
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuichi Takahashi | -- | 3083 | 2024-01-18 08:53:21 | | | |
| 2 | Mona Zou | Meta information modification | 3083 | 2024-01-19 10:13:38 | | |
Video Upload Options
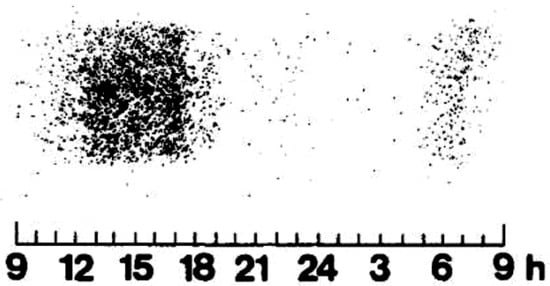
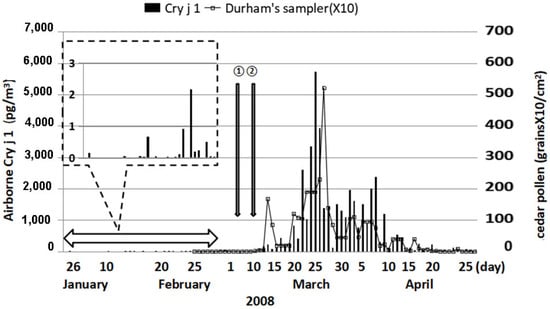
About 40% of cedar pollinosis patients living in the Yamagata Prefecture showed pollinosis symptoms before the first day of the pollen season, which was determined by Durham samplers, the standard sampler for pollen information in Japan. The amount of Cry j 1 (major cedar pollen allergen) per cedar pollen is reported to be six pg. This amount is difficult to measure using the ELISA method. It revealed that Cry j 1 exists in orbicles and tapetum. It is presumed that it is smaller than pollen, so it comes from a place where cedar are already in bloom. It is desirable to obtain real-time information on an hourly basis. Currently, information from automatic cedar pollen monitors is becoming main-stream. However, this monitor may count during snowfalls, Asian dust flying, etc., even when there was no apparent pollen examined with a microscope.
1. Do Cedar Pollen Allergens Appear before Cedar Pollen in the Air?
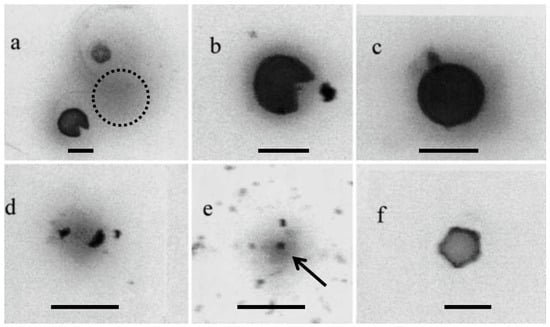
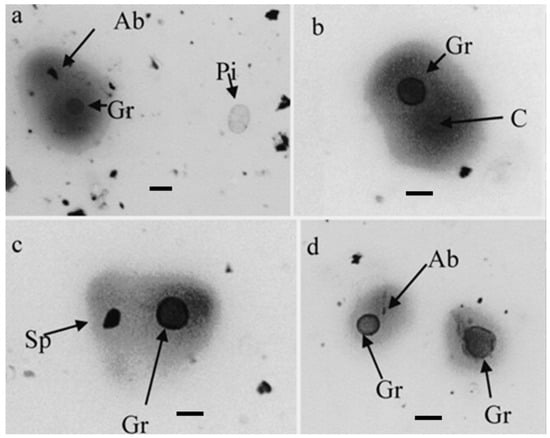
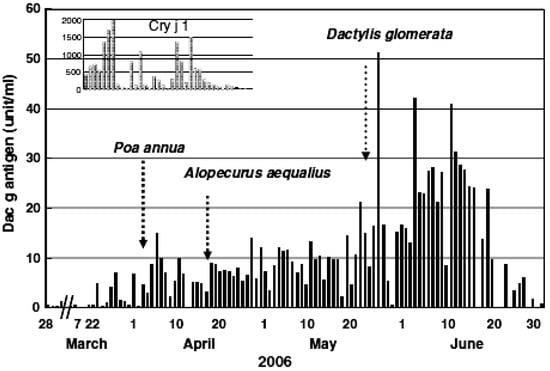
2. Particles Containing Cry j 1 Other Than Cedar Pollen Present in the Air
3. Ultrasensitive Measurement Method for Cry j 1 (ESR Radical Immunoassay)
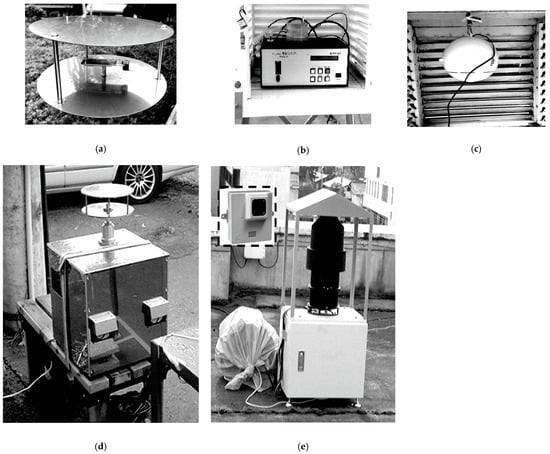
4. Detailed Pollen Monitoring by Real-Time Pollen Monitors
References
- Agarwal, M.K.; Swanson, M.C.; Reed, D.E.; Yunginger, J.W. Airborne ragweed allergens: Association with various particle sizes and short ragweed plant parts. J. Allergy Clin. Immunol. 1984, 72, 687–693.
