Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sheema Mir | -- | 2148 | 2024-01-18 02:45:40 | | | |
| 2 | Catherine Yang | Meta information modification | 2148 | 2024-01-18 02:47:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rowan, S.; Mohseni, N.; Chang, M.; Burger, H.; Peters, M.; Mir, S. Tick-Borne Diseases in the United States. Encyclopedia. Available online: https://encyclopedia.pub/entry/53999 (accessed on 08 February 2026).
Rowan S, Mohseni N, Chang M, Burger H, Peters M, Mir S. Tick-Borne Diseases in the United States. Encyclopedia. Available at: https://encyclopedia.pub/entry/53999. Accessed February 08, 2026.
Rowan, Sean, Nazleen Mohseni, Mariann Chang, Hannah Burger, Mykah Peters, Sheema Mir. "Tick-Borne Diseases in the United States" Encyclopedia, https://encyclopedia.pub/entry/53999 (accessed February 08, 2026).
Rowan, S., Mohseni, N., Chang, M., Burger, H., Peters, M., & Mir, S. (2024, January 18). Tick-Borne Diseases in the United States. In Encyclopedia. https://encyclopedia.pub/entry/53999
Rowan, Sean, et al. "Tick-Borne Diseases in the United States." Encyclopedia. Web. 18 January, 2024.
Copy Citation
Tick-borne diseases (TBDs) have become a significant public health concern in the United States over the past few decades. The increasing incidence and geographical spread of these diseases have prompted the implementation of robust surveillance systems to monitor their prevalence, distribution, and impact on human health.
tick-borne diseases
TBD
surveillance
ticks
1. Lyme Disease
Lyme disease is the most common TBD in the United States [1]. The bacteria Borrelia burgdorferi is the etiological agent of the infection and is transmitted to humans and animals through Ixodes ticks. There are approximately 476,000 diagnosed cases in the United States per year with Ixodes scapularis being the main vector of the infection [2]. Most cases of Lyme disease within the United States of America (USA) are in the mid-Atlantic, Northeast, and Upper Midwest regions [3]. Figure 1 gives the annual reported cases of Lyme disease for the year 2021 in the USA, according to the Centers for Disease Control and Prevention (CDC) (data from some reporting areas may be incomplete due to the 2019 coronavirus disease (COVID-19) pandemic). The most common clinical manifestation of Lyme disease is erythema