Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alena Kalendová | -- | 3479 | 2024-01-17 11:28:27 | | | |
| 2 | Rita Xu | Meta information modification | 3479 | 2024-01-18 03:00:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kalendova, A.; Kupkova, J.; Urbaskova, M.; Merinska, D. Polymer/Clay Nanocomposites. Encyclopedia. Available online: https://encyclopedia.pub/entry/53965 (accessed on 06 March 2026).
Kalendova A, Kupkova J, Urbaskova M, Merinska D. Polymer/Clay Nanocomposites. Encyclopedia. Available at: https://encyclopedia.pub/entry/53965. Accessed March 06, 2026.
Kalendova, Alena, Jana Kupkova, Martina Urbaskova, Dagmar Merinska. "Polymer/Clay Nanocomposites" Encyclopedia, https://encyclopedia.pub/entry/53965 (accessed March 06, 2026).
Kalendova, A., Kupkova, J., Urbaskova, M., & Merinska, D. (2024, January 17). Polymer/Clay Nanocomposites. In Encyclopedia. https://encyclopedia.pub/entry/53965
Kalendova, Alena, et al. "Polymer/Clay Nanocomposites." Encyclopedia. Web. 17 January, 2024.
Copy Citation
Clays and clay minerals are common natural materials, the unique properties of which have attracted the interest of the industry, especially because these materials are easily available, cheap, and non-toxic. Clays and clay minerals are widely used in many applications, such as in ceramic production, in the clarification of liquids, pollutant adsorbers, filler in composites and nanocomposites, soil amendments, in pharmacy, etc.
clay
nanofiller
ceramics
nanocomposite
1. Introduction
In the last century, the research in the area of nanotechnology started new technological development. The term “nanotechnology” was used first by the Japanese scientist Norio Taniguchi in 1974 [1]. Nanotechnology works in the area of incredibly small dimensions, in the range of 1–100 nm. A nanometer (nm) is 10−9 m, smaller than the wavelength of visible light and a hundred-thousandth the width of a human hair. In some senses, nanoscience and nanotechnologies are not new. Nanostructured and nanocomposite materials are commonly found in nature and living beings (such as bone) [2]. Also, chemists make polymers, which are large molecules made up of nanoscale subunits [3].
“Synthetic” polymer/clay nanocomposites have their origin in the pioneering research at Toyota Central Research Laboratories. In addition, the first practical application of nanocomposites, the nylon/montmorillonite timing belt cover on the Toyota Camry automobile, relates to this company as well. Nowadays, the PA/clay nanocomposites have the largest commercial presence in the application field. The global nanocomposites market size was USD 5.6 billion in 2022 and is likely to reach USD 18.3 billion by 2031 [4]. The market growth is attributed to the rapid increase in the use of nanocomposites in biomedical applications and packaging. Further, rising demand for nanocomposites has been recorded also in the sectors of aviation, sporting goods, and automobile parts [4].
Despite the development in the area of polymer nanocomposites and their expanding use in different applications, research in polymer science and nanotechnology continues. Especially, it is necessary to understand the principles of nanocomposite behavior at the nanometric level.
Clays and clay minerals have been accompanying humans since the dawn of history. Clays and clay minerals, raw or after modification, have great importance in a wide variety of applications mainly due to their abundant, inexpensive, inertness, stability, reactivity, and environmentally friendly [5][6]. Clays are naturally occurring materials consisting primarily of the various clay minerals content and degree of purity [5][7][8][9]. Clay minerals belong to the phyllosilicate group with a layered structure and with one dimension in the nanometer range [5][9]. The principal building elements of the clay minerals are two-dimensional sheets of silicon-oxygen tetrahedral and two-dimensional sheets of aluminum- or magnesium-oxygen-hydroxyl octahedral [10]. Individual clay minerals, such as kaolin, clay, bentonite, and vermiculite, differ significantly in their composition and crystal structure which causes different physical and chemical properties (e.g., particle size, surface chemistry, surface area, viscosity, plasticity, absorption, and adsorption) [7][8][11]. In many cases, the clays or clay minerals can be modified to obtain improved mechanical, thermal, structural, or functional properties.