- Rantio-Lehtimäki, A.; Viander, M.; Koivikko, A. Airborne birch pollen antigens in different particle sizes. Clin. Exp. Allergy 1994, 24, 23–28.
- Yli-Panula, E.; Takahashi, Y.; Rantio-Lehtimäki, A. Comparison of direct immuno staining and electroimmunoassay for analysis of airborne grass-pollen antigens. Allergy 1997, 52, 541–546.
- Spieksma, F.T.M.; Nikkel, B.; Dijikman, J.H. Seasonal appearance of grass pollen allergen in cultural, micronic aerosol of various size fractions, relationship with airborne grass pollen concentration. Clin. Exp. Allergy 1995, 25, 234–239.
- Takahashi, Y.; Sasaki, K.; Nakamura, S.; Miki-Hiroshige, H.; Nitta, H. Aerodynamic size distribution of the parti- cles emitted from the flowers of allergologically important plants. Grana 1995, 34, 45–49.
- Agarwal, M.K.; Yunginger, J.W.; Swanson, M.C.; Reed, C.E. An immunochemical method to measure atmospheric allergens. J. Allergy Clin. Immunol. 1981, 68, 194–200.
- Agarwal, M.K.; Yunginger, J.W.; Swanson, M.C. Immunochemical quantitation of airborne short ragweed, Alternaria, antigen E, and Alt-I allergens: A two-year prospective study. J. Allergy Clin. Immunol. 1983, 72, 40–45.
- Takahashi, Y.; Sakaguchi, M.; Inouye, S.; Miyazawa, H.; Imaoka, K.; Katagiri, S. Existense of exine free airborne allergen particles of Japanese cedar (Cryptomeria japonica) pollen. Allergy 1991, 46, 588–593.
- Takahashi, Y.; Sakaguchi, M.; Von-Pfaler, M.; El-Ghazaly, G. Relationship between numbers of birch pollen and dif- ferent particle sizes of the pollen antigens (Bet v) in the air in Stockholm, Sweden. Allergol. Int. 2003, 52, 111–114.
