the liver; LTH—ligamentum teres hepatis; QL—quadrate lobe; CaL—caudate lobe; FL—incised falciform ligament; CL—coronary ligament of the left liver lobe; GSL—gastrosplenic ligament; HGL—hepatogastric ligament; LV—ligamentum venosum; MP—Morison’s pouch; WF—Winslow’s foramen; CBD—common bile duct; CHD—common hepatic duct; CD—cystic duct; RGA—right gastric artery; LHA—left hepatic artery; RHA—right hepatic artery; GDA—gastroduodenal artery; PHA—proper hepatic artery; CHA—common hepatic artery; CT—celiac trunk; SA—splenic artery; LIPA—left inferior phrenic artery; RIPA—right inferior phrenic artery; AA—abdominal aorta; SMA—superior mesenteric artery; IVC—inferior vena cava; SV—splenic vein; PV—portal vein; SMV—superior mesenteric vein; IVM—inferior mesenteric vein; P—pancreas; S—stomach; Cr—cranial; Ca—caudal; L—left; R—right.
2.1.3. Vessels
The celiac trunk (CT), also referred to as the celiac axis, is the first visceral anterior branch of the abdominal aorta. It arises immediately after the aortic hiatus at the level of the T12/L1 vertebral bodies. The CT is approximately 1.5–2 cm long. It runs horizontally and above the splenic vein before trifurcating into the left gastric artery, splenic artery and common hepatic artery. This trifurcation is referred to as the “true” tripod because all three arteries share a common origin. When one of these arteries originates before the other two along the course of the CT, it is termed a “false” tripod. The left gastric artery is the smallest branch of the CT and lies slightly cranial to the remaining two arteries [11][15][17]. It passes between the two leaves of the lesser omentum to run along the lesser curvature of the stomach. The splenic artery, the largest branch, is slightly to the left of the common hepatic artery. The splenic artery is a tortuous branch and follows a leftward course slightly above the neck and tail of the pancreas. At the level of the neck of the pancreas, the artery runs horizontally before ascending and turning more laterally to terminate in the hilum of the spleen. The splenic artery gives off branches such as the left gastroepiploic artery and short gastric arteries. The common hepatic artery runs on the superior part of the duodenum. It divides into the gastroduodenal, proper hepatic and right gastric arteries [11][15][18]. The gastroduodenal artery is the first branch that runs caudally and supplies the pylorus, pancreas and duodenum. The right gastric artery follows a caudal course and passes within the two leaves of the lesser omentum along the lesser curvature of the stomach. The proper hepatic artery arises just after the origin of the gastroduodenal and right gastric arteries. It runs cranially and becomes a part of the portal triad between the two leaves of the HDL. The proper hepatic artery divides into the left and right hepatic arteries at the level of the porta hepatis [11][15][18].
The portal vein is the main vessel entering the liver, responsible for carrying about 75% of the blood flow. It arises from the confluence of the superior mesenteric vein and the splenic vein. The true origin of the portal vein begins immediately after the splenic–mesenteric confluence, which is located anterior to the IVC and posterior to the neck of the pancreas at the level of the second lumbar vertebra [19][20]. Three drainage patterns of the inferior mesenteric vein have been identified: into the splenic vein (type 1a), the superior mesenteric vein (type 1b) or the confluence of superior mesenteric and splenic vein (type 2) [21]. Notably, in the majority of cases, the inferior mesenteric vein and the left gastric vein drain into the splenic vein [11][15]. The portal vein enters the HDL and divides into left and right branches at the level of the porta hepatis [11][15].
2.1.4. Porta Hepatis
The porta hepatis (PH) is a transverse nonperitoneal fissure located on the inferior surface of the liver from the gallbladder neck to the fissure for the ligamentum teres hepatis and ligamentum venosum. The PH is also delimited by the quadrate lobe in front and from the caudate process at the back. The lesser omentum connects to the PH margins. Moving from posterior to anterior, the left and right portal veins and the left and right hepatic arteries enter the PH. Conversely, some lymph nodes emerge from PH along with the left and right hepatic ducts [11][15][22].
The vessels of the OB are shown in Figure 2.
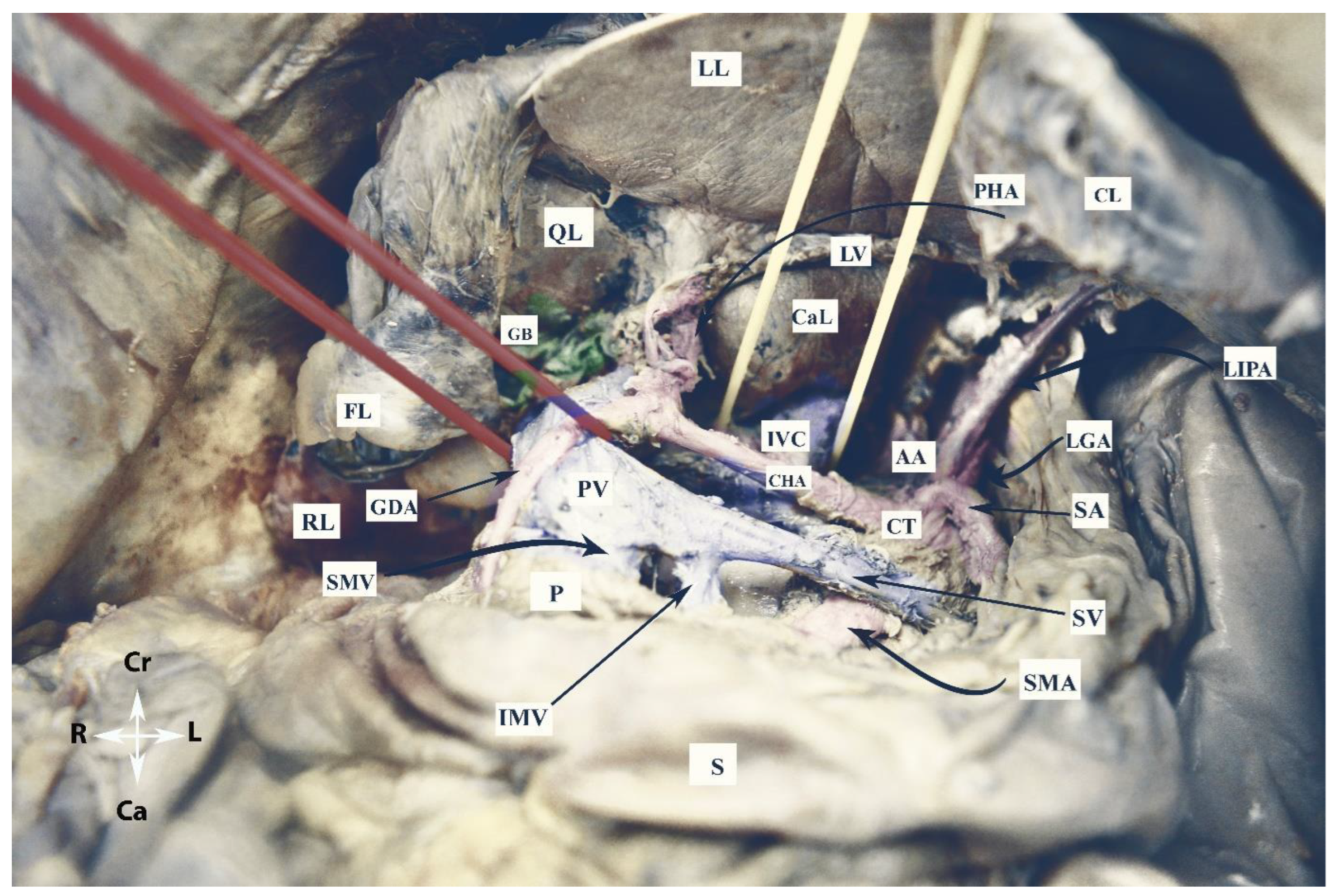
Figure 2. Vessels of the omental bursa (embalmed cadaver, authors’ own material). RL—right lobe of the liver; LL—left lobe of the liver; QL—quadrate lobe; CaL—caudate lobe; CL—coronary ligament of left lobe; FL—incised falciform ligament; LV—ligamentum venosum; S—stomach; P—pancreas; GB—gallbladder; CT—celiac trunk; AA—abdominal aorta; CHA—common hepatic artery; SA—splenic artery; GDA—gastroduodenal artery; LGA—left gastric artery; LIPA—left inferior phrenic artery; PV—portal vein; IVC—inferior vena cava; IMV—inferior mesenteric vein; SMV—superior mesenteric vein; PV—portal vein; SV—splenic vein; Cr—cranial; Ca—caudal; L—left; R—right.
