Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hei Sung Kim | -- | 2130 | 2024-01-18 18:21:40 | | | |
| 2 | Mona Zou | Meta information modification | 2130 | 2024-01-19 10:23:08 | | | | |
| 3 | Mona Zou | Meta information modification | 2130 | 2024-01-19 10:23:35 | | | | |
| 4 | Mona Zou | -31 word(s) | 2099 | 2024-01-24 08:58:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kim, S.; Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. Encyclopedia. Available online: https://encyclopedia.pub/entry/54066 (accessed on 07 February 2026).
Kim S, Woo YR, Cho SH, Lee JD, Kim HS. Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. Encyclopedia. Available at: https://encyclopedia.pub/entry/54066. Accessed February 07, 2026.
Kim, Suyeon, Yu Ri Woo, Sang Hyun Cho, Jeong Deuk Lee, Hei Sung Kim. "Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology" Encyclopedia, https://encyclopedia.pub/entry/54066 (accessed February 07, 2026).
Kim, S., Woo, Y.R., Cho, S.H., Lee, J.D., & Kim, H.S. (2024, January 18). Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. In Encyclopedia. https://encyclopedia.pub/entry/54066
Kim, Suyeon, et al. "Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology." Encyclopedia. Web. 18 January, 2024.
Copy Citation
Bleomycin and 5-fluorouracil (5-FU) are widely used in various dermatological disorders. Both drugs are well-recognized as antineoplastic drugs and exert their effect by blocking the cell cycle. Topical and intralesional formulations are available and have been studied in both non-neoplastic and cancerous lesions. However, data comparing the effect of bleomycin and 5-FU in the dermatological disorders are limited.
5-fluorouracil
bleomycin
dermatology
injection
topical
1. Hypertrophic Scar and Keloid
1.1. Efficacy
Hypertrophic scar and keloid are both abnormal tissue reactions following inflammation, trauma, or surgery. While a regular wound healing process recruits inflammatory cells, keratinocytes, and fibroblasts, its excessive and prolonged activation can lead to the formation of hypertrophic scars and keloids.
Since bleomycin induces the apoptosis of keratinocytes and inhibits collagen synthesis, it has also been applied to hypertrophic scars and keloids via intralesional administration. A meta-analysis study on the efficacy of bleomycin identified that bleomycin had a greater effect than other treatments, including triamcinolone (TAC) and 5-FU [1].
5-FU also exerts its effect on scars through its antimetabolic activity and the suppression of fibroblast proliferation [2]. Three randomized controlled trials (RCTs) reported 5-FU to be comparable to TAC [3][4]. In addition, one RCT and one comparative study showed that the combination of intralesional TAC with 5-FU was superior to 5-FU alone [5][6].
1.2. Dosage and Techniques
As for bleomycin, intradermal injection and the multipuncture method are effective [7]. Bleomycin is most often diluted with normal saline or distilled water to a concentration of 1.0 to 1.5 mg/mL per dosage [8]. A combination with intralesional TAC can be a better option on refractory keloid than using bleomycin alone (0.3 mL of diluted bleomycin mixed with 0.3 mL of TAC 40 mg/mL and 0.3 mL of lidocaine) [9].
For 5-FU, it is manufactured at a concentration of 50 mg/mL and 0.2–0.4 mL is usually injected per/cm2 area [4][7]. A combination with intralesional TAC can be a better treatment option than using 5-FU alone (0.9 mL of 50 mg/mL 5-FU mixed with 0.1 mL of TAC 40 mg/mL) [5][6].
A comparative study showed that intralesional bleomycin resulted in greater improvements in hypertrophic scars than intralesional 5-FU injection [10]. The depth of 5-FU and bleomycin injection should be aimed at the mid-dermis to avoid necrosis or ulceration [8]. Injections at 2–4-week intervals are recommended. Figure 1 demonstrates the treatment of a truncal keloid with seven sessions of bleomycin injection at 3–4-week intervals. A final follow-up was performed after six months with no sign of recurrence.