- Schumacher, M.J.; Griffith, R.D.; O’Rouke, M.K. Recognition of pollen and other particulate aeroantigens by immunoblot microscopy. J. Allergy Clin. Immunol. 1988, 82, 608–616.
- Takahashi, Y.; Nagoya, T.; Watanabe, M.; Inouye, S.; Sakaguchi, M.; Katagiri, S. A new method of counting airborne Japanese cedar (Cryptomeria japonica) pollen allergens by immunoblotting. Allergy 1993, 48, 94–98.
- Takahashi, Y.; Sakaguchi, M.; Inouye, S.; Nagoya, T.; Watanabe, M.; Yasueda, H. Confirmation of the airborne pollen antigen carrying particles by immunoblotting. Allerg. Immunol. 1993, 25, 132–136.
- Takahashi, Y.; Sakaguchi, M.; Inouye, S.; Yasueda, H.; Shida, T.; Katagiri, S. Airborne grass pollen antigens in a grass- land as studied by immunoblotting with anti-Lol p1 antibody. Grana 1993, 32, 302–307.
- Takahashi, Y.; Nilsson, S.; Berggren, B. Aeroallergen immunoblotting with human IgE antibody. Grana 1995, 34, 357–360.
- Suzuki, S.; Takahashi, Y.; Yasueda, H.; Saito, A. Measurement of airborne Cladosporium spp. antigens by aeroallergen immunoblotting and the seasonal fluctuation. Arerugi 2008, 57, 1175–1181.
- Sakaguchi, M. Measurement of indoor airborne mite allergens. Allergol. Int. 2005, 54, 35–38.
- Kawashima, S.; Takahashi, Y.; Aikawa, S. An attempt of applying the image processing for the automatic estimation of sampled airborne pollen. Arerugi 1995, 44, 1150–1158.
- Razmovski, V.O.; Meara, T.S.M.; Tovey, E.R. A new method for simultaneous immunodetection and morphologic identification of individual source of pollen allergens. J. Allergy Clin. Immunol. 2000, 105, 725–731.
- Takahashi, Y.; Nagoya, T.; Ohta, N. Identification of airborne pollen and airborne particles with pollen allergens (Cry j 1, Dac g) by aeroallergen immunoblotting technique. Arerugi 2002, 51, 609–614.
- Takahashi, Y.; Aoyama, M.; Abe, E.; Aita, T.; Kawashima, S.; Ohta, N.; Sakaguchi, M. Development of electron spin resonance radical immunoassay for measurement of airborne orchard grass (Dactylis glomerata) pollen antigens. Aerobiologia 2008, 24, 53–59.
- Takahashi, Y.; Mizoguchi, J.; Katagiri, S.; Sakaguchi, M.; Inouye, S.; Ishikawa, M.; Tonosaki, A.; Iwao, F. Development and distribution of the major pollen allergen (Cry j 1) in the male flower buds of Japanese cedar (Cryptomeria japonica). Arerugi 1989, 38, 1354–1358.
- Yasueda, H.; Yui, Y.; Shimizu, T.; Shida, T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) pollen. J. Allergy Clin. Immunol. 1993, 71, 77–86.
- Sakaguchi, M.; Inouye, S.; Taniai, M.; Ando, S.; Usui, M.; Matuhasi, T. Identification of the second major allergen of Japanese cedar pollen. Allergy 1994, 45, 309–312.
- Miki-Hiroshige, H.; Nakamura, S.; Yasueda, H.; Shida, T.; Takahashi, Y. immunochemical localization of the allergenic proteins in the pollen of Cryptomeria japonica. Sex. Plant Reprod. 1994, 7, 95–100.
- Nakamura, S. Immunocytochemical localization of the allergenic proteins of Japanese cedar pollen and their origin. Kenbikyo (Microscope) 2006, 42, 50–54.