migrans, a rash on the skin about 10 cm in size, which occurs in approximately 80% of patients affected by Lyme disease. Other clinical manifestations that occur weeks to months after the initial tick transmission include Lyme arthritis, neuroborreliosis, and borrelial lymphocytoma [4]. Another disease agent that is closely related to the bacteria causing Lyme disease is Borrelia mayonii, transmitted to humans by the blacklegged (deer) tick. This was first identified in Minnesota in 2013 [5].
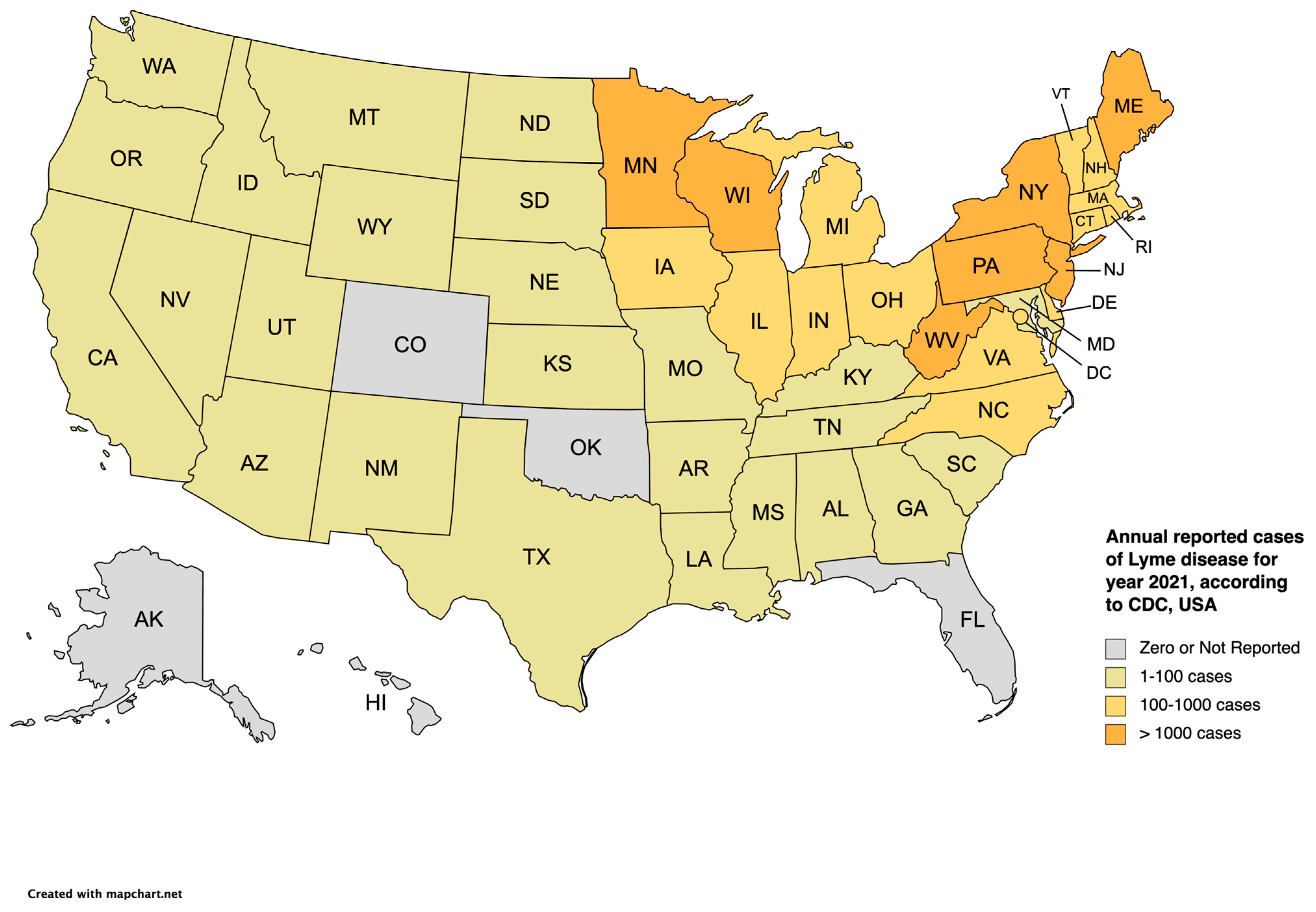
Figure 1. Annual reported cases of Lyme disease according to the CDC in the United States [6] for the year 2021.
2. Anaplasmosis
Anaplasma (A) phagocytophilum is the etiological agent for human granulocytic anaplasmosis (HGA) [7]. A. phagocytophilum is a bacteria transmitted by the blacklegged tick, Ixodes scapularis [8]. A. phagocytophilum primarily targets neutrophils and is geographically prominent in the northeastern and north central states as well as northern California [9]. With approximately 4151 cases reported per year, there is still a steady increase in cases within these regions [10]. The geographic distribution for HGA is very similar to Lyme disease because both diseases share the same primary hosts and primary vectors. The six states with the highest incidence for HGA are Rhode Island, Minnesota, Connecticut, Wisconsin, New York, and Maryland [11]. In New York, anaplasmosis has become the second most common TBD [12]. With the continuing expansion of the blacklegged tick in non-endemic regions of the USA, A. phagocytophilum, and other pathogens that this tick carries, will eventually become a greater burden to human health. The blacklegged tick can carry and transmit a variety of different pathogens and with an increase in this tick in endemic areas, patients may carry co-infections and experience more severe illnesses that require more extensive treatments [13]. A lack of awareness and under reporting of these TBDs, like anaplasmosis, can lead to undiagnosed and untreated cases in both endemic and novel regions of the disease [12]. Clinical manifestations of anaplasmosis include fever, headache, leukopenia, thrombocytopenia, and myalgias. Rashes are uncommon in HGA and occur in less than 10% of patients. Neurological manifestations are also uncommon in HGA [9].
