
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Merab Tsagareli | -- | 3087 | 2024-01-18 09:13:00 | | | |
| 2 | Lindsay Dong | -44 word(s) | 3043 | 2024-01-19 03:38:50 | | |
Video Upload Options
Itch (pruritus) is a sensation in the skin that provokes the desire to scratch. The sensation of itch is mediated through a subclass of primary afferent sensory neurons, termed pruriceptors, which express molecular receptors that are activated by itch-evoking ligands. Also expressed in pruriceptors are several types of Transient Receptor Potential (TRP) channels. TRP channels are a diverse class of cation channels that are responsive to various somatosensory stimuli like touch, pain, itch, and temperature. In pruriceptors, TRP channels can be activated through intracellular signaling cascades initiated by pruritogen receptors and underly neuronal activation.
1. Introduction
2. The Transient Receptor Potential (TRP) Channels and Itch
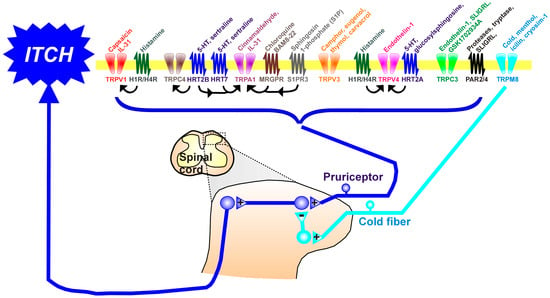
2.1. TRP Cation Channel, Subfamily A, Member 1 (TRPA1) in Acute Itch
TRPA1 in Chronic Itch
2.2. TRP Cation Channel, Subfamily V (Vanilloid), Member 1 (TRPV1) in Acute Itch
TRPV1 in Chronic Itch
2.3. TRP Cation Channel, Subfamily V (Vanilloid), Member 2 (TRPV2)
2.4. TRP Cation Channel, Subfamily V (Vanilloid), Member 3 (TRPV3)
2.5. TRP Cation Channel, Subfamily V (Vanilloid), Member 4 (TRPV4)
2.6. TRP Cation Channel, Subfamily C (Canonical), Members 3,4 (TRPC3 and TRPC4)
2.7. TRP Cation Channel, Subfamily M (Melastatin), Member 8 (TRPM8)
3. Conclusions
Emerging evidence clearly implicates TRP channels, Mrgprs, and PARs in a variety of itch-inducing mechanisms relevant to diseases that produce chronic itch. Since these channels and receptors are peripherally expressed and can mediate both inflammation and itch, they represent promising targets for the development of antipruritic pharmaceuticals. Several TRPV1 antagonists are in development for the treatment of pruritus, but with mixed results. Despite the strong preclinical evidence for TRPA1, there is a current lack of reports regarding pharmaceutical development for this promising target. More studies, including clinical trials of promising pharmaceutical agents that act at TRP channels, Mrgprs, and PARs, are sorely needed since most types of chronic itch are poorly treated by current therapeutics. Moreover, as we learn more about the central itch circuitry, it is hoped that pharmaceutical development will target other receptors, such as Gastrin Releasing Peptide (GRP)-expressing spinal neurons known to be involved in the central transmission of itch signals to relieve itch.
References
- Halvorsen, J.A.; Dalgard, F.; Thoresen, M.; Bjertness, E.; Lien, L. Itch and pain in adolescents are associated with suicidal ideation: A population-based cross-sectional study. Acta Derm.-Venereol. 2012, 92, 543–546.
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138.
- Drucker, A.M.; Wang, A.R.; Li, W.Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The burden of atopic dermatitis: Summary of a report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30.
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212.
- Krueger, G.G.; Bergstresser, P.R.; Lowe, N.J.; Voorhees, J.J.; Weinstein, G.D. Psoriasis. J. Am. Acad. Dermatol. 1984, 11, 937–947.
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gold, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2005, 55, 490–500.
- Thorpe, K.E.; Florence, C.S.; Joski, P. Which medical conditions account for the rise in health care spending? Health Aff. 2004, 23 (Suppl. 1), Suppl Web Exclusives. W4–437–45.
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714.
- Martins, M.S.; Almeida, I.F.; Cruz, M.T.; Sousa, E. Chronic pruritus: From pathophysiology to drug design. Biochem. Pharmacol. 2023, 212, 115568.
- Tsagareli, M.G.; Carstens, E.E. Allodynia and hyperalgesia in pruritus: Involvement of TRP channels. In Histaminergic and Non-Histaminergic Itch; Tsagareli, M.G., Follansbee, T., Eds.; Nova Science: New York, NY, USA, 2023; Chapter 1; pp. 1–23.