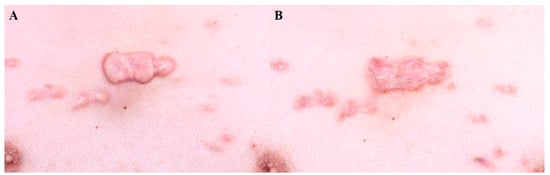
Figure 1. Truncal keloid, (A) before treatment and (B) after 7 sessions of bleomycin injection (every 4 weeks).
2. Wart
2.1. Efficacy
Wart is an abnormal epidermal proliferation caused by human papillomavirus (HPV) infection in keratinocytes. Many topical and intralesional agents have been studied for the treatment of warts, since cryotherapy alone is often not satisfactory in terms of efficacy.
Since bleomycin blocks the cell cycle and cleaves DNA in viruses, it has been applied to warts since the 1970s [8]. Two RCTs reported that intralesional bleomycin had significantly higher cure rates than cryotherapy [11][12]. In addition, it had superior effects on recalcitrant warts [12][13]. A comparative study showed that intralesional bleomycin injection had higher clearance rates of warts compared to 5-FU and Candida albicans antigen injection [14].
5-FU exerts its effects on warts by disrupting viral DNA synthesis. Two RCTs evaluated the efficacy of intralesional 5-FU and local anesthesia (4 mL of 50 mg/mL 5-FU, 1 mL of a mixture of 20 mg/mL lidocaine, and 0.0125 mg/mL epinephrine), comparing it with saline injection with the successful treatment of 65% of the warts [15][16]. However, an RCT reported that combining topical 5-FU with cryotherapy had no additional benefit when compared to cryotherapy alone [17].
2.2. Dosages and Techniques
Bleomycin is usually diluted with normal saline or distilled water at a concentration of 0.5 or 1.0 U/mL [13]. Typically, 0.2 mL is injected into 5 mm warts and up to 1 mL in larger warts [11]. Intralesional injection is the most widely chosen administration technique, but the multipuncture method or a microneedling pen can be applied to reduce pain [18]. According to a prospective study, a local electroporation procedure after intralesional bleomycin injection showed a higher cure rate than bleomycin alone [19].
For 5-FU, intralesional injection (50 mg/mL) into the wart is often recommended. Two RCTs showed that topical 5-FU under occlusion or in combination with salicylic acid could enhance the effect of 5-FU cream [20][21].
Unlike hypertrophic scars, the injections on warts should be aimed at the superficial dermis, since epidermal necrosis is important for eliminating the wart [8]. Injections at 2–4-week intervals are recommended. Figure 2 demonstrates the disappearance of periungual warts following two sessions of bleomycin injection at an interval of 2 weeks.
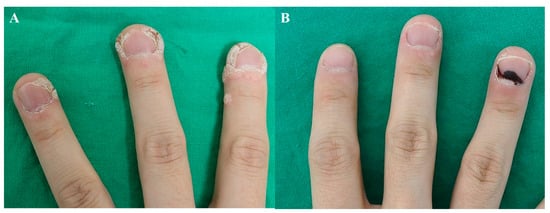
Figure 2. Periungual warts, (A) before treatment and (B) after 2 sessions of bleomycin injection (2 weeks apart).
3. Skin Cancer
3.1. Efficacy
Complete excision remains the mainstay of treatment for skin cancer [22]. However, for patients who are not optimal candidates for surgery due to old age or co-morbidities, local bleomycin or 5-FU can be a viable option. Since bleomycin and 5-FU block the cell cycle and disrupt DNA synthesis, trials on skin cancer have been conducted. Overall, data are limited on the clinical efficacy of bleomycin and 5-FU on skin cancer and only 5% 5-FU cream is FDA-approved for the treatment of actinic keratosis and superficial basal cell carcinoma [22].