2.1.5. Lymph Nodes
Celiac lymph nodes are situated near the origin of the CT. These nodes are terminal, as they collect lymph from nodes located near the common hepatic, splenic and left gastric vessels. Celiac lymph nodes also drain lymph from most internal organs (liver, gallbladder, stomach, spleen and pancreas) into the cisterna chyli. Right and left small intestinal lymph nodes originate from the celiac nodes and form the small intestinal lymphatic trunk [5][11][15][23][24][25]. The number of celiac nodes varies from 3 to 15 [24][25].
The number of hepatic lymph nodes is variable. They can be divided into hepatic nodes (receiving lymph from the celiac nodes and located near the hepatic artery), subpyloric nodes (four or five nodes near the gastroduodenal artery) and cystic nodes (located at the neck of the gallbladder) [24][25]. The hepatic lymph nodes can be identified in both the PH and HDL [11][15]. The drainage of the hepatic lymph nodes can be classified into superficial and deep lymphatic networks. The superficial is later separated into three main groups, the most common being that passing through the HDL and gastrohepatic ligament. The deep pathway drains the lymph nodes at the liver hilum, and then from the hepatic lymph nodes to the nodes at the HDL. The latter can be divided into two chains—the posterior periportal chain and the hepatic artery chain. The hepatic chain drains into the celiac lymph nodes and then into the cisterna chyli [23][25].
2.2. Omental Bursa and Ovarian Cancer
The spread of OC into the OB occurs primarily by two routes—transcoelomic (peritoneal) spread or progressive lymph node involvement [5].
2.2.1. Transcoelomic Metastases
There are mainly two hypotheses that have been described for the transcoelomic metastasis model in OC. The “seed and soil” theory explains that tumor cells detach from the primary tumor and circulate within the peritoneal cavity through peritoneal fluid before seeding intraperitoneally. The peritoneal fluid and OC cells flow in particular directions in a clockwise rotation—influenced by gravity, they tend to accumulate in the most dependent sites. Subsequently, the intraperitoneal fluid follows a cephalad direction towards the upper abdomen due to the movement of the diaphragm and peristalsis of the bowels. However, anatomical limitations restrict their movement within certain parts of the peritoneal cavity. On the right side, peritoneal fluid passes from the pelvis through the right paracolic gutter, Morison’s pouch, and the OB via the foramen of Winslow. Intraperitoneal fluid flow also reaches the right subphrenic space, including the liver capsule and the diaphragm. However, the falciform ligament limits the flow from the right to the left subphrenic space. Conversely, on the left side, peritoneal fluid is confined by the phrenicocolic ligament to the left paracolic gutter at the level of the inframesocolic recces [26][27][28].
The “metaplasia theory” postulates that metastatic omental sites in OC are not true metastases, but rather a synchronous malignant transformation due to the common lineage between omentum and ovarian epithelium [26][28].
However, both theories are insufficient to fully explain the pathogenesis of peritoneal metastases in OC. The “seed and soil” theory does not explain the different distribution patterns of peritoneal carcinomatosis (some patients have more peritoneal disease in the upper abdomen than in the pouch of Douglas), whereas the “metaplasia” theory implies that ovarian peritoneal carcinomatosis spreads randomly in the abdominal cavity [26][28].
In AEOC, the OB is often affected via the transcoelomic route. Peritoneal metastases in the lesser sac can be found in the following anatomical structures: HDL, PH, medial aspect of Winslow’s foramen, caudate lobe of the liver, parietal peritoneum covering the posterior border of the lesser sac, fissure for ligamentum venosum, subpyloric space, peritoneum over the transverse mesocolon, and posterior surface of the hepatogastric and gastrocolic ligaments [5][13]. The subpyloric space is a cul-de-sac, located below the pylorus. Due to gravity, ovarian tumor cells accumulate in this space along with the peritoneal fluid or ascites [29].
As previously mentioned, the peritoneal spread of OC to the lesser sac is possible only through the foramen of Winslow, which is a connection between the lesser sac and the peritoneal cavity. Thus, transcoelomic lesser sac metastases are absent in cases of adhesions and occlusion of the epiploic foramen (e.g., as a result of previous surgeries in the upper abdomen, with cholecystectomy being the most common cause of adhesions and obliteration of the foramen). Transcoelomic spread to the OB is also linked with conditions such as ascites, peritoneal carcinomatosis, high peritoneal cancer index (PCI), involvement of Morison’s pouch and diaphragmatic dissemination [5]. However, tumor spread into the lesser sac does not consistently follow expected patterns. Supragastric lesser sac metastases are observed in 70% of patients with a normal supracolic omentum. Interestingly, the lesser omentum can remain unaffected in about one-fifth of patients with lesser sac metastases [13]. These findings show that there is no fully reliable predictor for the transcoelomic spread of OC to the OB. Hence, a thorough assessment of the lesser sac is imperative in every patient undergoing cytoreductive surgery for AEOC. If upper abdominal disease is detected, the OB should always be opened and checked. Moreover, the surgeon must be careful of the adhesions because they may contain metastatic nodules.
The percentage of lesser sac peritoneal carcinomatosis has been estimated in a few reports; the majority of studies combine descriptions of both peritoneal and lymph metastases [5][13][29]. Mukhopadhyay et al. reported lesser sac peritoneal metastases in 64% of patients with AEOC. Celiac lymph node metastases were excluded from the study. A PCI equal to or greater than 17 and involvement of Morison’s pouch were identified as the strongest multivariate predictors for lesser sac involvement [13]. Raspagliesi et al. found that 67% of women with AEOC had OB involvement, either peritoneal or lymphatic [5]. The authors specifically estimated transcoelomic dissemination to the OB in 59% of patients, with 81% having supragastric lesser sac involvement and 19% having peritoneal dissemination at the HDL [5]. Tozzi et al. investigated the dissemination of PH and hepato-celiac lymph nodes in 216 patients with AOC. Among these, 31 patients (14.3%) had a tumor on both anatomical sides, and out of these, 18 (8.3%) patients had only HDL involvement [30].
Transcoelomic tumor dissemination of the OB is shown in Figure 3.