Clays and clay minerals have many industrial applications such as ceramics [12][13], paper coatings [14][15], pesticides [16], paints [17][18], pharmaceuticals [19][20], agriculture [21][22], construction industry [23], ion exchangers, separators, plastics [24][25][26], cosmetics [27], insulations [28], and electrical applications. Clay minerals can also be used as a good adsorbent for water purification due to lamellar structure, high cation exchange capacity, pore size distribution, and large surface area [29]. Moreover, considerable attention obtained the clay composite or nanocomposite materials which can be incorporated into many areas such as biomedical, construction, automobile, remediation technology, petroleum industry, wastewater, treatment, aerospace, and nanotechnology [30].
In ceramic technology, the most important and most widely used natural raw materials also belong to clay minerals such as kaolinite, illite, montmorillonite, talc, pyrophyllite, and serpentine [31]. Generally, ceramic materials show a combination of useful properties such as high strength and stiffness at very high temperatures, chemical inertness, and low density. Their applications are restricted owing to their brittle behavior. Cordierite, enstatite, and forsterite are silicate materials that form the main components of the ternary system MgO-Al2O3-SiO2. One of the methods for the synthesis of these ceramic types is the sintering of natural raw materials including clay and clay minerals, especially kaolinite, talc, and a combination of both minerals. The important properties relating to applications of clay minerals in the ceramic industry are plasticity, chemical and mineralogical composition, thermal properties, color, and mechanical strength after firing [32]. Extensive research is also carried out in the field of ceramics. Nanoceramics have emerged as valuable materials in biomedicine and medical technology (orthopedics and bone tissue engineering). In bone repair, nanoceramics serve as nano scaffolds to support and facilitate bone growth. Applications in energy storage, coating systems, environmental technology (water treatment), chemistry, construction, electronics, and batteries have also been reported [33].
2. Polymer/Clay Nanocomposites
2.1. Nanofiller Sources
Nanoparticles have at least one characteristic length scale that is of the order of nanometers and can range from isotropic to highly anisotropic needle-like to sheet-like elements. These nanoelements can lead to ultra-large interfacial areas between the constituents. In addition, the distance between the nanoelements begins to approach molecular dimensions at extremely low loading of the nanoparticles. This large internal interfacial area and the nanoscopic dimensions between constituents differentiate polymer nanocomposites from traditional composites [34]. Nanofillers can be different types of materials like carbon nanotubes, fullerenes, carbon black, polyhedral oligomeric silsesquioxanes (POSS), silica, and phyllosilicates (montorillonite, halloysite, and vermiculite). MXene, nanofibers, metals, and their oxides are also used. One of the most common sources of nanofillers is a clay mineral called montmorillonite (MMT).
Montmorillonite
Montmorillonite has become one of the most widely used minerals as nanofillers, because of the versatility of reactions, layered morphology with a high aspect ratio, and large specific area, which offers substantial cation exchange capacities. Additionally, MMT is commercially available.
MMT is a naturally occurring mineral derived from the weathering of volcanic ash. This mineral belongs to the clay minerals of the smectite family. MMT represents 2:1 layered aluminous-silicate. The suggested crystallographic structure for montmorillonite is in Figure 1 [35]. Isomorphous substitutions of Si4+ for Al3+ in the tetrahedral lattice and of Al3+ for Mg2+ in the octahedral sheet cause an excess of negative charges within the montmorillonite layers. All atoms may also be replaced by Fe3+, Ti, Ni, Zn, Cr, and Mn [36]. In many minerals, an atom of lower positive valence replaces one of higher valence. These negative charges are counterbalanced by cations such as Ca2+ and Na+ situated between the layers.