3. Babesiosis
Babesia microti, an intraerythrocytic protozoan, is the main etiological agent of human tick-borne babesiosis [14]. Infection by the Babesia species occurs primarily through Ixodes ticks and the primary reservoir species in the USA is the white-footed mouse [15]. The Babesia species is endemic to the northeastern and upper Midwestern states, but its geographical range has expanded past these endemic regions and can range from Maine to Maryland. Most cases of transmission occur in the summer due to the increased number of ticks during this time [16]. The number of reported cases of babesiosis with Babesia microti is approximately 2000 cases per year [10]. Ticks are infected with the Babesia species by ingesting host erythrocytes that contain the pathogen. The parasite reaches the tick’s salivary glands and is transmitted transstadially from one tick stage to the next or transovarially to the eggs [17].Clinical signs of babesiosis include fever, chills, sweats, anorexia, headache, myalgia, nausea, and arthralgia. Laboratory indicators, which can include low hemoglobin and low hematocrit levels, reflect hemolytic anemia due to the Babesia species’ invasion of red blood cells. Immunocompromised and elderly patients may experience more severe infections which include respiratory distress, pulmonary edema, congestive heart failure, renal failure, or splenic rupture. Co-infections of Babesia with other tick-borne infections may increase the severity of acute symptoms [16].
4. Powassan Virus Disease
Powassan virus disease was initially discovered in a town in Ontario, Canada when it was isolated from the brain of a 5-year-old boy who died from severe encephalitis [18]. Like dengue, yellow fever, and West Nile encephalitis, Powassan virus belongs to the vector-borne flavivirus group [19]. This flavivirus is encoded by a single polypeptide that replicates within host cells [20]. The primary vectors responsible for transmitting the disease are hard-bodied Ixodes ticks, particularly I. cookei (the groundhog tick) and I. scapularis [19]. Unlike many flaviviruses that are transmitted by mosquitoes, there have been no reported cases of Powassan virus disease transmitted by mosquitoes, nor is there evidence supporting their competence as vectors for viral transmission. Interestingly, Powassan virus can be transmitted in as little as 15 min of tick feeding [20]. This quick transmission is attributed to the virus already being present in the salivary glands during feeding, unlike other non-viral TBDs that reside in the tick’s midgut [21]. The quick transmission along with the neurological sequelae of Powassan virus disease are devastating, with a mortality rate of 10% [20][21]. Given the severity of this disease, there is a pressing need for its detection and ongoing research in the development of diagnostic tools for global surveillance purposes.
5. Ehrlichiosis
Ehrlichia (E) chaffeensis and Ehrlichia ewingii are causative agents of human tick-borne ehrlichiosis. These agents of ehrlichiosis are intracellular Gram-negative bacteria that replicate in granulocytes of the host. E. chaffeensis is the etiological agent of human monocytic ehrlichiosis (HME) while E. ewingii is the etiological agent of human Ewingii ehrlichiosis (HEE) [9]. Infection of HME is primarily through the Lone Star Tick, Amblyomma americanum. E. chaffeensis is geographically located in the southeast, south central, and Midwest states and targets monocytes and macrophages in infected individuals [9]. Ehrlichia chaffeensis is reported to cause 1377 cases annually of HME [10]. Ehrlichia ewingii differs by primarily targeting neutrophils in infected patients and is most prominent in the southeast, south central, and Midwestern states. Clinical manifestations are similar to anaplasmosis, which includes fever, headache, leukopenia, thrombocytopenia, myalgias, and arthralgias. Rashes occur in approximately 10% of cases and manifest on the face, palms, and soles of the feet. Neurological manifestations are identified in about 20% of patients with HME [Ismail, 2010 #5736]. Since 2009, more than 115 instances of ehrlichiosis attributed to E. muris eauclairensis have also been detected in patients in the Upper Midwest. The tick species responsible for spreading this new subspecies is Ixodes scapularis. In terms of clinical presentation, it typically mirrors the symptoms associated with infections induced by E. chaffeensis and E. ewingii [22].