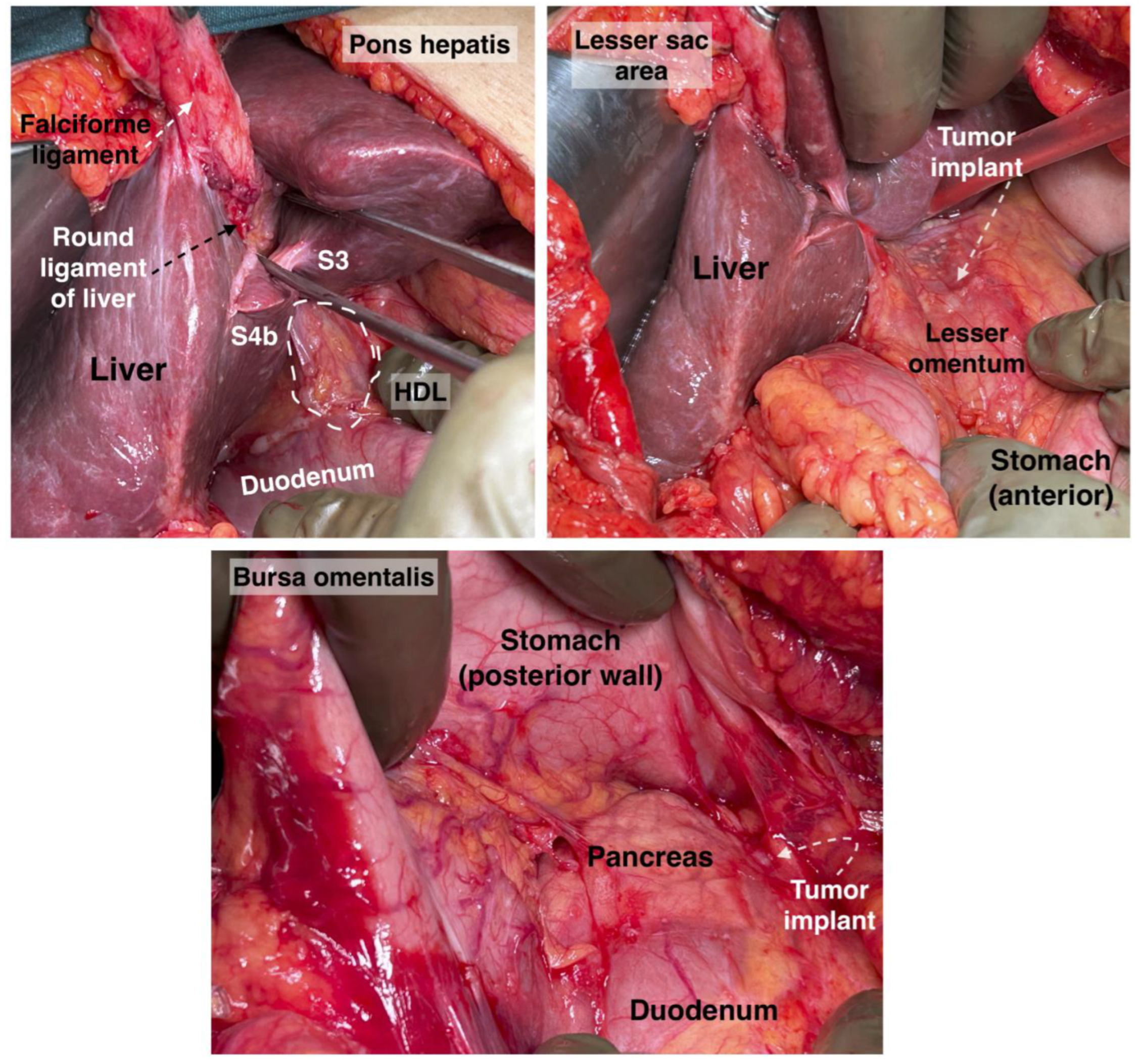
Figure 3. Pons hepatis, hepatoduodenal ligament, lesser sac area and bursa omentalis. Tumor implants will be found in those zones. S: segment, HDL: hepatoduodenal ligament. Surgical archive of author IS.
2.2.2. Omental Bursa Lymph Node Metastases
The other pathway of OC dissemination into the lesser sac is through the lymphatics. The following lymph nodes could be metastatic: triad (hepatoduodenal), portal and celiac lymph nodes (CLNs). The real incidence of CLN involvement is unclear as systematic lymph node dissection is not routinely performed in this region [5][7][30][31][32][33]. Studies have shown that patients with AOC only benefit from the removal of bulky nodes as part of optimal cytoreduction [34]. Therefore, the majority of studies included the rate of metastases among patients with suspicious CLNs [7][30][31][32][33]. Angeles et al. reported on 150 patients with AOC who underwent optimal cytoreduction. Seventeen (11.3%) women had CLN metastases [7]. Raspagliesi et al. reported on 3 patients (8%) with bulky metastatic CLNs among 37 women with AOC [5]. Gallota et al. observed metastases to the hepato-celiac lymph nodes in 52.9% of 85 patients who underwent hepato-celiac lymph node dissection. However, in their study, the hepato-celiac lymph nodes included also the portal and celiac triad lymph nodes [35]. Martinez et al. dissected CLNs in 41 women and found CLN metastases in 23 (56.1%) of the patients. However, the estimated percentage in this study could not be accurate as the authors included women with recurrent disease [32]. Patients with metastatic CLNs have higher PCI and more frequent PH involvement, as well as more frequent involvement of the mesenteric and paraaortic lymph nodes [5][7][32][33][35]. Martinez et al. reported that 81.1% of patients with CLN involvement had metastatic paraaortic lymph nodes [33]. Similarly, Angeles et al. observed that all patients with CLN metastases had metastatic disease of the paraaortic lymph nodes. The authors additionally found that more than 80% of patients with hepatic and lung recurrence had CLN involvement [7]. Martinez et al. reported that metastases to the CLNs are associated with extensive upper abdominal disease, hepatic metastases and a median PCI of 21. The authors also found that 20% of patients with CLN involvement had suspicious mediastinal lymph nodes on imaging tests (CT and PET-CT) [33]. Therefore, CLN dissection should be performed after a thorough preoperative evaluation of the mediastinal lymph nodes. The prognostic impact of CLN involvement is unfavorable as it is associated with decreased disease-free survival (DFS) and reduced overall survival (OS) due to short-term recurrences, increased risk of lymph node progression and resistance to platinum-based chemotherapy [7][32][33]. Furthermore, it is important to note that patients with CLN involvement experience poor outcomes even after undergoing optimal cytoreduction [7][32]. These findings prompt us to question the appropriateness of assigning these OC cases to FIGO stage IIIC [7][33][36][37]. In fact, there is a growing suggestion that this stage should be re-evaluated and possibly divided based on a distinct consideration of metastases to infrarenal lymph nodes and CLNs [7][33]. Notably, some experts have taken this notion a step further, advocating for classifying CLN metastases in line with FIGO stage IVB, a classification similar to that of patients with cardiophrenic lymph node involvement [7].
Comparably to the metastatic CLNs, the rate of metastases to the portal and triad lymph nodes in AOC patients is also hard to estimate. Donato et al. reported a rate of 4.5% for portal node metastases among 55 patients with AEOC and hepatobiliary involvement [31]. Song et al. identified portal lymph node involvement in 1.9% of patients undergoing primary cytoreduction for OC. However, recurrence rates in these nodes were notably as high as 16.7% [38]. Tozzi et al. observed hepato-celiac lymph node metastases in 16.1% of patients with AEOC and macroscopic disease at the PH and found PH and hepato-celiac lymph node involvement in approximately 15% of studied AOC cases [30]. In cases of paraaortic and mesenteric metastatic lymph nodes, it is essential to assess portal and triad lymph nodes during surgery [35]. The presence of portal or triad lymph node involvement is associated with poorer prognosis compared to uninvolved nodes. Involvement of lymph nodes in these regions serves as an indicator of disease severity, decreased DFS and reduced OS [31][35][39]. Retrospective data indicate that hepato-celiac lymph node metastases independently predict decreased progression-free survival (PFS) [35].
The sensitivity and specificity of different imaging modalities for detecting metastatic lymph nodes in the OB vary in the medical literature. One study reported good sensitivity (77%) but low specificity in detecting CLN metastases on CT, highlighting the importance of radiologist expertise (with nonexpert sensitivity at 20%) [7]. Another study indicated that pre-operative CT missed detecting most cases of PH and CLN metastases [5]. Retrospective data suggested that positron emission tomography (PET) CT scans are more sensitive than CT for detecting hepato-celiac lymph nodes and PH peritoneal dissemination [31]. However, another retrospective study reported low sensitivity in the detection of CLN metastases using preoperative PET-CT and CT scans [33]. Nevertheless, a different retrospective study found that a combination of preoperative CT and diagnostic laparoscopy detected all cases of hepato-celiac lymph node metastases and PH peritoneal involvement, with CT alone missing the disease in these particular regions in 31% of cases [30]. Nowadays, oncogynecologists deal with tumor peritoneal implants or lymph node dissemination in the omental bursa. An exception is transcoelomic or lymph node dissemination of the porta hepatis, where an interdisciplinary surgical approach with biliary surgeons is required [30][31][38].
CLN metastases are shown in Figure 4.