3.2. Actinic Keratosis
Topical 5-FU has been widely used to treat AK with sufficient clinical data on its efficacy [23]. Two RCTs identified that combining topical 5-FU with cryotherapy resulted in a higher clearance of AK than cryotherapy alone [24][25]. The study also recommended the once-daily application of a 0.5% formulation, as it offers similar efficacy to 5% 5-FU cream while causing less irritation, thereby increasing patient compliance [25]. In countries where 5-FU cream is not available, and in cases of full-thickness irregularity (Bowen’s disease) and fewer lesions, intralesional 5-FU can be attempted.
3.3. Basal Cell Carcinoma
The FDA has approved 5% 5-FU cream for superficial BCC and has numerous data to support its effect [22][26]. However, there are few trials and case studies on the use of intralesional 5-FU on BCC. Miller et al. (2009) [26] conducted an RCT using an investigational agent (i.e., intralesional 5-FU (30 mg/mL)/epinephrine (0.1 mg/mL) gel) for superficial and nodular BCC. Although this gel formulation is not yet commercially available, the results were promising.
There have been two clinical trials on bleomycin-mediated electrochemotherapy (ECT) for BCC [27][28] where electroporation was used to increase the penetration of bleomycin into cancer cells. Glass et al. (1997) [27] laid out a protocol for injecting 0.5 to 1 mg of bleomycin depending on the tumor size, followed by the delivery of an electric pulse. In this study, most patients (94%) required only a single session of ECT. The second ECT study offered treatment every 3 weeks until complete remission was achieved, with the mean follow-up period being 8.6 months [28]. Recently, the intralesional delivery of bleomycin via micro-infusion (MMP®) was introduced and was found to be effective in BCC [29]. There is a case report in which BCC was cleared with intralesional bleomycin injection alone [30].
3.4. Squamous Cell Carcinoma and Keratoacanthoma
Topical 5-FU (5%) has been applied to Bowen’s disease (SCC in situ) [31][32]. However, more research is needed since the data are scarce and outdated.
The use of intralesional 5-FU has been widely studied for SCC and KA [33]. One study involving 172 SCC lesions showed a high cure rate of SCC with intralesional 5-FU injection, where 158 (92%) showed clinical resolution after treatment [34]. In a case series of 20 SCC patients, intralesional 5-FU injection was said to remove the tumor in 19 patients (95%) without any recurrence [35]. In addition, the successful use of 5-FU for SCC on cosmetically sensitive areas has been reported [36][37]. Therefore, intralesional 5-FU may be considered a promising alternative to surgery in patients with KAs, and even for invasive SCC on cosmetically sensitive areas.
Since there are few reports of KA and SCC treated with intralesional bleomycin, further studies are needed to confirm its efficacy. Considering that bleomycin has a stronger effect than 5-FU, it is expected to provide more positive results.
3.5. Metastatic Melanoma
5-FU and bleomycin are rarely applied to primary melanoma. However, they may be applied to control cutaneous metastasis and for palliative treatment. Intralesional bleomycin ECT has shown an effect in controlling metastatic melanoma where intralesional bleomycin ECT was superior to intralesional bleomycin alone [38][39].
3.6. Dosages and Techniques
The dose and number of treatments vary depending on the tumor size. Generally, 0.5 to 2 mL injections at an interval of 1 week until complete clearance is recommended [22]. Here, complete resolution is claimed when no tumor cells are found in a follow-up biopsy. The concentration of bleomycin ranged from 0.5 to 1.5 U/mL, whereas that of 5-FU was fixed at 50 mg/mL [22]. Bleomycin-mediated ECT was the most recommended [38].
In summary, although surgery remains the treatment of choice for skin cancer, the intralesional injection of 5-FU or bleomycin can be considered in special circumstances (i.e., old age, debilitating condition) for BCC, SCC, and melanoma skin metastasis, under close supervision. Figure 3 demonstrates the regression of Bowen’s disease on a cosmetically sensitive area in an 81-year-old woman. After eight sessions of 5-FU injection, each spaced 2–3-weeks apart, a follow-up biopsy confirmed the disappearance of atypical cells.