6. Spotted Fever Rickettsioses
Rocky Mountain spotted fever (RMSF) is one of the most common and severe tick-borne rickettsial infections in North America [23]. It is caused by the Gram-negative obligate intracellular bacterium Rickettsia rickettsii, leading to an acute febrile illness. Numerous genera and species of ixodid ticks are known to carry rickettsiae naturally. However, transmission most often occurs after a bite from ticks such as the American dog tick (Dermacentor variabilis), the Rocky Mountain wood tick (Dermacentor andersoni), or the brown dog tick (Rhipicephalus sanguineus) [24][25]. Other hematophagous arthropods like lice, mites, mosquitoes, and fleas can also serve as vectors for Rickettsial diseases [26][27]. RMSF is frequently misdiagnosed due to its initial presentation of nonspecific symptoms, including fever, headache, rash, myalgia, and nausea. The disease progresses rapidly and can be fatal if not detected and treated within the first five days of illness [23]. While the immuno-fluorescence assay (IFA), which compares IgG titers between acute and convalescent samples, is considered the gold standard for detection, it is important to note that most diagnostic results are not available within the first five days of illness, which coincides with the ideal treatment period. Consequently, the primary approach to diagnosis remains primarily clinical [23]. The geographical range of recognized tick-associated rickettsiae has expanded since the 1980s. Global surveillance reports these infections in livestock worldwide, with wildlife infections being more frequently reported in Europe and Africa. Additionally, there is a higher frequency of tick-associated rickettsiae infections in dogs and cats in North America. Notably, national surveillance in the United States has identified most cases in states such as Oklahoma, Arkansas, Missouri, Tennessee, and North Carolina [23]. The neglect of rickettsial infections manifests as under-recognition, leading to under-treatment due to a lack of available diagnostics, rather than the absence of an effective specific treatment [27]. This under-recognition significantly hampers the prompt administration of antibiotic treatment, resulting in mortality rates rising as high as 20–30% [25][27]. Moreover, this limitation impairs our ability to accurately assess the global burden of RMSF.
R. parkeri rickettsiosis, also known as American boutonneuse fever, is a tick-borne illness caused by the bacterium Rickettsia parkeri. It is primarily transmitted to humans through the bite of infected Amblyomma maculatum ticks, commonly referred to as Gulf Coast ticks. This disease is prevalent in certain regions of the United States, particularly in the southeastern states [28]. Pacific Coast tick fever, on the other hand, is a term that can refer to several different rickettsial diseases transmitted by ticks on the western coast of the United States. These diseases are caused by various species of Rickettsia, including Rickettsia philipii. The ticks responsible for transmitting these pathogens are primarily found in the western coastal states [29]. Both R. parkeri rickettsiosis and Pacific Coast tick fever share similarities in their mode of transmission through tick bites and can lead to a range of symptoms, including fever, rash, and other flu-like symptoms [29].
7. Other Tick-Borne Diseases (TBDs)
There are several lesser-known TBDs of concern in the USA, such as tularemia, Colorado tick fever, tick-borne relapsing fever, Heartland virus, and Bourbon virus. Tularemia, caused by the bacterium Francisella tularensis, can be transmitted through tick and insect bites, handling infected tissue, consuming undercooked meat from infected mammals, and rarely through inhalation of bacteria. It typically results in ulceroglandular disease, characterized by painful regional lymphadenopathy and a cutaneous eschar at the site of the tick bite [30].