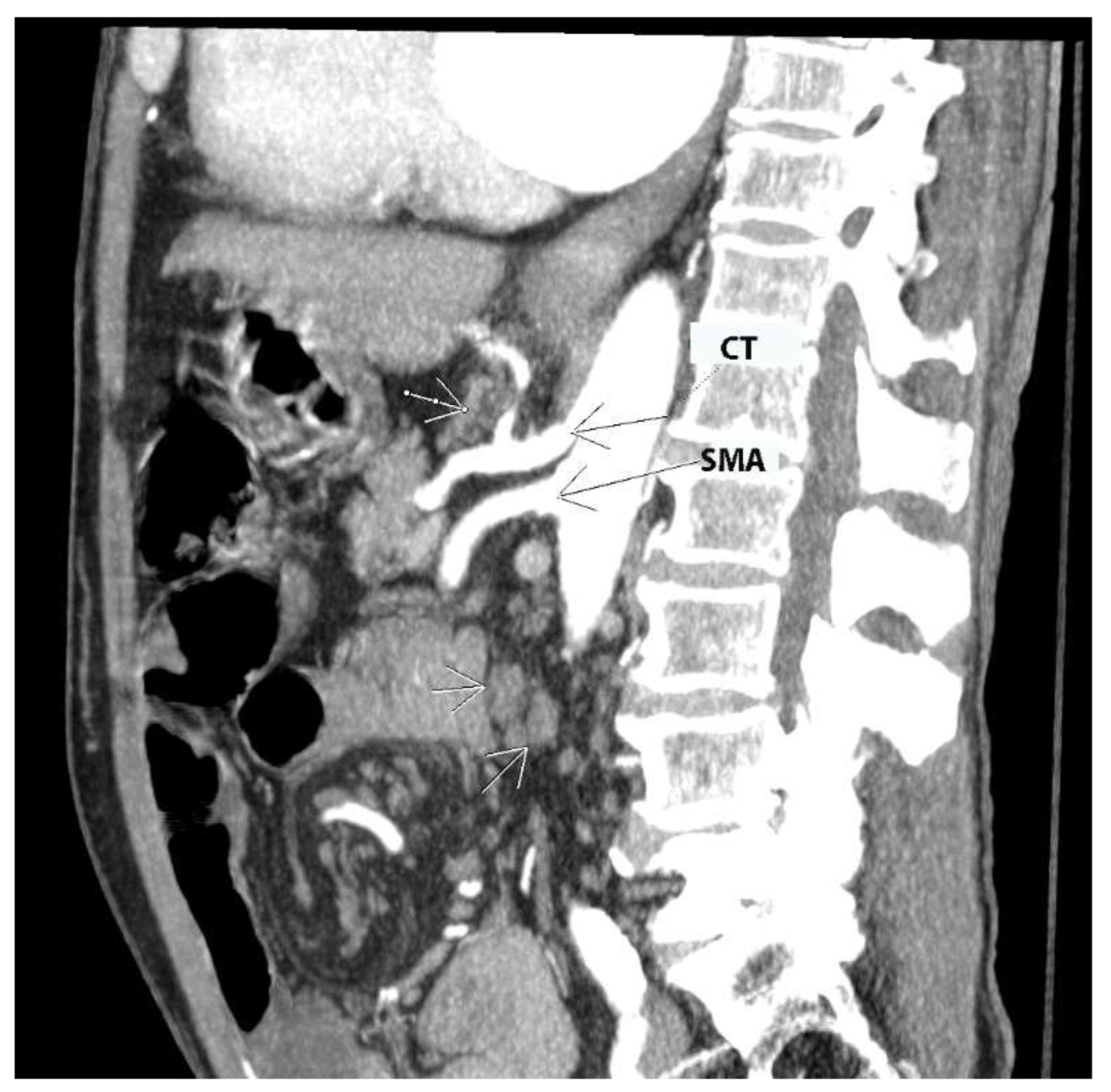
Figure 4. Contrast-enhanced CT in the sagittal plane (authors’ own material). Arrows point to pathologic lymph nodes in celiac and superior mesenteric stations. CT—celiac trunk; SMA—superior mesenteric artery.
2.3. Surgical Approaches to the Omental Bursa
2.3.1. Dissection of the Hepatogastric Ligament (Pars Flaccida)
To gain access to the supragastric part of the OB, the stomach should be retracted to the left, exposing the superior part of the pancreas. The hepatogastric ligament lies between the visceral surface of the left liver lobe and the lesser curvature of the stomach. The left section of the gastrohepatic ligament is thinner than other parts of the ligament because there is almost no fatty tissue between the peritoneal layers. It is also referred to as pars flaccida of the lesser omentum. This is the preferred anatomical entry point to the OB [12][15]. This approach enables access to the supragastric part of the OB. It is important to note potential anatomical variations of the celiac trunk. Particular attention is warranted for cases of a left hepatic artery arising from the left gastric artery (incidence 12–34%), and in rare instances, a common hepatic artery originating from the left gastric artery. In such scenarios, the anomalous hepatic artery crosses the supragastric part of the OB through the midline [40].
2.3.2. Dissection of the Gastrocolic Ligament
The gastrocolic ligament extends from the inferior two-thirds of the greater curvature of the stomach to the transverse mesocolon. On the left, it continues as the gastrosplenic ligament, whereas on the right, it is limited by the gastroduodenal junction. Four layers of the peritoneum that enclose the stomach and the transverse mesocolon/colon are part of the greater omentum. The layers that descend to form the greater omentum later fuse to become the two layers of the gastrocolic ligament at the level of the transverse mesocolon. The anterior layer of the gastrocolic ligament attaches to the greater curvature of the stomach, and the posterior layer attaches to the transverse mesocolon. In adults, the two layers on the right side of the ligament are in close proximity to each other and the transverse mesocolon. On the left side, there is a distance between the two layers of the ligament. Therefore, the left side of the gastrocolic ligament is the preferable point of dissection and entry to the OB [10][11][12][15][41].
While these two approaches are frequently employed in OC surgery, additional techniques will also be discussed. It is important for oncogynecologists to possess a basic familiarity with various surgical accesses to the OB.
2.3.3. Anterior Trans-Omentum Approach
This approach represents a direct transection of the greater omentum at the level of the greater curvature of the stomach [42]. It is suitable for patients with a normal body mass index and a greater omentum characterized by minimal adipose tissue. However, injury to the gastroepiploic vessels can potentially compromise the dissection.
2.3.4. Dissection of the Gastrosplenic Ligament
The gastrosplenic ligament forms through the lateral fusion of the peritoneal layers of the greater omentum. It is a thin attachment between the left part of the great stomach curvature and the hilum of the spleen. During dissection of the gastrosplenic ligament, the surgeons should be aware of the short gastric vessels and the left gastroepiploic vessels [10][11][12][15].
2.3.5. Trans-Mesocolic Dissection
In this technique, the transverse mesocolon is dissected above the inferior border of the pancreas. Surgeons should be aware of the possible presence of the Moskowitz artery, an anatomical variation found in up to 17% of patients. This artery, also known as the meandering mesenteric artery, forms a collateral pathway between the left colic and middle colic arteries, passing above the inferior border of the pancreas [42][43][44].
The different surgical approaches are shown in Figure 5.