- Suárez-Cervera, M.; Takahashi, Y.; Vega-Maray, A.; Seoane-Camba, J.A. Immunocytochemical localization of Cry j 1, the major allergen of Cryptomeria japonica (Taxodiaceae), in Cupressus arizonica and Cupressus sempervirens (Cupressaceae) pollen grains. Sex Plant Reprod. 2003, 16, 9–15.
- El-Ghazaly, G.; Takahashi, Y.; Nilsson, S.; Grafstrom, E.; Berggren, B. Orbicles in Betula pendula and their possible role in allergy. Grana 1996, 34, 300–3004.
- Smiljanic, D.; Apostolovic, S.; Trifunovic, J.; Ognjenovic, M.L.; Mihajlovic, L.; Burazer, M.; van Hage, T.; Velickovic, C. Subpollen particles are rich carriers of major short ragweed allergens and NADH dehydrogenases: Quantitative proteomic and allergomic study. Clin. Exp. Allergy 2017, 47, 815–828.
- Visez, N.; Chassard, G.; Azarkan, N.; Naas, O.; Sénéchal, H.; Sutra, J.-P.; Poncet, P.; Choël, M. Wind-induced mechanical rupture of birch pollen: Potential implications for allergen dispersal. J. Aerosol Sci. 2015, 89, 77–84.
- Schäppi, G.; Taylor, P.E.; Staff, I.A.; Suphioglu, C.; Knox, R.B. Source of Bet v 1 loaded inhalable particles from birch revealed. Sex. Plant Reprod. 1997, 10, 315–323.
- Schäppi, G.; Taylor, P.; Staff, I.; Rolland, J.; Suphioglu, C. Immunologic significance of respirable atmospheric starch granules containing major birch allergen Bet v 1. Allergy 1999, 54, 478–483.
- El-Ghazaly, G.; Nakamura, S.; Takahashi, Y.; Cresti, M.; Walles, B.; Milanesi, C. Localization of the major allergen Bet v 1 in Betula pollen using monoclonal antibody labeling. Grana 1996, 35, 369–374.
- El-Ghazaly, G.; Moate, R.; Cresti, M.; Walles, B.; Takahashi, Y.; Ferreira, F.; Obermeyer, G. Localization and release of antigens from tapetum and pollen grains of Betula pendula. Protoplasma 1999, 208, 37–46.
- Sénéchal, H.; Visez, N.; Charpin, D.; Shahali, Y.; Peltre, G.; Biolley, J.-P.; Lhuissier, F.; Couderc, R.; Yamada, O.; Malratdomenge, A.; et al. A Review of the effects of major atmospheric pollutants on pollen. grains, pollen content, and allergenicity. Sci. World J. 2015, 2015, 940243.
- Singh, M.B.; Hough, T.; Theerakulpist, P.; Avjioglu, A.; Davies, S.; Smith, P.M.; Taylor, P.; Simpson, R.J.; Ward, L.D.; McCluskey, J.; et al. Isolation of cDNA encoding a newly identified major allergic protein of rye-grass pollen: Intercellular targeting to amyloplast. Proc. Natl Acad. Sci. USA 1991, 88, 1384–1388.
- Taylor, P.E.; Flagan, R.C.; Miguel, A.G.; Valenta, R.; Glovsky, M.M. Birch pollen rupture and the release of aerosols of respirable allergens. Clin. Exp. Allergy 2004, 34, 1591–1596.
- Taylor, P.E.; Jacobson, K.W.; House, J.M.; Glovsky, M.M. Links between pollen, atopy and the asthma epidemic. Int. Arch. Allergy Immunol. 2007, 144, 162–170.
- Suphioglu, C.; Singh, M.B.; Taylor, P.; Bellomo, R.; Holmes, P.; Puy, R.; Knox, R.B. Mechanism of grass pollen-induced asthma. Lancet 1992, 339, 567–572.