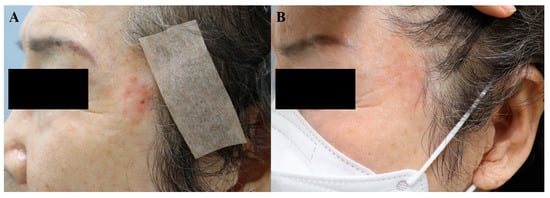
Figure 3. Bowen’s disease, (A) before treatment and (B) after 8 sessions of 5-FU injection (at an interval of 2–3 weeks).
4. Vitiligo
4.1. Efficacy
Vitiligo is an autoimmune disease mediated by T cells which results in the destruction of melanocytes and skin depigmentation. Treatment is based on the extent and activity of the disease, with phototherapy and topical agents (i.e., topical calcineurin inhibitors, topical steroids) being the mainstay of therapy.
Topical 5-FU was first introduced for the treatment of vitiligo by Tsuki and Hamada (1983) [40]. 5-FU induces the proliferation of melanocytes in the hair follicles, which migrate to the epidermis and produce melanin [41]. Many acknowledge that superficial wounding via dermabrasion or fractional CO2 or erbium: YAG laser can enhance the penetration of 5-FU cream. One RCT suggested 5-FU with microneedling to have higher efficacy compared to 5-FU cream alone in vitiligo (p = 0.03) [41]. This study included patients only with stable vitiligo and the regimen required they apply topical 5% 5-FU twice daily for 14 days. Another RCT suggested that topical 5-FU following erbium: YAG (2940 nm) laser is more effective than 5-FU cream alone [42]. Instead of applying the 5-FU cream, the 5-FU solution (50 mg/mL) can be sprayed on the superficial wounds.
Intralesional 5-FU injection is another option for vitiligo. One RCT suggested that 5-FU injection was more effective than intralesional triamcinolone (p = 0.025 in the face, p = 0.043 in acral lesions) [43]. In this study, patients with stable vitiligo were recruited, and 0.1–0.2 mL of 5-FU (50 mg/mL) was injected per point, spaced 1 cm apart. A total of four intradermal injections were performed at an interval of 2 weeks.
4.2. Dosage and Techniques
Most studies included patients with at least one year of stable disease. Topical 5-FU combined with dermabrasion or laser treatment is a widely used technique, but intralesional 5-FU can be another option. Once-daily application is recommended for 5-FU cream, whereas the intralesional injection of 5-FU is performed every 2 weeks. Studies of vitiligo treated with intralesional bleomycin are lacking but may be a viable option. Figure 4 demonstrates an improvement of facial vitiligo after four sessions of CO2 fractional laser plus bleomycin, where bleomycin solution was sprayed on the wounded site every 4–8 weeks.
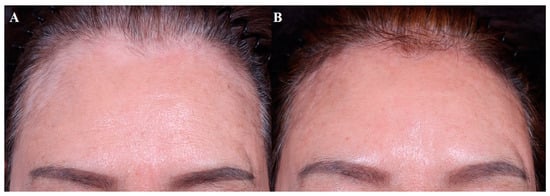
Figure 4. Facial vitiligo, (A) before treatment and (B) after 4 sessions combined treatment with CO2 fractional laser and bleomycin (each performed every 4–8 weeks).
5. Vascular Anomalies
5.1. Efficacy
In accordance with the International Society for the Study of Vascular Anomalies (ISSVA) classification updated in 2014, vascular anomalies can be divided into vascular malformations and vascular tumors. Infantile hemangioma and congenital hemangioma are included in vascular tumor and vascular malformations and are subdivided into capillary, lymphatic, venous, and arterial malformations. Hemangioma is the result of endothelial hyperplasia, while vascular malformation is the result of a vascular system error during embryonic development.