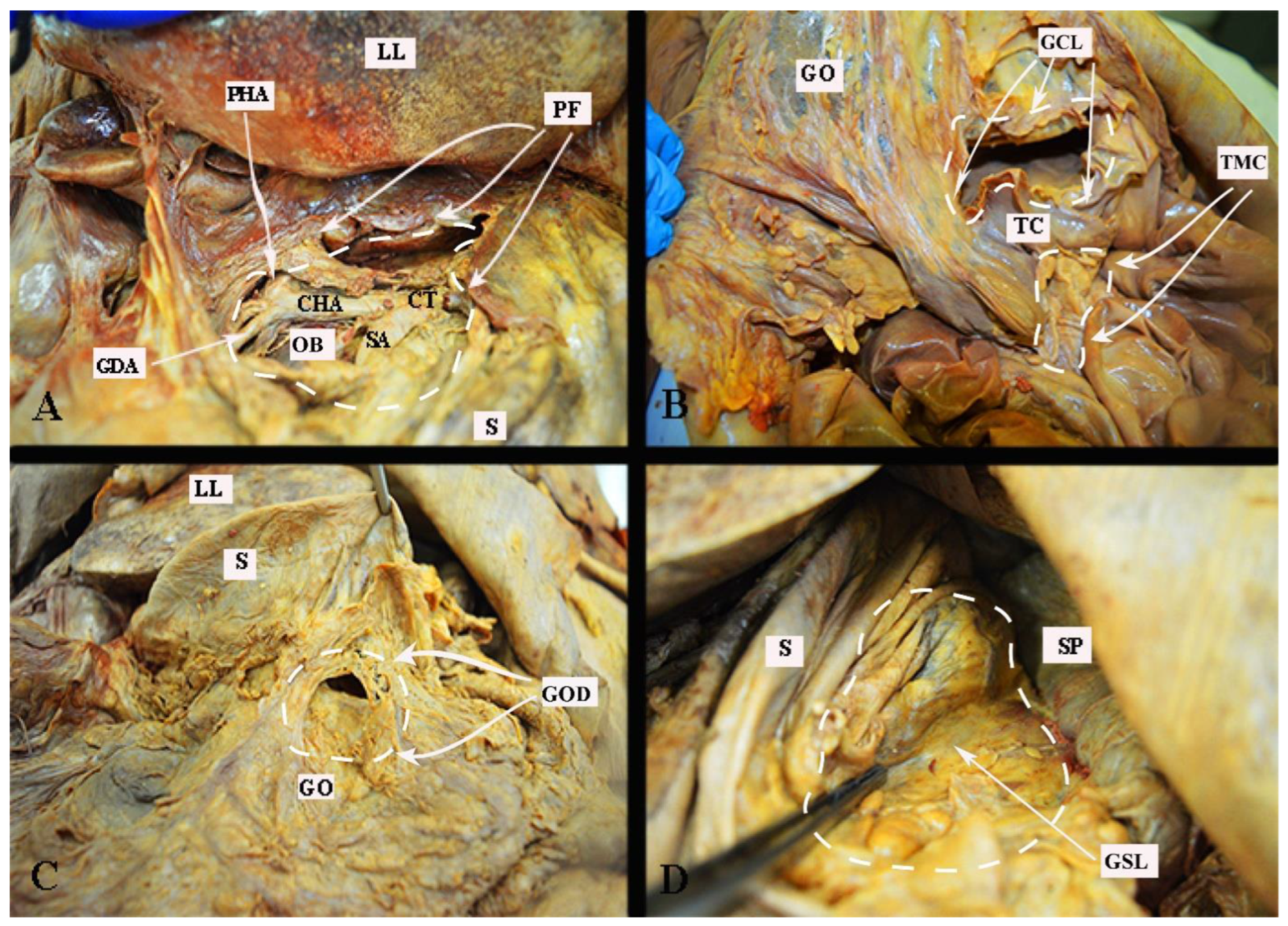
Figure 5. Surgical approaches to the omental bursa (embalmed cadaver, authors’ own material). (A) Dissection through the pars flaccida. (B) Trans-mesocolic approach and dissection of the gastrocolic ligament. (C) Direct dissection over the greater omentum just below the great curvature of the stomach. (D) Dissection of the gastrosplenic ligament. PF—pars flaccida; LL—left lobe of the liver; OB—omental bursa (supragastric part); GDA—gastroduodenal artery; CHA—common hepatic artery; CT—celiac trunk; SA—splenic artery; S—stomach; PHA—proper hepatic artery; GCL—gastrocolic ligament; TC—transverse colon; GO—greater omentum; TMC—trans-mesocolic dissection; GOD—greater omentum dissection just below its attachment to the great stomach curvature; SP—spleen; GSL—gastrosplenic ligament.
2.3.6. Kocher Maneuver
Dissection of the gastrocolic and gastrohepatic ligaments provides good access to the OB and celiac trunk but is insufficient for dissection of the HDL and PH. To fully expose these structures, a maneuver for the mobilization of the duodenum and head of the pancreas was first described by Theodor Kocher. This approach to duodeno-pancreatic mobilization, commonly used in visceral surgeries such as the Whipple procedure, or in emergent surgeries for retroperitoneal hemorrhage, and can also be beneficial in gynecologic-oncological procedures in cases of tumor dissemination involving the PH, HDL, and suprarenal lymph nodes. The Kocher maneuver starts with the medialization of the first, second and proximal third portions of the duodenum. A vertical incision of the parietal peritoneum is made 1–2 cm lateral to the second part of the duodenum. The incision extends perpendicularly between the lateral aspect of the epiploic foramen and the inferior duodenal flexure. The procedure continues with a gentle dissection of the fascia of Toldt (the peritoneal adhesion plane between the visceral peritoneum of the ascending mesocolon and the retroperitoneum), which is located lateral to the duodenum and the head of the pancreas. A further avascular plane containing loose connective tissue and allowing for easy and bloodless dissection is situated below the duodenum and the head of the pancreas and corresponds to the fusion fascia of Treitz (the adhesion plane between the visceral peritoneum of the duodenum and pancreas and the retroperitoneum). Both structures are covered from above by the visceral peritoneum and the fusion fascia of Fredet (the plane between the ascending mesocolon and the visceral duodenal–pancreatic peritoneum) [45][46][47][48][49].
The Kocher maneuver allows access to the infrahepatic IVC, duodenum, abdominal aorta, superior mesenteric artery, posterior surface of the head of the pancreas, right renal hilum and HDL. The limit of the dissection is the medial aspect of the IVC, determined by identifying the left renal vein. The inferior mesenteric vein has also been described as the medial limit. The dissection of the peritoneum can be carried out cranially up to the retrohepatic IVC, thereby enabling the dissection of the posterior part of the PH [5][30][46][47][48]. The precise incision point for entry is critical, as a more lateral incision could open the renal fascia and lose the right plane of dissection. Conversely, injury to the duodenum and vessels is possible if the incision is made more medially than usual [47]. The Kocher maneuver is often combined with the Cattel—Braasch maneuver, which represents a mobilization of the ascending colon from the retroperitoneum after dissection of Toldt’s fascia [46]. Currently, the Kocher maneuver is also performed by oncogynecologists [47]. The Kocher maneuver and fascias near the duodenum and pancreas head are depicted in Figure 6, Figure 7 and Figure 8.
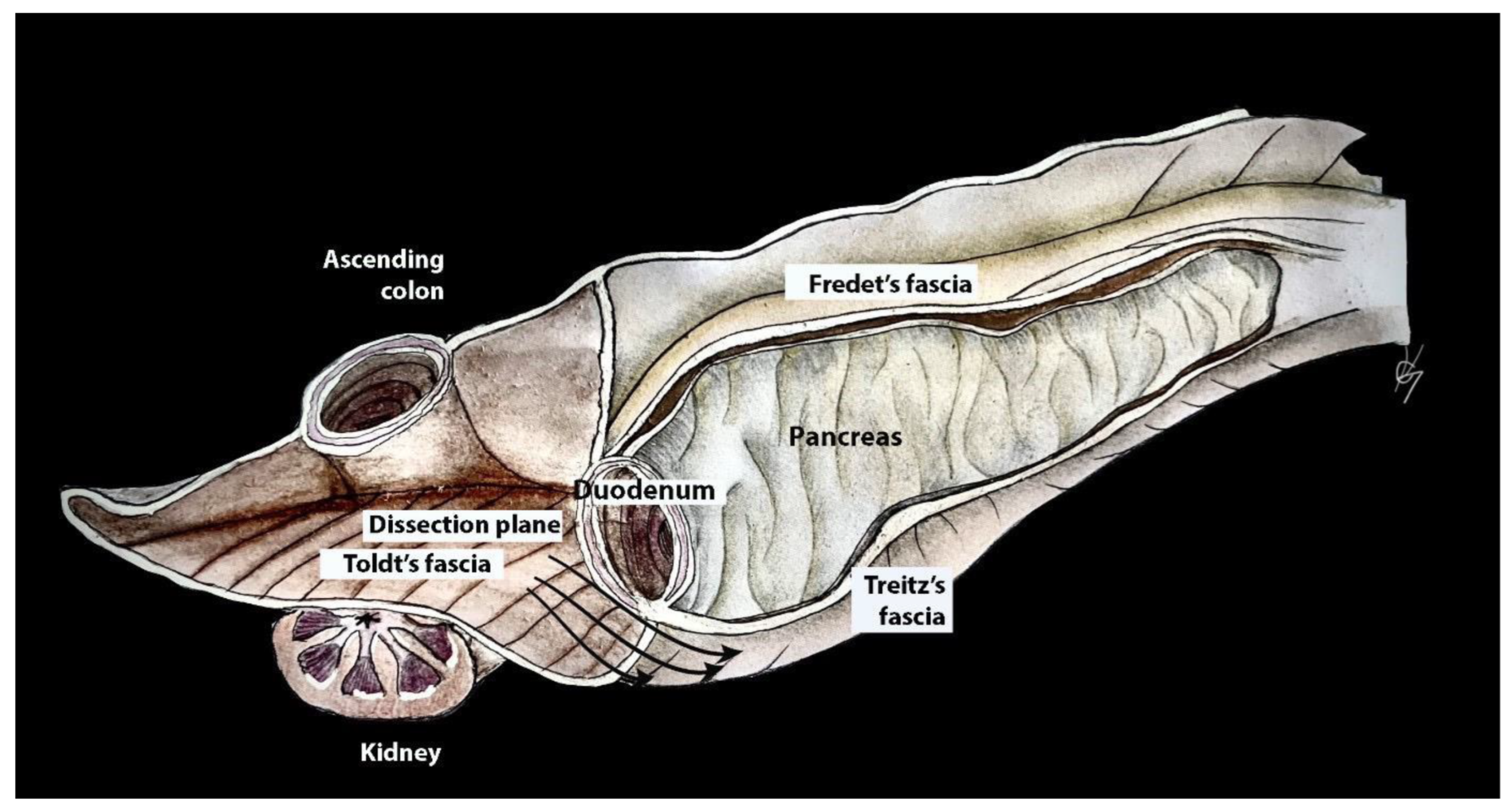
Figure 6. Dissection plane between the fusion fascia of Toldt and fusion fascia of Treitz during the Kocher maneuver (authors’ own material—modified from reference [48]).