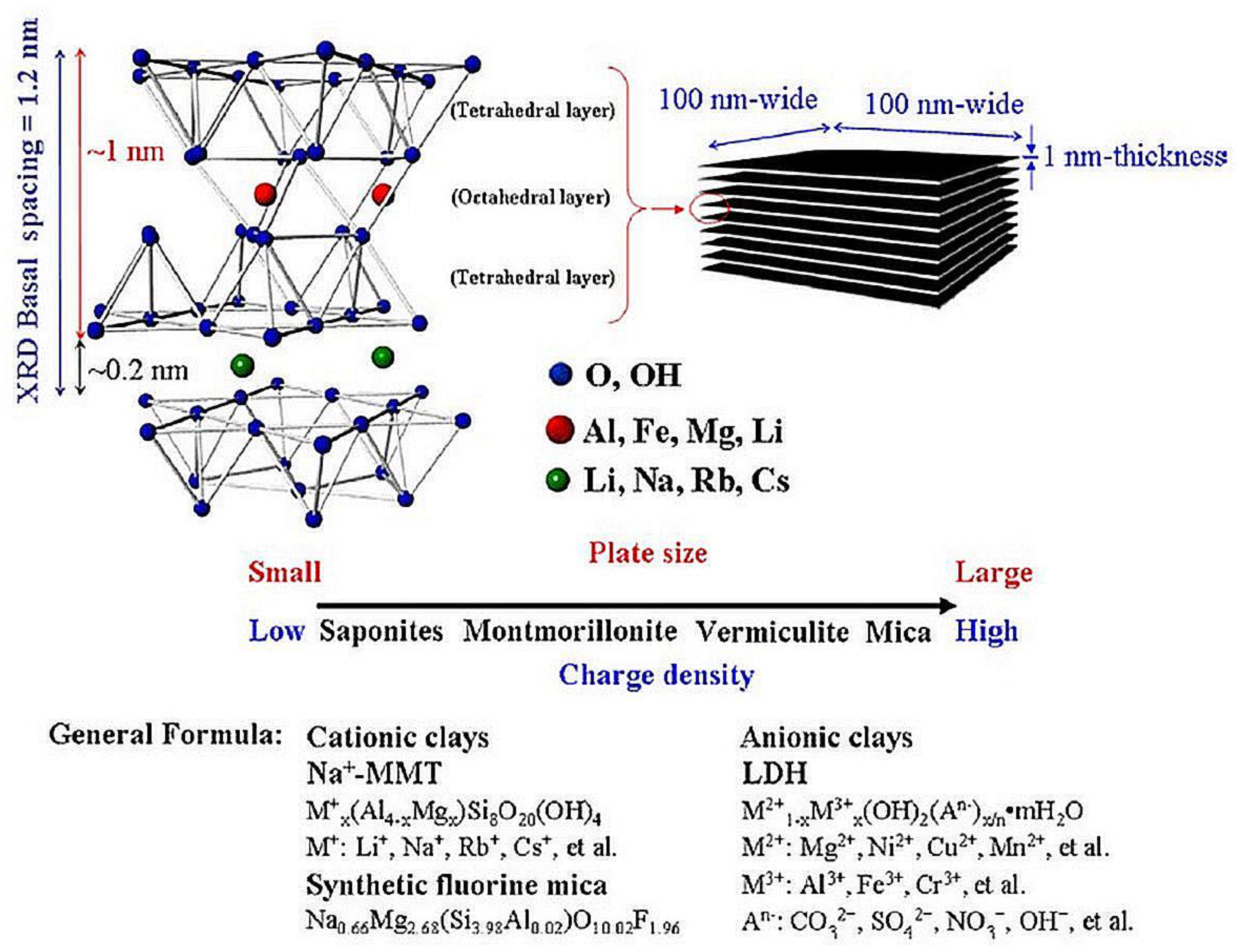
Figure 1. Chemical structure of smectite clay.
2.2. Clay Organophilization
The main difficulties for polymer/clay nanocomposites relate to the hygroscopic character of clay and clay minerals. It is relatively simple to disperse clay in water or water-soluble polymer monomers, but clay dispersed in a high molecular hydrophobic polymer is like trying to mix oil in water. The reason for such behavior is the different nature of these two materials as was mentioned above. Organophilic polymers are not miscible with pristine hydrophilic clay represented in the case by montmorillonite (MMT). This phenomenon is attributed to the higher surface energy of MMT compared to a macromolecular matrix which tends to create a stronger cohesive interaction between clay layers and further hamper the inclusion of polymer chains into the interlamellar region of MMT [37][38][39][40][41]. Therefore, surface modification of MMT plays a very important role in clay incorporation into the polymer matrix. The process leading to nanocomposite is called delamination or exfoliation of montmorillonite to individual sheets. On the other hand, the hydrophilic feature of the MMT surface permits water and other polar molecules to intercalate into the galleries within clay layers [37].
The clay modification process, called intercalation (1 chemical agent) or co-intercalation (2 or more chemical agents), can be defined as the reversible inclusion of a molecule or ion into filler with a layered structure. In the case of layered aluminous silicates (phylosilicates), intercalation means intercalant penetration into the clay-layered structure. The result of such a successful process is an increase in d-spacing, Figure 2. Next, the changes depend on the type of used intercalant, its concentration, and the length of the chain. The clay affinity to the polymer surface is influenced and the intercalation more compatible montmorillonite-polymer interface can be then developed.