Intralesional bleomycin has been used in these diseases, with its sclerosing and antineoplastic effect, which induces apoptosis in immature proliferating cells [7].
5.2. Hemangioma
As hemangiomas often leave scars, early intervention is becoming increasingly popular over the ‘wait and see’ approach. While vascular lasers and oral propranolol are the first-line treatment options for hemangioma, intralesional bleomycin is applied to patients who show resistance and in individuals who would benefit from additional treatment. One RCT suggested that intralesional bleomycin was more effective than triamcinolone by 87.5% (excellent response + good response) (p = 0.037) in reducing the size of the hemangioma [44].
5.3. Vascular Malformation
Although surgery and ethanol injection are the preferred options for vascular malformation, diffuse microcystic malformations can benefit from bleomycin injection. A systematic review identified bleomycin sclerotherapy to be effective in lymphatic malformations (84%) and venous malformations (87%) [45]. While bleomycin is comparable to other sclerosants in terms of efficacy, it has a far better safety profile, which makes it a suitable treatment option. The size of a vascular malformation can be assessed clinically or combined with radiologic images including Doppler ultrasonography and MRI [45].
References
- Kim, W.I.; Kim, S.; Cho, S.W.; Cho, M.K. The efficacy of bleomycin for treating keloid and hypertrophic scar: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 3357–3366.
- Huang, L.; Wong, Y.; Cai, Y.; Lung, I.; Leung, C.; Burd, A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br. J. Dermatol. 2010, 163, 1181–1185.
- Searle, T.B.; Al-Niaimi, F.M.; Ali, F.R. 5-Fluorouracil in Dermatology: The Diverse Uses Beyond Malignant and Premalignant Skin Disease. Dermatol. Surg. 2021, 47, e66–e70.
- Hietanen, K.; Järvinen, T.; Huhtala, H.; Tolonen, T.; Kuokkanen, H.; Kaartinen, I. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections—A randomized controlled trial. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 4–11.
- Khalid, F.A.; Mehrose, M.Y.; Saleem, M.; Yousaf, M.A.; Mujahid, A.M.; Rehman, S.U.; Ahmad, S.; Tarar, M.N. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: Randomized control trial. Burns 2019, 45, 69–75.
- Davison, S.P.; Dayan, J.H.; Clemens, M.W.; Sonni, S.; Wang, A.; Crane, A. Efficacy of Intralesional 5-Fluorouracil and Triamcinolone in the Treatment of Keloids. Aesthet. Surg. J. 2009, 29, 40–46.
- Saitta, P.; Krishnamurthy, K.; Brown, L.H. Bleomycin in Dermatology: A Review of Intralesional Applications. Dermatol. Surg. 2008, 34, 1299–1313.
- Bik, L.; Sangers, T.; Greveling, K.; Prens, E.; Haedersdal, M.; van Doorn, M. Efficacy and tolerability of intralesional bleomycin in dermatology: A systematic review. J. Am. Acad. Dermatol. 2020, 83, 888–903.
- Mozafari, N.; Mollaabasi, F.; Mansouri, P.; Robati, R.M. The Combined Application of Bleomycin and Triamcinolone for Treating Refractory Keloids. Dermatol. Surg. 2023; Epub ahead of print.
- Kabel, A.M.; Sabry, H.H.; Sorour, N.E.; Moharm, F.M. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J. Dermatol. Dermatol. Surg. 2016, 20, 32–38.
- Dhar, S.; Rashid, M.; Islam, A.; Bhuiyan, M. Intralesional bleomycin in the treatment of cutaneous warts: A randomized clinical trial comparing it with cryotherapy. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 262–267.
- Adalatkhah, H.; Khalilollahi, H.; Amini, N.; Sadeghi-Bazargani, H. Compared therapeutic efficacy between intralesional bleomycin and cryotherapy for common warts: A randomized clinical trial. Dermatol. Online J. 2007, 13, 4.