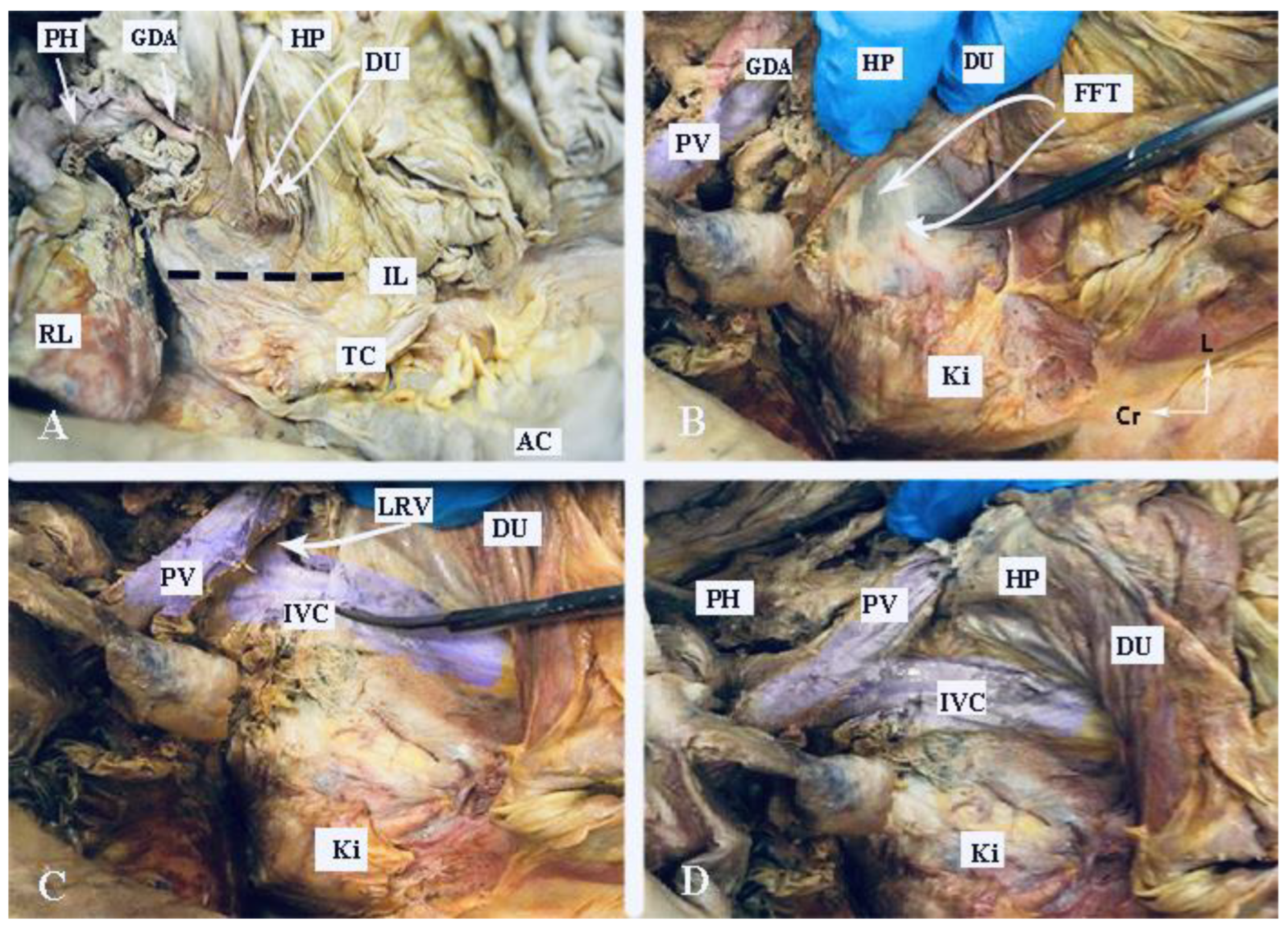
Figure 7. Kocher maneuver (embalmed cadaver, authors’ own material). (A) The duodenum and stomach are retracted medially. The incision line of the peritoneum is indicated by the interrupted black line. (B) Dissection of the fusion fascia of Treitz. (C) Mobilization of the duodenum and pancreatic head at the level of the left renal vein. The IVC is identified. (D) Anatomical structures after completion of the Kocher maneuver. PH—porta hepatis, GDA—gastroduodenal artery; HP—head of the pancreas; DU—duodenum; IL—incision line; RL—right lobe of the liver; TC—transverse colon; AC—ascending colon; PV—portal vein; Ki—kidney; FFT—fusion fascia of Treitz; LRV—left renal vein; Cr—cranial; L—left.