Colorado tick fever (CTF) is a rare viral disease transmitted primarily through the bite of an infected Rocky Mountain wood tick, Dermacentor andersoni. These ticks are found in the western United States and Canada at elevations of 4000–10,000 feet, usually in grassy areas near sage or other brush [31]. CTF symptoms include fever, headache, myalgia, and fatigue, which can persist for several weeks. Although the reported cases of CTF in the United States are relatively low, it remains a cause of febrile illness in the Rocky Mountain regions [31]. Tick-borne relapsing fever (TBRF) is transmitted by Ornithodoros ticks, commonly infected with Borrelia hermsii and Borrelia turicata. Unlike other ticks found in tall brush and grass, Ornithodoros tick species that spread TBRF live in rodent burrows [30]. The highest concentrations of TBRF in the United States are primarily found in the western states, including the Cascade, San Bernardino, Sierra Nevada, and Rocky Mountain ranges [32]. TBRF symptoms typically appear approximately seven days after exposure and include high fever, headache, muscle and joint aches, and nausea. The fever lasts for about three days, accompanied by episodes of rigors, increased heart rate, and elevated blood pressure. Profuse sweating and a decline in fever then follows. It is crucial to consider TBRF in patients experiencing recurrent fevers, as untreated cases can lead to cardiac and neurological complications with potential long-term effects [33]. While these diseases are rare, other emerging threats in North America should also be noted. Borrelia miyamotoi, a tick-borne pathogen that infects ticks along with other pathogens, has recently been identified as a cause of relapsing fever. It may share a similar geographic range with Borrelia burgdorferi, the bacterium responsible for Lyme disease, as they both infect the same Ixodes ticks [19][30]. Southern tick-associated rash illness, spread by the Lone Star tick, is an emerging infection in the southeastern United States. The infectious cause of this illness is unknown, and it presents with a rash like erythema migrans [30].
Heartland virus and Bourbon virus are both tick-borne illnesses caused by viruses. Heartland virus was first identified in the United States in 2009, and it is primarily transmitted by the Lone Star tick. It can lead to flu-like symptoms and, in severe cases, may result in hospitalization [34]. Bourbon virus, discovered in 2014, is another tick-borne virus found in the Midwest and southern United States. It can cause fever, fatigue, rash, and other flu-like symptoms. Although it is rare, Bourbon virus can lead to severe illness and, in some cases, fatalities. Both viruses serve as a reminder of the potential health risks associated with tick bites, emphasizing the importance of preventive measures and seeking medical attention if symptoms arise after possible exposure to ticks [35]. Improvements are needed in diagnostic tools to detect and differentiate all tick-borne viral diseases, regardless of their prevalence within the region.
References
- Mead, P.S. Epidemiology of Lyme disease. Infect. Dis. Clin. N. Am. 2015, 29, 187–210.
- Hook, S.A.; Jeon, S.; Niesobecki, S.A.; Hansen, A.P.; Meek, J.I.; Bjork, J.K.H.; Dorr, F.M.; Rutz, H.J.; Feldman, K.A.; White, J.L.; et al. Economic Burden of Reported Lyme Disease in High-Incidence Areas, United States, 2014–2016. Emerg. Infect. Dis. 2022, 28, 1170–1179.
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024.
- Chomel, B. Lyme disease. Rev. Sci. Tech. 2015, 34, 569–576.
- Minnesota Department of Health. About Borrelia Mayonii. Available online: https://www.health.state.mn.us/diseases/bmayonii/basics (accessed on 17 September 2023).
- CDC. Lyme Disease Surveillance Data. Available online: https://www.cdc.gov/lyme/datasurveillance/surveillance-data.html (accessed on 17 September 2023).
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032.
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309.
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292.
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18.
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms 2022, 10, 1433.
- Russell, A.; Prusinski, M.; Sommer, J.; O’Connor, C.; White, J.; Falco, R.; Kokas, J.; Vinci, V.; Gall, W.; Tober, K.; et al. Epidemiology and Spatial Emergence of Anaplasmosis, New York, USA, 2010–2018. Emerg. Infect. Dis. 2021, 27, 2154–2162.
- Schotthoefer, A.M.; Meece, J.K.; Fritsche, T.R. A clinical, diagnostic, and ecologic perspective on human anaplasmosis in the Upper Midwest. WMJ 2014, 113, 107–114.
- Bhanot, P.; Parveen, N. Investigating disease severity in an animal model of concurrent babesiosis and Lyme disease. Int. J. Parasitol. 2019, 49, 145–151.