- Lewis, T.G.; Nydorf, E.D. Intralesional bleomycin for warts: A review. J Drugs Dermatol. 2006, 5, 499–504.
- Hodeib, A.A.E.; Al-Sharkawy, B.G.; Hegab, D.S.; Talaat, R.A.Z. A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J. Dermatol. Treat. 2021, 32, 663–668.
- Yazdanfar, A.; Farshchian, M.; Fereydoonnejad, M.; Farshchian, M. Treatment of common warts with an intralesional mixture of 5-fluorouracil, lidocaine, and epinephrine: A prospective placebo-controlled, double-blind randomized trial. Dermatol. Surg. 2008, 34, 656–659.
- Isçimen, A.; Aydemir, E.H.; Göksügür, N.; Engin, B. Intralesional 5-fluorouracil, lidocaine and epinephrine mixture for the treatment of verrucae: A prospective placebo-controlled, single-blind randomized study. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 455–458.
- Luk, N.M.; Tang, W.Y.M.; Tang, N.L.S.; Chan, S.W.; Wong, J.K.W.; Hon, K.L.E.; Lo, K.K. Topical 5-fluorouracil has no additional benefit in treating common warts with cryotherapy: A single-centre, double-blind, randomized, placebo-controlled trial. Clin. Exp. Dermatol. 2006, 31, 394–397.
- Amer, M.; Diab, N.; Ramadan, A.; Galal, A.; Salem, A. Therapeutic evaluation for intralesional injection of bleomycin sulfate in 143 resistant warts. J. Am. Acad. Dermatol. 1988, 18, 1313–1316.
- Pasquali, P.; Frietes-Martinez, A.; Gonzalez, S.; Spugnini, E.P.; Baldi, A. Successful treatment of plantar warts with intralesional bleomycin and electroporation: Pilot prospective study. Dermatol. Pract. Concept. 2017, 7, 21–26.
- Salk, R.S.; Grogan, K.A.; Chang, T.J. Topical 5% 5-fluorouracil cream in the treatment of plantar warts: A prospective, randomized, and controlled clinical study. J. Drugs Dermatol. 2006, 5, 418–424.
- IsËik, S.; Koca, R.; Sarici, G.; Altinyazar, H.C. A comparison of a 5% potassium hydroxide solution with a 5-fluorouracil and salicylic acid combination in the treatment of patients with anogenital warts: A randomized, open-label clinical trial. Int. J. Dermatol. 2014, 53, 1145–1150.
- Good, L.M.; Miller, M.D.; High, W.A. Intralesional agents in the management of cutaneous malignancy: A review. J. Am. Acad. Dermatol. 2011, 64, 413–422.
- Jorizzo, J.; Weiss, J.; Furst, K.; VandePol, C.; Levy, S.F. Effect of a 1-week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: A randomized, vehicle-controlled clinical trial. Arch. Dermatol. 2004, 140, 813–816.
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: A comparison of clinical and his-tological outcomes including 1-year follow-up. Br. J. Dermatol. 2007, 157 (Suppl. S2), 34–40.
- Loven, K.; Stein, L.; Furst, K.; Levy, S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin. Ther. 2002, 24, 990–1000.
- Miller, B.H.; Shavin, J.S.; Cognetta, A.; Taylor, J.; Salasche, S.; Korey, A.; Orenberg, E.K. Nonsurgical treatment of basal cell carcinomas with intralesional 5-fluorouracil/epinephrine injectable gel. J. Am. Acad. Dermatol. 1997, 36, 72–77.
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599.
- Rodríguez-Cuevas, S.; Barroso-Bravo, S.; Almanza-Estrada, J.; Cristóbal-Martínez, L.; González-Rodríguez, E. Electrochemotherapy in Primary and Metastatic Skin Tumors: Phase II Trial Using Intralesional Bleomycin. Arch. Med. Res. 2001, 32, 273–276.