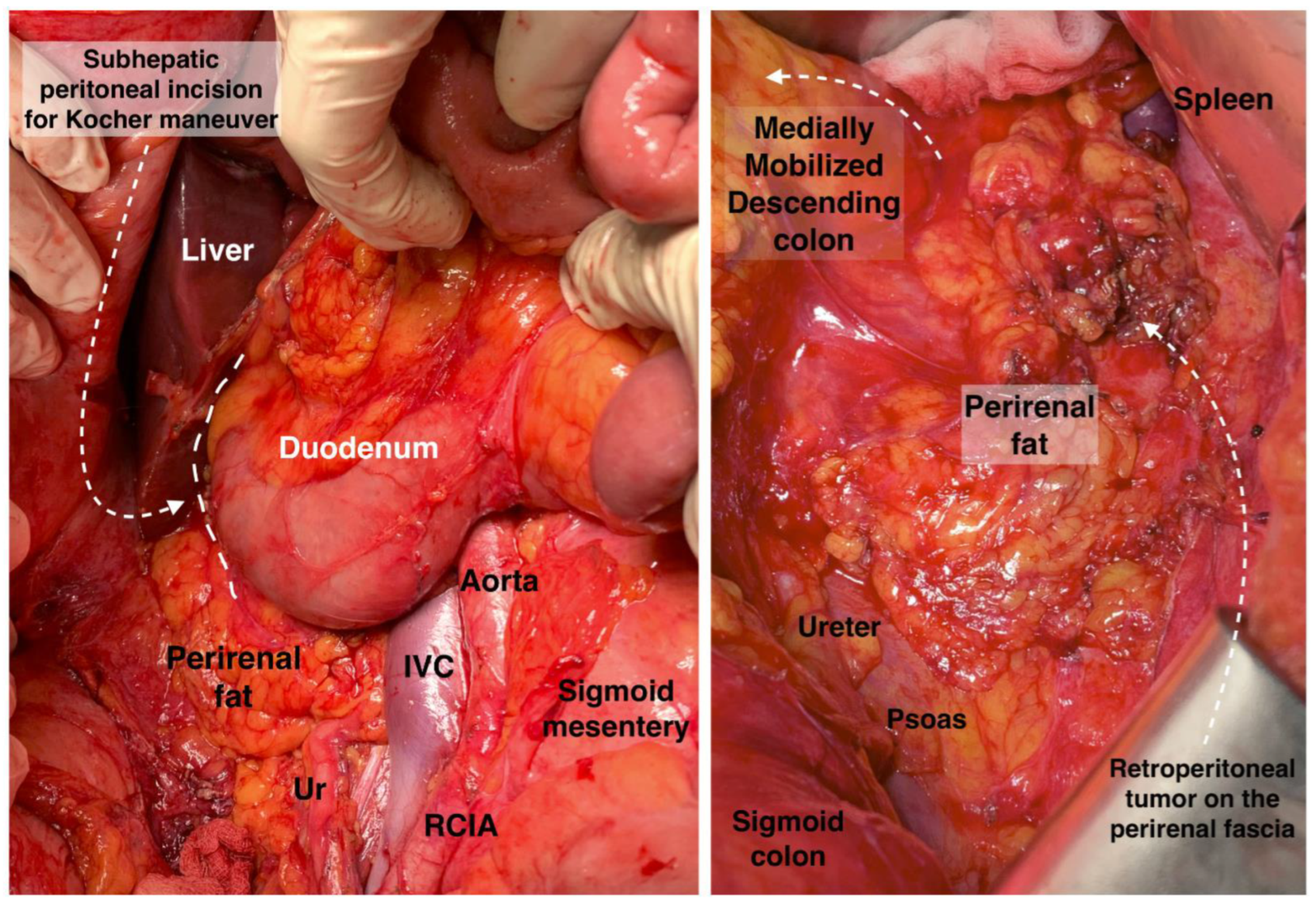
Figure 8. Anatomy for Kocher maneuver and retroperitoneal access on the (right) and (left) side. Ur: ureter, IVC: inferior vena cava, RCIA: right common iliac artery. Surgical archive of author IS.
2.3.7. Dissection of Portal, Celiac and Triad Lymph Nodes
The full exposure of the OB is achieved through the dissection of the gastrocolic and gastrohepatic ligaments and the Kocher maneuver. The HDL is isolated using a vessel loop through the foramen of Winslow, enabling traction of the ligament. For hepatic resections, this vessel loop can additionally control the vascular flow of the liver (Pringle maneuver); the loop can close the hepatoduodenal vascular flow for up to 25–30 min [18][30]. Dissection begins with peritonectomy of the HDL within a tumor-free zone. The hepatic artery and common bile duct are dissected. The artery is identified after cranial retraction of the stomach and gentle caudal retraction of the pancreas. The peritoneum is dissected between the superior part of the duodenum and the ligamentum teres hepatis. The HDL is retracted medially with the vessel loop, and the posterior peritoneum of the ligament is dissected. The portal vein is identified, and all structures of the portal triad are visualized and mobilized. Enlarged lymph nodes at the PH, proper hepatic artery and common bile duct are meticulously separated and dissected [30][33][35]. Suspicious lymph nodes between the portal vein and infrahepatic IVC are removed after gentle medial traction of the portal vein with the vessel loop. In cases of other enlarged lymph nodes, the dissection proceeds in a retrograde manner along the gastroduodenal artery, right gastric artery and common hepatic arteries. This pathway leads to the celiac trunk, where enlarged lymph nodes are also resected. There are various dissection techniques; however, all authors start dissection immediately after the identification of all anatomical structures within the OB, which will prevent inadvertent injuries and enable immediate actions for bleeding complications. Starting lymphadenectomy from the arteries and using them as landmarks during dissection is a commonly employed approach in oncogynecology [30][33][35]. Some surgeons perform cholecystectomy for better exposure of the right side of the HDL and PH [33]. It should be stressed that in approximately 3.5% of cases, the common hepatic artery may originate from the superior mesenteric artery [50].
References
- Sehouli, J.; Senyuva, F.; Fotopoulou, C.; Neumann, U.; Denkert, C.; Werner, L.; Gülten, O.Ö. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J. Surg. Oncol. 2009, 99, 424–427.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30.
- Bristow, R.E.; Karlan, B.Y.; Chi, D.S. Surgery for Ovarian Cancer, 3rd ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2016.
- Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Poynor, E.A.; Aghajanian, C.; Jarnagin, W.R.; DeMatteo, R.P.; D’Angelica, M.I.; Barakat, R.R.; et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 1083–1090.
- Raspagliesi, F.; Ditto, A.; Martinelli, F.; Haeusler, E.; Lorusso, D. Advanced ovarian cancer: Omental bursa, lesser omentum, celiac, portal and triad nodes spread as cause of inaccurate evaluation of residual tumor. Gynecol. Oncol. 2013, 129, 92–96.
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244.
- Angeles, M.A.; Ferron, G.; Cabarrou, B.; Balague, G.; Martínez-Gómez, C.; Gladieff, L.; Pomel, C.; Martinez, A. Prognostic impact of celiac lymph node involvement in patients after frontline treatment for advanced ovarian cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 1410–1416.
- Ren, Y.; Li, R.; Feng, H.; Xie, J.; Gao, L.; Chu, S.; Li, Y.; Meng, F.; Ning, Y. Single-cell sequencing reveals effects of chemotherapy on the immune landscape and TCR/BCR clonal expansion in a relapsed ovarian cancer patient. Front. Immunol. 2022, 13, 985187.
- Taskiran, C.; Vatansever, D.; Giray, B.; Eraslan, A.; Tanju, S.; Arvas, M.; Balik, E. Upper abdominal debulking surgery for ovarian cancer total colectomy, total peritonectomy, and extended upper abdominal debulking surgery. Int. J. Gynecol. Cancer 2020, 30, 1648–1649.
- Elmohr, M.M.; Blair, K.J.; Menias, C.O.; Nada, A.; Shaaban, A.M.; Sandrasegaran, K.; Elsayes, K.M. The Lesser Sac and Foramen of Winslow: Anatomy, Embryology, and CT Appearance of Pathologic Processes. AJR. Am. J. Roentgenol. 2020, 215, 843–851.
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175.
- Brenkman, H.J.F.; van der Wielen, N.I.; Ruurda, J.P.; van Leeuwen, M.S.; Scheepers, J.J.G.; van der Peet, D.L.; van Hillegersberg, R.; Bleys, R.L.A.W.; Cuesta, M.A. Surgical anatomy of the omental bursa and the stomach based on a minimally invasive approach: Different approaches and technical steps to resection and lymphadenectomy. J. Thorac. Dis. 2017, 9 (Suppl. S8), S809–S816.
- Mukhopadhyay, A.; Bizzarri, N.; Bradbury, M.; Sinha, S.; Bhaumik, J.; Helm, C.W. Metastatic Involvement of Lesser Sac in Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 293–301.
- Yoo, E.; Kim, J.H.; Kim, M.-J.; Yu, J.-S.; Chung, J.-J.; Yoo, H.-S.; Kim, K.W. Greater and Lesser Omenta: Normal Anatomy and Pathologic Processes1. RadioGraphics 2007, 27, 707–720.
- Netter, F.H. Atlas of Human Anatomy, 2nd ed.; 18 Guilford Press: New York, NY, USA, 2000.
- Röthlin, M.; Largiadèr, F. The anatomy of the hepatoduodenal ligament in laparoscopic sonography. Surg. Endosc. 1994, 8, 173–180.
- Juszczak, A.; Mazurek, A.; Walocha, J.A.; Pasternak, A. Coeliac trunk and its anatomic variations: A cadaveric study. Folia Morphol. 2021, 80, 114–121.