- Wang, Q.; Gong, X.; Suzuki, M.; Lu, S.; Sekiguchi, K.; Nakajima, D.; Miwa, M. Size-segregated allergenic particles released from airborne Cryptomeria japonica pollen grains during the Yellow Sand events within the pollen scattering seasons. Asian J. Atmos. Environ. 2003, 7, 191–198.
- Aita, T.; Mogami, K.; Takahashi, Y.; Abe, E.; Aoyama, M. A survey of the Cryptomeria japonica pollen and the allergen of Cryptomeria japonica (Cry j 1) in Yamagata-City. Rep. Yamagata Prefectual Inst. Public Health 2008, 41, 20–22.
- Watanabe, M.; Tamura, M.; Nagoya, T.; Takahashi, Y.; Katagiri, S. An enzyme-linked immune-sorbent assay for the quantitation of the major allergen from Japanese cedar (Cryptomeria japonica) pollen, Cry j 1, using monoclonal antibody. Arerugi 1992, 41, 1535–1539.
- Buters, J.T.M.; Antunes, C.; Galveias, A.; Bergmann, K.C.; Thibaudon, M.; Galan, C.; Schmidt-Weber, C.; Oteros, J. Pollen and spore monitoring in the world. Clin. Transl. Allergy 2018, 8, 9.
- Aoyama, M.; Takahashi, Y. Development of super-sensitive radical immunoassay for Cry j 1. Arerugi 2004, 53, 1089–1090.
- Buters, J.T.M.; Thibaudon, M.; Smith, M.; Kennedy, R.; Rantio-Lehtimäki, A.; Albertini, R.; Reese, G.; Weber, B.; Galan, C.; Brandao, R.; et al. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos. Environ. 2012, 55, 496–505.
- Kawashima, S.; Clot, B.; Fujita, T.; Takahashi, Y.; Nakamura, K. An algorithm and a device for counting airborne pollen automatically using laser optics. Atmos. Environ. 2007, 41, 7987–7993.
- Kawashima, S.; Thibaudon, M.; Matsuda, S.; Fujita, T.; Lemonis, N.; Clot, B.; Oliver, G. Automated pollen monitoring system using laser optics for observing seasonal changes in the concentration of total airborne pollen. Aerobiologia 2017, 33, 351–362.
- Mitsumoto, K.; Yabusaki, K.; Aoyagi, H. Classification of pollen species using autofluorescence image analysis. J. Biosci. Bioeng. 2009, 107, 90–94.
- Mitsumoto, K.; Yabusaki, K.; Kobayashi, K.; Aoyagi, H. Development of a novel real-time pollen-sorting counter using species-specific pollen autofluorescence. Aerobiologia 2010, 26, 99–111.
- Miki, K.; Kawashima, S.; Fujita, T.; Nakamura, K.; Clot, B. Effect of micro-scale wind on the measurement of airborne pollen concentrations using volumetric methods on a building rooftop. Atmos. Environ. 2017, 158, 1–10.
- Oteros, J.; Pusch, G.; Weichenmeier, I.; Heimann, U.; Möller, R.; Röseler, S.; Traidl-Hoffmann, C.; Schmidt-Weber, C.; Buters, J.T.M. Automatic and online pollen mon itoring. Allergy Immunol. 2015, 167, 158–166.
- Šaulien, I.; Šukien, L.; Daunys, G.; Valiulis, G.; Vaitkevičius, L.; Matavulj, P.; Brdar, S.; Panic, M.; Sikoparija, B.; Clot, B.; et al. Automatic pollen recognition with the Rapid-E particle counter: The first-level procedure, experience and next steps. Atmos. Meas. Tech. 2018, 12, 3435–3452.
- Takahashi, Y.; Ohashi, T.; Nagoya, T.; Sakaguchi, M.; Yasueda, H.; Nitta, H. Possibility of real-time measurement of an airborne Cryptomeria japonica pollen allergen based on the principle of the surface plasmon resonance. Aerobiologia 2001, 17, 313–318.