- Vander Does, A.; Ju, T.; Mohsin, N.; Chopra, D.; Yosipovitch, G. How to get rid of itching. Pharmacol. Ther. 2023, 243, 108355.
- Ständer, S.; Weisshaar, E.; Mettang, T.; Szepietowski, J.C.; Carstens, E.; Ikoma, A.; Bergasa, N.V.; Gieler, U.; Misery, L.; Wallengren, J.; et al. Clinical classification of itch: A position paper of the International Forum for the Study of Itch. Acta Derm.-Venereol. 2007, 87, 291–294.
- Sun, S.; Dong, X. TRP channels and itch. Semin. Immunopathol. 2016, 38, 293–307.
- Tsagareli, M.G.; Nozadze, I. An overview on transient receptor potential channels superfamily. Behav. Pharmacol. 2020, 31, 413–434.
- Sanjel, B.; Shim, W.-S. Molecules that Channel stimulus into pruritus. In Histaminergic and Non-Histaminergic Itch; Tsagareli, M.G., Follansbee, T., Eds.; Nova Science: New York, NY, USA, 2023; Chapter 4; pp. 59–92.
- Hu, Z.; Zhang, Y.; Yu, W.; Li, J.; Yao, J.; Zhang, J.; Wang, J.; Wang, C. Transient receptor potential ankyrin 1 (TRPA1) modulators: Recent update and future perspective. Eur. J. Med. Chem. 2023, 257, 115392.
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602.
- Hill, R.Z.; Morita, T.; Brem, R.B.; Bautista, D.M. S1PR3 mediates itch and pain via distinct TRP channel-dependent pathways. J. Neurosci. 2018, 38, 7833–7843.
- Wilson, S.R.; Nelson, A.M.; Batia, L.; Morita, T.; Estandian, D.; Owens, D.M.; Lumpkin, E.A.; Bautista, D.M. The ion channel TRPA1 is required for chronic itch. J. Neurosci. 2013, 33, 9283–9294.
- Wilzopolski, J.; Kietzmann, M.; Mishra, S.K.; Stark, H.; Bäumer, W.; Rossbach, K. TRPV1 and TRPA1 channels are both involved downstream of histamine-induced itch. Biomolecules 2021, 11, 1166.
- Xie, B.; Li, X.Y. Inflammatory mediators causing cutaneous chronic itch in some diseases via transient receptor potential channel subfamily V member 1 and subfamily A member 1. J. Dermatol. 2019, 46, 177–185.
- Yu, H.; Usoskin, D.; Nagi, S.S.; Hu, Y.; Kupari, J.; Bouchatta, O.; Cranfill, S.L.; Gautam, M.; Su, Y.; Lu, Y.; et al. Single-Soma Deep RNA Sequencing of Human Dorsal Root Ganglion Neurons Reveals Novel Molecular and Cellular Mechanisms Underlying Somatosensation. bioRxiv 2023.
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic dermatitis in children: Clinical features, pathophysiology, and treatment. Immunol. Allergy Clin. N. Am. 2015, 35, 161–183.
- Shahwan, K.T.; Kimball, A.B. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: A meta-analysis. J. Am. Acad. Dermatol. 2017, 76, 1198–1200.e1.
- Huet, F.; Faffa, M.S.; Poizeau, F.; Merhand, S.; Misery, L.; Brenaut, E. Characteristics of pruritus in relation to self-assessed severity of atopic dermatitis. Acta. Derm. Venereol. 2019, 99, 279–283.
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155.
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of pain and itch by TRP channels. Neurosci. Bull. 2017, 34, 120–142.
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295.
- Li, M.; Hener, P.; Zhang, Z.; Kato, S.; Metzger, D.; Chambon, P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11736–11741.
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.B.; Wong, J.F.; Stucky, C.L.; et al. HTR7 mediates serotonergic acute and chronic itch. Neuron 2015, 87, 124–138.
- Oh, M.-H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13–induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382.
- Zeng, D.; Chen, C.; Zhou, W.; Ma, X.; Pu, X.; Zeng, Y.; Zhou, W.; Lv, F. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. 2021, 266, 118906.
- Sanjel, B.; Kim, B.H.; Song, M.H.; Carstens, E.; Shim, W.S. Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons. Br. J. Pharmacol. 2022, 179, 2193–2207.
- Afzal, R.; Shim, W.S. Activation of serotonin receptor 2 by glucosylsphingosine can be enhanced by TRPA1 but not TRPV1: Implication of a novel glucosylsphingosine-mediated itch pathway. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184014.