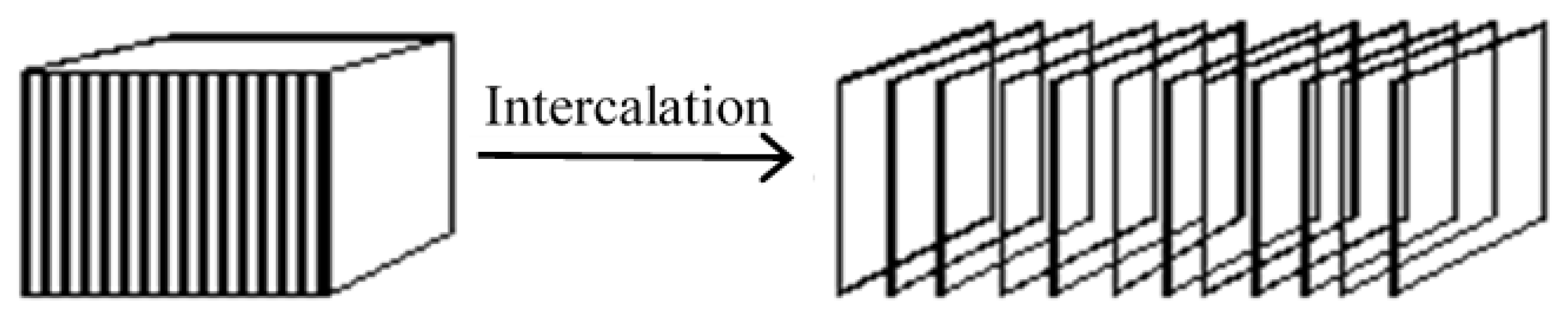
Figure 2. Process of montmorillonite intercalation.
Amino acids were employed as the first intercalants in the synthesis of nanocomposites (polyamide 6-clay hybrids) [42]. Numerous other kinds of intercalant agents have been used in the synthesis of nanocomposites today’s. The most popular are cationic surfactants, such as alkylammonium ions, because they can be exchanged easily with the ions situated between the clay layers. All the commercial types of montmorillonites are based on these substances. This kind of surfactant consists of two distinct parts, a positively charged hydrophilic head and a hydrophobic hydrocarbon chain tail. After modification, cationic surfactant molecules attach to the inner and outer surface of clay minerals and thus alter the surface properties of clay minerals from hydrophilic to hydrophobic [43]. In addition, silanes have been used because of their ability to react with the hydroxyl groups situated at the surface and the edges of the clay layers.
Smectite organically intercalated structures were first studied by Lagaly and Weis in 1969. After modification, several arrangements inside the interlayer are possible Figure 3. Lagaly and Weis found two possible arrangements of organic molecules in the interlayer, namely lateral and perpendicular (paraffin) [44][45].