- Ramirez, P.T.; Frumovitz, M.; Abu-Rustum, N.R. Principles of Gynecologic Oncology Surgery; Elsevier: Amsterdam, The Netherlands, 2018; p. 5. ISBN 978032342878.
- Shrikantaiah, V.C.; Basappa, M.; Hazrika, S.; Ravindranath, R. Study of surgical anatomy of portal vein of liver segments by cast method and its clinical implications. Anat. Cell Biol. 2018, 51, 232–235.
- Mazziotti, A.; Grazi, G.L. Surgical Anatomy of the Portal System. In Portal Hypertension; Springer: Berlin/Heidelberg, Germany, 2000; pp. 51–56.
- Kanasker, N.; Sonje, P.; Vatsalaswamy, P. Study of Variations in the Draining Pattern of Inferior Mesenteric Vein with Its Surgical Importance. Int. J. Anat. Res. 2018, 6, 4897–4900.
- Neginhal, D.D.; Kulkarni, U.K. Normal anatomy of porta hepatis—A cadaveric study. Natl. J. Clin. Anat. 2019, 8, 22.
- Ahluwalia, N.; Nassereddin, A.; Futterman, B. Anatomy, Abdomen and Pelvis: Celiac Trunk; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459241/ (accessed on 19 September 2022).
- Mehta, K.; Tubbs, R.S. Lymphatics of the Abdomen. In Surgical Anatomy of the Lateral Transpsoas Approach to the Lumbar Spine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 147–149.
- Harisinghani, M.G. Abdominal Lymph Node Anatomy. In Atlas of Lymph Node Anatomy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 59–88.
- Mei, S.; Chen, X.; Wang, K.; Chen, Y. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer Cell Int. 2023, 23, 11.
- Pannu, H.K.; Oliphant, M. The subperitoneal space and peritoneal cavity: Basic concepts. Abdom. Imaging 2015, 40, 2710–2722.
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet. Oncol. 2006, 7, 925–934.
- Sugarbaker, P.H. The subpyloric space: An important surgical and radiologic feature in pseudomyxoma peritonei. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2002, 28, 443–446.
- Tozzi, R.; Traill, Z.; Garruto Campanile, R.; Ferrari, F.; Soleymani Majd, H.; Nieuwstad, J.; Hardern, K.; Gubbala, K. Porta hepatis peritonectomy and hepato-celiac lymphadenectomy in patients with stage IIIC-IV ovarian cancer: Diagnostic pathway, surgical technique and outcomes. Gynecol. Oncol. 2016, 143, 35–39.
- Di Donato, V.; Giannini, A.; D’Oria, O.; Schiavi, M.C.; Di Pinto, A.; Fischetti, M.; Lecce, F.; Perniola, G.; Battaglia, F.; Berloco, P.; et al. Hepatobiliary Disease Resection in Patients with Advanced Epithelial Ovarian Cancer: Prognostic Role and Optimal Cytoreduction. Ann. Surg. Oncol. 2021, 28, 222–230.
- Martínez, A.; Pomel, C.; Filleron, T.; De Cuypere, M.; Mery, E.; Querleu, D.; Gladieff, L.; Poilblanc, M.; Ferron, G. Prognostic relevance of celiac lymph node involvement in ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 48–53.
- Martinez, A.; Pomel, C.; Mery, E.; Querleu, D.; Gladieff, L.; Ferron, G. Celiac lymph node resection and porta hepatis disease resection in advanced or recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2011, 121, 258–263.
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832.
- Gallotta, V.; Ferrandina, G.; Vizzielli, G.; Conte, C.; Lucidi, A.; Costantini, B.; De Rose, A.M.; Di Giorgio, A.; Zannoni, G.F.; Fagotti, A.; et al. Hepatoceliac Lymph Node Involvement in Advanced Ovarian Cancer Patients: Prognostic Role and Clinical Considerations. Ann. Surg. Oncol. 2017, 24, 3413–3421.
- Menczer, J. Is there a revision needed of the current FIGO staging system? Acta Obstet. Gynecol. Scand. 2017, 96, 1159–1161.
- Prader, S.; Harter, P.; Grimm, C.; Traut, A.; Waltering, K.U.; Alesina, P.F.; Heikaus, S.; Ataseven, B.; Heitz, F.; Schneider, S.; et al. Surgical management of cardiophrenic lymph nodes in patients with advanced ovarian cancer. Gynecol. Oncol. 2016, 141, 271–275.
- Song, Y.J.; Lim, M.C.; Kang, S.; Seo, S.S.; Kim, S.H.; Han, S.S.; Park, S.Y. Extended cytoreduction of tumor at the porta hepatis by an interdisciplinary team approach in patients with epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 253–257.
- Huang, H.; Wei, R.; Long, Y.; Mo, Y.; Xie, Y.; Yao, D. Hepatic Hilar Lymph Node Resection in Cytoreductive Surgery for Advanced Ovarian Cancer: A Necessity or Not? Cancer Manag. Res. 2021, 13, 7981–7988.
- Abid, B.; Douard, R.; Chevallier, J.M.; Delmas, V. L’artère hépatique gauche, variations anatomiques et implications cliniques . Morphologie 2008, 92, 154–161.
- Gore, R.M.; Newmark, G.M.; Thakrar, K.H.; Mehta, U.K.; Berlin, J.W. Pathways of abdominal tumour spread: The role of the subperitoneal space. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2009, 9, 112–120.
- Garcia-Granero, A.; Romaguera, V.P.; Millan, M.; Pellino, G.; Fletcher-Sanfeliu, D.; Frasson, M.; Flor-Lorente, B.; Ibañez-Canovas, N.; Saenz, O.C.; Sánchez-Guillén, L.; et al. A video guide of five access methods to the splenic flexure: The concept of the splenic flexure box. Surg. Endosc. 2020, 34, 2763–2772.
- Garcia-Granero, A.; Sánchez-Guillén, L.; Carreño, O.; Sancho Muriel, J.; Alvarez Sarrado, E.; Fletcher Sanfeliu, D.; Flor Lorente, B.; Frasson, M.; Martinez Soriano, F.; Garcia-Granero, E. Importance of the Moskowitz artery in the laparoscopic medial approach to splenic flexure mobilization: A cadaveric study. Tech. Coloproctology 2017, 21, 567–572.
- Karatay, E.; Javadov, M. The importance of the Moskowitz artery as a lesser-known collateral pathway in the medial laparoscopic approach to splenic flexure mobilisation and its evaluation with preoperative computed tomography. Wideochirurgia Inne Tech. Maloinwazyjne 2021, 16, 305–311.
- Fletcher-Sanfeliu, D.; García-Granero, Á.; Doménech Dolz, A.; Pellino, G.; Orbis, F.; Arroyo, A.; Valverde-Navarro, A.A.; Ortí, L.S.; Martín-González, I. Surgical anatomy applied to transperitoneal approaches of the abdominal aorta and visceral trunks. Dynamic article. Anatomía quirúrgica aplicada a abordajes transperitoneales de la aorta abdominal y los troncos viscerales. Artículo dinámico. Cir. Esp. 2021, 99, 562–571.
- Livani, A.; Angelis, S.; Skandalakis, P.N.; Filippou, D. The Story Retold: The Kocher Manoeuvre. Cureus 2022, 14, e29409.
- Song, Y.J.; Suh, D.S.; Kim, K.H.; Na, Y.J.; Lim, M.C.; Park, S.Y. Suprarenal lymph node dissection by the Kocher maneuver in the surgical management of ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, 29, 647–648.
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon. Med. Sch. 2022, 89, 246–254.
- Garcia-Granero, A.; Pellino, G.; Frasson, M.; Fletcher-Sanfeliu, D.; Bonilla, F.; Sánchez-Guillén, L.; Dolz, A.D.; Romaguera, V.P.; Ortí, L.S.; Martinez-Soriano, F.; et al. The fusion fascia of Fredet: An important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg. Endosc. 2019, 33, 3842–3850.
- Chen, H.; Yano, R.; Emura, S.; Shoumura, S. Anatomic variation of the celiac trunk with special reference to hepatic artery patterns. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2009, 191, 399–407.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
989
Revisions:
2 times
(View History)
Update Date:
22 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