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R., 3rd; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469.
- Vannier, E.G.; Diuk-Wasser, M.A.; Ben Mamoun, C.; Krause, P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015, 29, 357–370.
- Beugnet, F.; Moreau, Y. Babesiosis. Rev. Sci. Tech. 2015, 34, 627–639.
- Mc, L.D.; Donohue, W.L. Powassan virus: Isolation of virus from a fatal case of encephalitis. Can. Med. Assoc. J. 1959, 80, 708–711.
- Della-Giustina, D.; Duke, C.; Goldflam, K. Underrecognized Tickborne Illnesses: Borrelia Miyamotoi and Powassan Virus. Wilderness Environ. Med. 2021, 32, 240–246.
- Kemenesi, G.; Banyai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019, 32, e00106-17.
- Pesko, K.N.; Torres-Perez, F.; Hjelle, B.L.; Ebel, G.D. Molecular epidemiology of Powassan virus in North America. J. Gen. Virol. 2010, 91, 2698–2705.
- Xu, G.; Foster, E.; Ribbe, F.; Hojgaard, A.; Eisen, R.J.; Paull, S.; Rich, S.M. Detection of Ehrlichia muris eauclairensis in Blacklegged Ticks (Ixodes scapularis) and White-Footed Mice (Peromyscus leucopus) in Massachusetts. Vector Borne Zoonotic Dis. 2023, 23, 311–315.
- Jay, R.; Armstrong, P.A. Clinical characteristics of Rocky Mountain spotted fever in the United States: A literature review. J. Vector Borne Dis. 2020, 57, 114–120.
- Azad, A.F.; Beard, C.B. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 1998, 4, 179–186.
- Snowden, J.; Simonsen, K.A. Rocky Mountain Spotted Fever (Rickettsia rickettsii); StatPearls: Treasure Island, FL, USA, 2023.
- Zhang, Y.Y.; Sun, Y.Q.; Chen, J.J.; Teng, A.Y.; Wang, T.; Li, H.; Hay, S.I.; Fang, L.Q.; Yang, Y.; Liu, W. Mapping the global distribution of spotted fever group rickettsiae: A systematic review with modelling analysis. Lancet Digit. Health 2023, 5, e5–e15.
- Salje, J.; Weitzel, T.; Newton, P.N.; Varghese, G.M.; Day, N. Rickettsial infections: A blind spot in our view of neglected tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009353.
- Herrick, K.L.; Pena, S.A.; Yaglom, H.D.; Layton, B.J.; Moors, A.; Loftis, A.D.; Condit, M.E.; Singleton, J.; Kato, C.Y.; Denison, A.M.; et al. Rickettsia parkeri Rickettsiosis, Arizona, USA. Emerg. Infect. Dis. 2016, 22, 780–785.
- Kim, H.K. Rickettsia-Host-Tick Interactions: Knowledge Advances and Gaps. Infect. Immun. 2022, 90, e0062121.
- Pace, E.J.; O’Reilly, M. Tickborne Diseases: Diagnosis and Management. Am. Fam. Physician 2020, 101, 530–540.
- Yendell, S.J.; Fischer, M.; Staples, J.E. Colorado tick fever in the United States, 2002–2012. Vector Borne Zoonotic Dis. 2015, 15, 311–316.
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E., Jr.; Borchardt, S.M. Tick-borne relapsing fever. Infect. Dis. Clin. N. Am. 2008, 22, 449–468.
- Roscoe, C.; Epperly, T. Tick-borne relapsing fever. Am. Fam. Physician 2005, 72, 2039–2044.
- Higuita, N.I.A.; Franco-Paredes, C.; Henao-Martinez, A.F. The expanding spectrum of disease caused by the Lone Star Tick, Amblyomma americanum. Infez. Med. 2021, 29, 378–385.
- Rupani, A.; Elshabrawy, H.A.; Bechelli, J. Dermatological manifestations of tick-borne viral infections found in the United States. Virol. J. 2022, 19, 199.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
859
Revisions:
2 times
(View History)
Update Date:
18 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