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563.
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Comm. 2017, 8, 980.
- Szepietowski, J.C.; Reich, A. Pruritus in psoriasis: An update. Eur. J. Pain 2016, 20, 41–46.
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-centric view. Int. J. Mol. Sci. 2018, 19, 171.
- Flutter, B.; Nestle, F.O. TLRs to Cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur. J. Immunol. 2013, 43, 3138–3146.
- Zhou, Y.; Han, D.; Follansbee, T.; Wu, X.; Yu, S.; Wang, B.; Shi, Z.; Domocos, D.T.D.T.; Carstens, M.; Carstens, E.; et al. Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice. J. Cell. Mol. Med. 2019, 23, 4819–4828.
- Follansbee, T.; Zhou, Y.; Wu, X.; Delahanty, J.; Nguyen, A.; Domocos, D.; Carstens, M.I.; Hwang, S.T.; Carstens, E. Signs of chronic itch in the mouse imiquimod model of psoriasiform dermatitis. Itch 2019, 4, e25.
- Caterina, M.J. How do you feel? A warm and touching 2021 Nobel tribute. J. Clin. Investig. 2021, 31, e156587.
- Kim, B.M.; Lee, S.H.; Shim, W.S.; Oh, U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci. Lett. 2004, 361, 159–162.
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335.
- Tsagareli, M.G.; Nozadze, I.; Tsiklauri, N.; Carstens, M.I.; Gurtskaia, G.; Carstens, E. Thermal hyperalgesia and mechanical allodynia elicited by histamine and non-histaminergic itch mediators: Respective involvement of TRPV1 and TRPA1. Neuroscience 2020, 449, 35–45.
- Belghiti, M.; Estévez-Herrera, J.; Giménez-Garzó, C.; González-Usano, A.; Montoliu, C.; Ferrer-Montiel, A.; Felipo, V.; Planells-Cases, R. Potentiation of the transient receptor potential vanilloid 1 channel contributes to pruritogenesis in a rat model of liver disease. J. Biol. Chem. 2013, 288, 9675–9685.
- Shirolkar, P.; Mishra, S.K. Role of TRP ion channels in pruritus. Neurosci. Lett. 2022, 768, 136379.
- Hui-Beckman, J.; Goleva, E.; Leung, D.Y.M.; Kim, B.E. The impact of temperature on the skin barrier and atopic dermatitis. Ann. Allergy Asthma Immunol. 2023, 131, P713–P719.
- Yun, J.W.; Seo, J.A.; Jang, W.H.; Koh, H.J.; Bae, I.H.; Park, Y.H.; Lim, K.M. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Investig. Dermatol. 2011, 131, 1576–1579.
- Yun, J.W.; Seo, J.A.; Jeong, Y.S.; Bae, I.H.; Jang, W.H.; Lee, J.; Kim, S.Y.; Shin, S.S.; Woo, B.Y.; Lee, K.W.; et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. 2011, 62, 8–15.
- Metz, M.; Krause, K.; Maurer, M.; Magerl, M. Treatment of notalgia paraesthetica with an 8% capsaicin patch. Br. J. Dermatol. 2011, 165, 1359–1361.
- Ansari, A.; Weinstein, D.; Sami, N. Notalgia paresthetica: Treatment review and algorithmic approach. J. Dermatol. Treat. 2019, 31, 424–432.
- Fernández-Carvajal, A.; Fernández-Ballester, G.; Ferrer-Montiel, A. TRPV1 in chronic pruritus and pain: Soft modulation as a therapeutic strategy. Front. Mol. Neurosci. 2022, 15, 930964.
- Lee, J.H.; Choi, C.S.; Bae, I.H.; Choi, J.K.; Park, Y.H.; Park, M. A novel, topical, nonsteroidal, TRPV1 antagonist, PAC-14028 cream improves skin barrier function and exerts anti-inflammatory action through modulating epidermal differentiation markers and suppressing Th2 cytokines in atopic dermatitis. J. Dermatol. Sci. 2018, 91, 184–194.
- Lee, Y.; Won, C.H.; Jung, K.; Nam, H.J.; Choi, G.; Park, Y.H.; Park, M.; Kim, B. Efficacy and safety of PAC-14028 cream–a novel, topical, nonsteroidal, selective TRPV 1 antagonist in patients with mild-to-moderate atopic dermatitis: A phase II b randomized trial. Br. J. Dermatol. 2019, 180, 1030–1038.