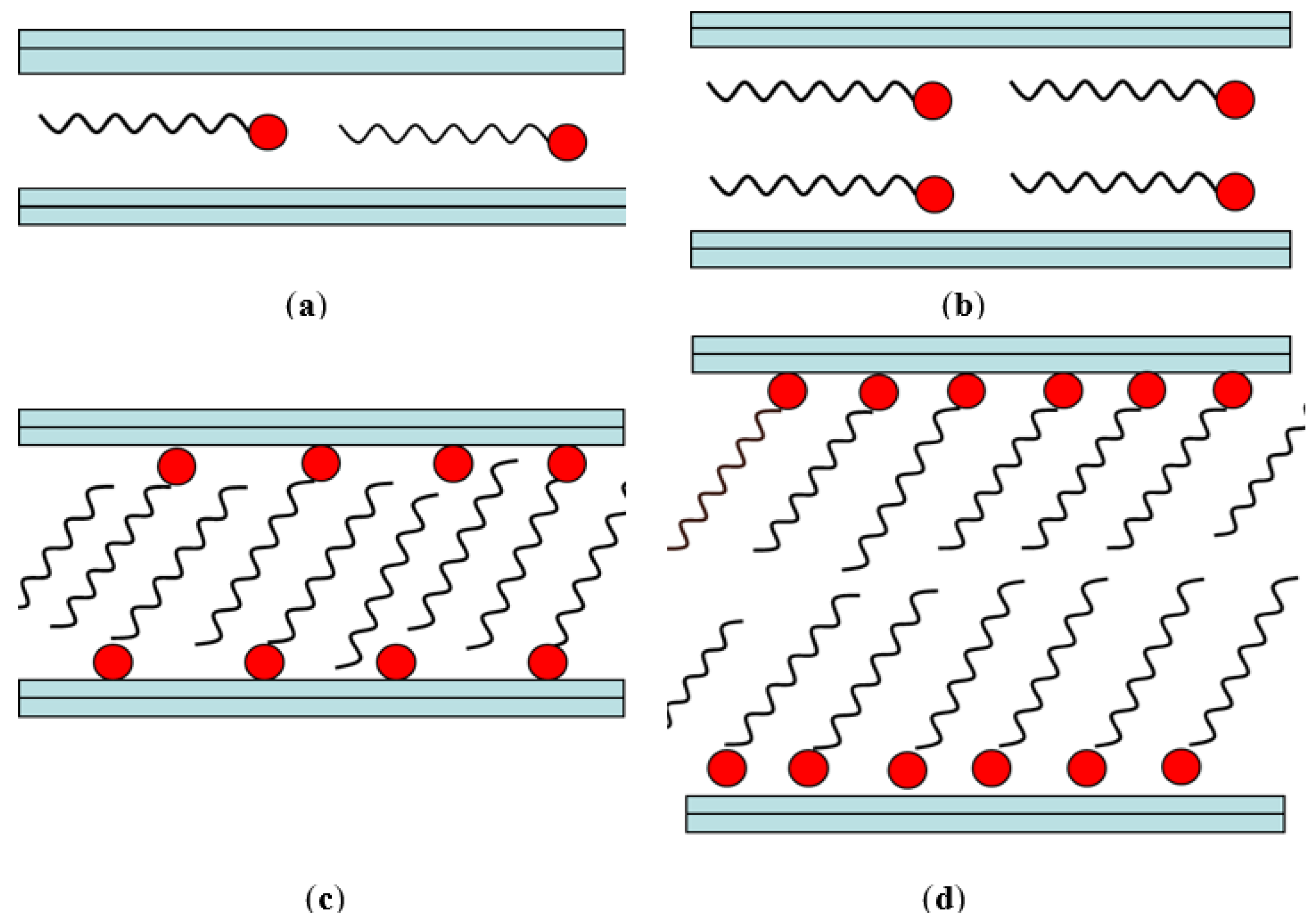
Figure 3. Alkyl chain arrangement in layered silicates: (a) lateral monolayer, (b) lateral bilayer, (c) paraffin (monolayer), and (d) paraffin type bilayer.
At present three basic methods of intercalation are performed:
2.2.1. Ion-Exchange Intercalation
A characteristic feature of smectites such as montmorillonite is their ability to sorb certain cations and retain them in an exchangeable state. It means that these intercalated cations can be exchanged by treatment of other cations in a water solution (wet method), Figure 4. The most common exchangeable cations are Na+, Ca2+, Mg2+, H+, K+, and NH4+. Indeed, if the clay is placed in a solution of a given electrolyte, an exchange occurs between the ions of the clay (X+) and those of the electrolyte (Y+):
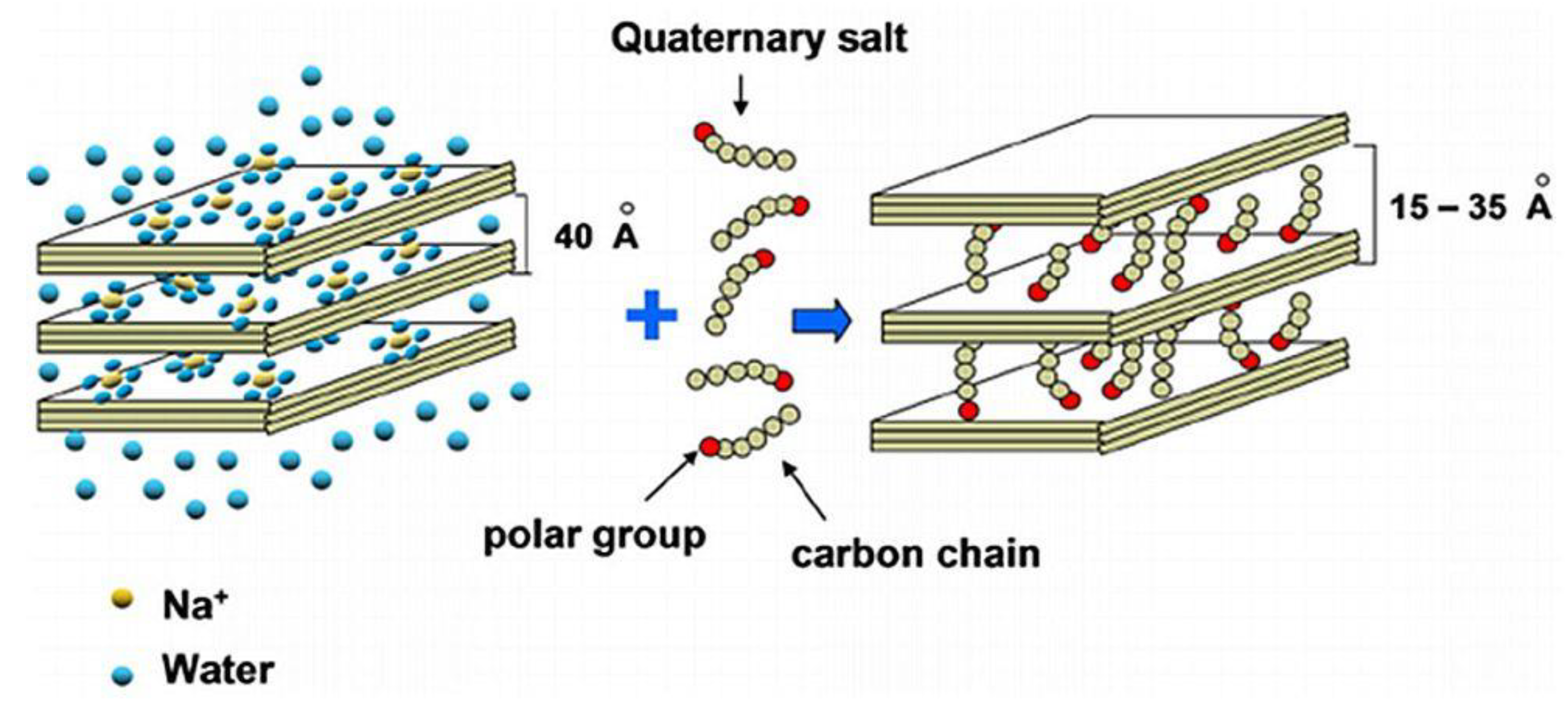
Figure 4. Development of organoclay treated with quaternary salt: ion-exchange intercalation method.
For given clay, the maximum number of cations that can be taken up is constant and known as the cation-exchange capacity (CEC). CEC is measured in milliequivalents per gram (meq/g) or more frequently per 100 g (meq/100 g). Cation-exchange capacity measurements are performed at a neutral pH of 7. The CEC of montmorillonite varies from 80 to 150 meq/100 g [49]. In the dependence on the CEC and the character of organocation, different structures can be created in the interlayer space. These structures could be helpful during the nanocomposite compounding and for the right choice of intercalant. The successful MMT intercalation and following exfoliation process during compounding is a good assumption for enhancement of final materials properties.
2.2.2. Ion-Dipole Intercalation
Ion-dipole method is based on ion-dipole interaction. This method differs from the ion-exchange approach in that the exchangeable cation (partial positive charge) remains on the clay surface, Figure 5. It means that it is not necessary to ablate any product of a chemical reaction. Next ion-dipole approach advantage relates to the non-water environment (dry method). As the intercalants, alkylamine (octadecylamine (ODA), dodecylamine (DDA)), and primary amine can be used. As the compatibilizer the common plastics processing aid, like plasticizers, lubricants, and other modifiers, could also be suitable [50][51][52]. The intercalate structure depends on the concentration of used organic intercalant, in the guest-guest and guest-host interactions. This technique is also possible to apply to process co-intercalation [53][54].