- Pacola, P.R.; Rostey, R.R.L.; Rizzo, F.d.F.A. Chemotherapeutical treatment of basal cell carcinoma with bleomycin via microinfusion of the drug into the skin (MMP®). An. Bras. Dermatol. 2023, 98, 587–594.
- Gyurova, M.S.; Stancheva, M.Z.; Arnaudova, M.N.; Yankova, R.K. Intralesional bleomycin as alternative therapy in the treatment of multiple basal cell carcinomas. Dermatol. Online J. 2006, 12, 25.
- Bargman, H.; Hochman, J. Topical treatment of Bowen’s disease with 5-Fluorouracil. J. Cutan. Med. Surg. 2003, 7, 101–105.
- Salim, A.; Leman, J.; McColl, J.; Chapman, R.; Morton, C. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br. J. Dermatol. 2003, 148, 539–543.
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557.
- Maxfield, L.; Shah, M.; Schwartz, C.; Tanner, L.S.; Appel, J. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J. Am. Acad. Dermatol. 2021, 84, 1696–1697.
- Luu, W.; McRae, M.Y. Intralesional 5-fluorouracil as a management for cutaneous squamous cell carcinomas: A rural Australian retrospective case series. Australas. J. Dermatol. 2023, 64, 556–559.
- Morse, L.G.; Kendrick, C.; Hooper, D.; Ward, H.; Parry, E. Treatment of squamous cell carcinoma with intralesional 5-Fluorouracil. Dermatol. Surg. 2003, 29, 1150–1153; discussion 1153.
- Leonard, A.L.; Hanke, C.W. Treatment of giant keratoacanthoma with intralesional 5-fluorouracil. J. Drugs Dermatol. 2006, 5, 454–456.
- Byrne, C.M.; Thompson, J.F.; Johnston, H.; Hersey, P.; Quinn, M.J.; Michael Hughes, T.; McCarthy, W.H. Treatment of metastatic mel-anoma using electroporation therapy with bleomycin (electrochemotherapy). Melanoma Res. 2005, 15, 45–51.
- Gaudy, C.; Richard, M.-A.; Folchetti, G.; Bonerandi, J.J.; Grob, J.-J. Randomized Controlled Study of Electrochemotherapy in the Local Treatment of Skin Metastases of Melanoma. J. Cutan. Med. Surg. 2006, 10, 115–121.
- Tsuji, T.; Hamada, T. Topically Administered Fluorouracil in Vitiligo. Arch. Dermatol. 1983, 119, 722–727.
- Adil, M.; Zahra, F.T.; Amin, S.S.; Mohtashim, M.; Bansal, R.; Khan, H.Q. Efficacy of topical 5% 5-fluorouracil with needling versus 5% 5-fluorouracil alone in stable vitiligo: A randomized controlled study. J. Cutan. Aesthet. Surg. 2020, 13, 197–203.
- Abdelwahab, M.; Salah, M.; Samy, N.; Rabie, A.; Farrag, A.R. Effect of Topical 5-Fluorouracil Alone versus Its Combination with Erbium:YAG (2940 nm) Laser in Treatment of Vitiligo. Clin. Cosmet. Investig. Dermatol. 2020, 13, 77–85.
- Zohdy, H.A.; Hussein, M.S. Intradermal injection of Fluorouracil versus triamcinolone in localized vitiligo treatment. J. Cosmet. Dermatol. 2019, 18, 1430–1434.
- Pandey, V.; Tiwari, P.; Sharma, S.; Kumar, R.; Singh, O. Role of intralesional bleomycin and intralesional triamcinolone therapy in residual haemangioma following propranolol. Int. J. Oral Maxillofac. Surg. 2018, 47, 908–912.
- Horbach, S.E.R.; Rigter, I.M.; Smitt, J.H.S.; Reekers, J.A.; Spuls, P.I.; van der Horst, C.M.A.M. Intralesional Bleomycin Injections for Vascular Malformations: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016, 137, 244–256.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
781
Revisions:
4 times
(View History)
Update Date:
24 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