- Park, C.W.; Kim, B.J.; Lee, Y.W.; Won, C.; Park, C.O.; Chung, B.Y.; Lee, D.H.; Jung, K.; Nam, H.-J.; Choi, G. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J. Allergy Clin. Immunol. 2022, 149, 1340–1347.e4.
- Sideris, N.; Paschou, E.; Bakirtzi, K.; Kiritsi, D.; Papadimitriou, I.; Tsentemeidou, A.; Sotiriou, E.; Vakirlis, E. New and upcoming topical treatments for atopic dermatitis: A review of the literature. J. Clin. Med. 2022, 11, 4974.
- Gunthorpe, M.J.; Hannan, S.L.; Smart, D.; Jerman, J.C.; Arpino, S.; Smith, G.D.; Brough, S.; Wright, J.; Egerton, J.; Lappin, S.C.; et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J. Pharmacol. Exp. Ther. 2007, 321, 1183–1192.
- Gibson, R.A.; Robertson, J.; Mistry, H.; McCallum, S.; Fernando, D.; Wyres, M.; Yosipovitch, G. A randomized trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS ONE 2014, 9, e100610.
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The genetics of chronic itch: Gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J. Investig. Dermatol. 2018, 138, 1311–1317.
- Zhang, D.; Spielmann, A.; Wang, L.; Ding, G.; Huang, F.; Gu, Q.; Schwarz, W. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol. Res. 2012, 61, 113–124.
- Guo, Y.; Song, Y.; Liu, W.; Wang, T.; Ma, X.; Yu, Z. Novel insights into the role of keratinocytes-expressed TRPV3 in the skin. Biomolecules 2023, 13, 513.
- Steinhoff, M.; Bíró, T.A. TR(i)P to pruritus research: Role of TRPV3 in inflammation and itch. J. Investig. Dermatol. 2009, 129, 531–535.
- Liedtke, W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J. Physiol. 2005, 567 Pt 1, 53–58.
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.; Hall, R.P.; Liedtke, W.B. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J. Biol. Chem. 2016, 291, 10252–10262.
- Kim, S.; Barry, D.M.; Liu, X.Y.; Yin, S.; Munanairi, A.; Meng, Q.T.; Cheng, W.; Mo, P.; Wan, L.; Liu, S.B.; et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal. 2016, 9, ra71.
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M.; et al. Involvement of TRPV4 in serotonin-evoked scratching. J. Investig. Dermatol. 2016, 136, 154–160.
- Zhang, Q.; Henry, G.; Chen, Y. Emerging role of transient receptor potential vanilloid 4 (TRPV4) ion channel in acute and chronic itch. Int. J. Mol. Sci. 2021, 22, 7591.
- Domocos, D.; Follansbee, T.; Nguyen, A.; Nguyen, T.; Carstens, M.I.; Carstens, E. Cinnamaldehyde elicits itch behavior via TRPV1 and TRPV4 but not TRPA1. Itch 2020, 5, e36.
- Liu, Y.; Liu, Y.; Limjunyawong, N.; Narang, C.; Jamaldeen, H.; Yu, S.; Patiram, S.; Nie, H.; Caterina, M.J.; Dong, X.; et al. Sensory neuron-expressed TRPC3 mediates acute and chronic itch. Pain 2023, 164, 98–110.
- Lee, S.H.; Cho, P.S.; Tonello, R.; Lee, H.K.; Jang, J.H.; Park, G.Y.; Hwang, S.W.; Park, C.K.; Jung, S.J.; Berta, T. Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J. Allergy Clin. Immunol. 2018, 142, 1349–1352.e16.
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58.
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715.
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Lim, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312.
- Palkar, R.; Ongun, S.; Catich, E.; Li, N.; Borad, N.; Sarkisian, A.; McKemy, D.D. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J. Investig. Dermatol. 2018, 138, 1391–1399.
- Kardon, A.P.; Polgár, E.; Hachisuka, J.; Snyder, L.M.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M.; et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82, 573–586.
- Kang, S.Y.; Choi, M.G.; Wei, E.T.; Selescu, T.; Lee, S.Y.; Kim, J.C.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPM8 agonist (cryosim-1) gel for scalp itch: A randomised, vehicle-controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e588–e589.
- Jung, M.J.; Kim, J.C.; Wei, E.T.; Selescu, T.; Chung, B.Y.; Park, C.W.; Kim, H.O. A randomized, vehicle-controlled clinical trial of a synthetic TRPM8 agonist (Cryosim-1) gel for itch. J. Am. Acad. Dermatol. 2021, 84, 869–871.