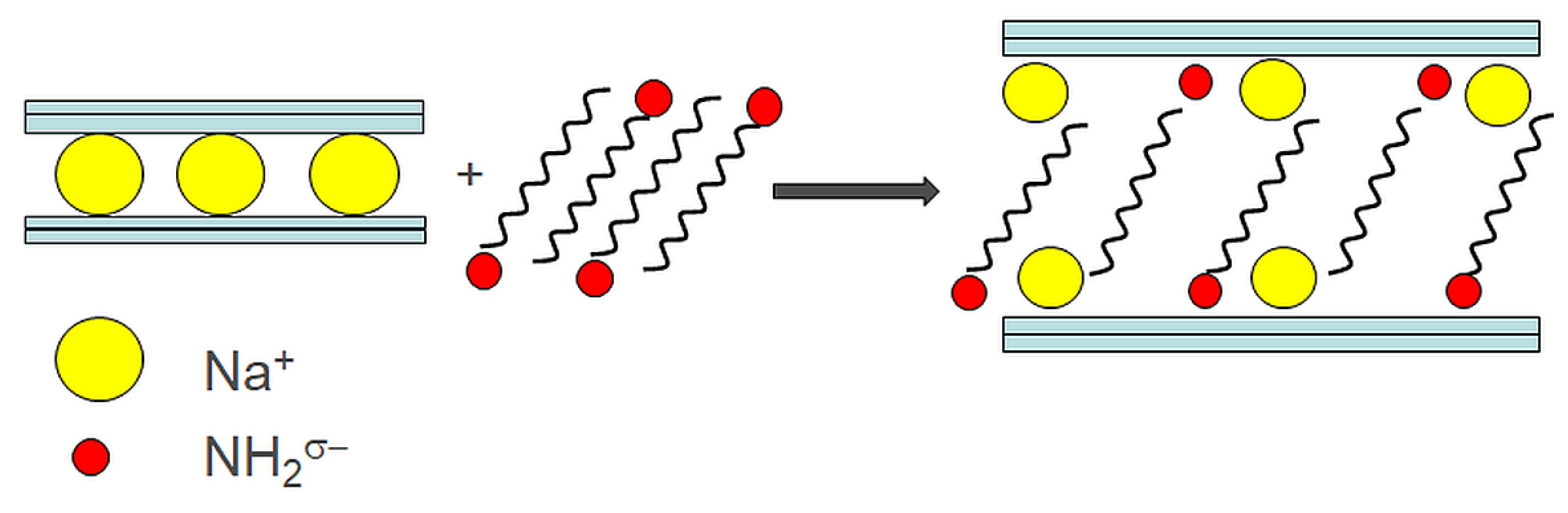
Figure 5. Ion-dipole intercalation method.
2.2.3. Grafting
The grafting approach has attracted scientists over the last 15 years. The method is based on the formation of covalent bonds between the montmorillonite platelet surface and the hydrophobic modifier. This method can improve the stability of organophilized clay surfaces. The most famous modifiers are silanes, therefore this process is called silanization or silylation. Alkoxysilane and chlorosilane are the most common intercalates. However, chlorosilane is not used too often due to its tendency to create HCl during the grafting process [55]. In addition to increased thermal stability, clay treatment by this approach offers irreversible coupling and tightly secures the agent on the clay surface, avoiding release into the environment to cause adverse effects [56]. Organosilanes also serve as a key bridge to enhance the interfacial interaction between the silylated-MMT and the polymer matrix owing to the reduced clay surface energy because of silane grafting, thus enabling better dispersibility of the reinforcing phase in the continuous matrix [37][57].
2.3. Clay Application in Nanocomposites
Generally, nanocomposites represent materials with multiphase ultrafine structures with at least one dimension 10−9 m. In the case of polymer/clay nanocomposites, they are formed through the connection of two different materials, organic (polymer) and inorganic (mineral). The next important features of polymer nanocomposites are low content of filler (1%–5%) compared to conventional composites (30%–50%), transparency, and large changes in material properties, like E-modulus, strength, shrinkage, density, chemical and fire resistance, etc.
The polymer nanocomposites could be divided into several groups according to the dimensions of the dispersed nanoscale:
One of the processing problems of nanocomposites is the nanofiller exfoliation during processing. Therefore, polymer/clay systems are divided into four general groups according to the nanofiller exfoliation level (Figure 6):
-
Microcomposite, where the clay acts as a conventional filler. The final material belongs to traditional composite materials.
-
Intercalated nanocomposite consists of a regular insertion of the polymer between the clay layers. The final material belongs to nanocomposites.
-
Intercalated and partially delaminated nanocomposites, an intermediate step between intercalated and exfoliated structure. The final material belongs to nanocomposites.
-
Exfoliated nanocomposite where the filler is delaminated to 1 nm-thick layers. The final material belongs to nanocomposites.
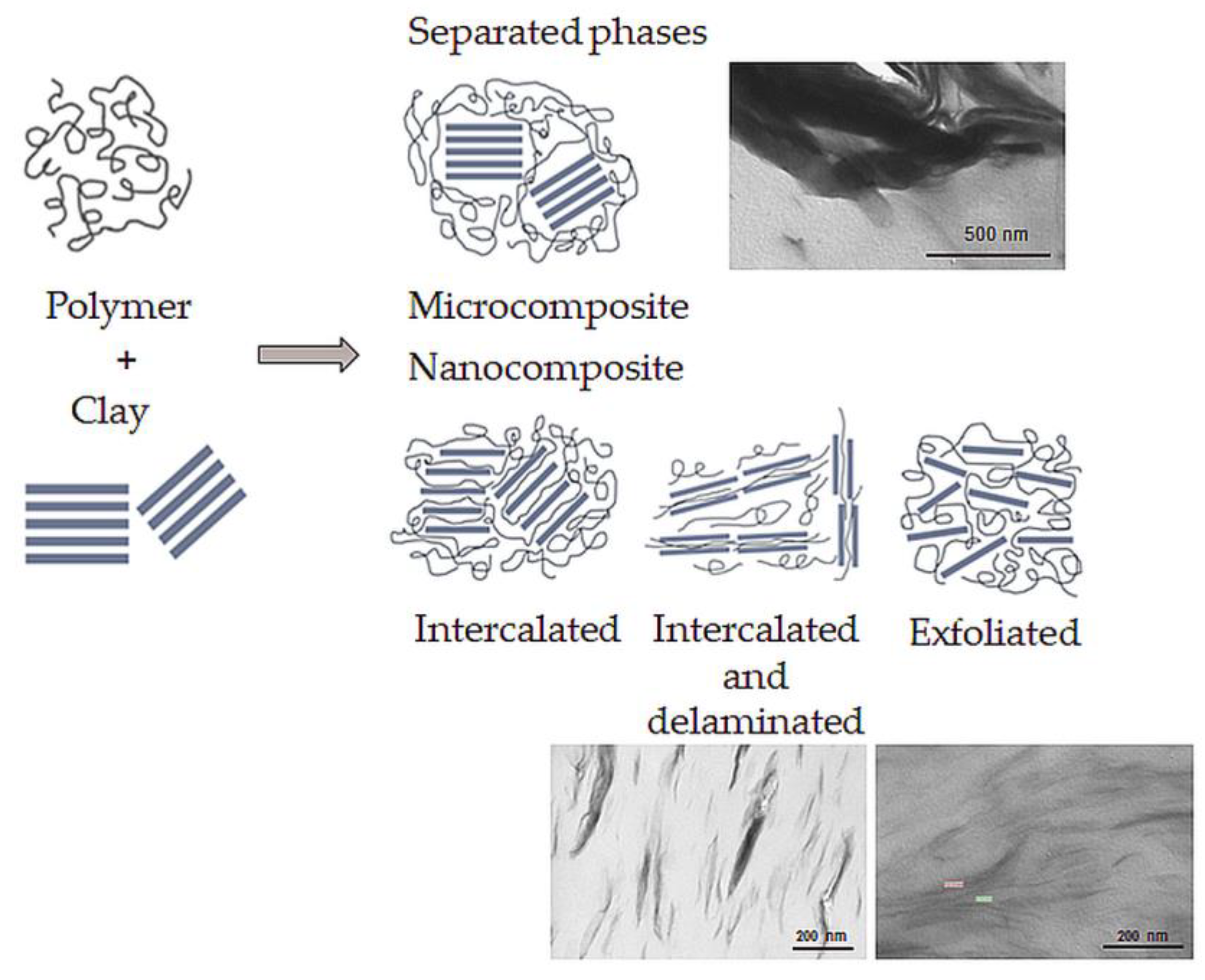
Figure 6. Polymer/clay nanocomposite classification.
2.3.1. Polymer/Clay Nanocomposite Processing
The final step of nanocomposite preparation is the organoclay mixing with polymer. Early experiments with clay-filled polymers required processing that was not commercially friendly, but this situation has changed. A primary difficulty is the proper dispersion of the filler in the polymer matrix. Without good dispersion and filler distribution, the high surface area is compromised, and the aggregates can act as defects, which limits their properties [2]. Several strategies have been considered to prepare polymer/montmorillonite nanocomposites (Figure 7) [75]:
-
In-situ polymerization method, intercalation of a suitable monomer followed by polymerization. The first method used to synthesize polymer/clay nanocomposites is based on polyamide 6.
-
Solution method, intercalation of dissolved polymer from a solution. The drawback of this method is the requirement of a suitable solvent. It has been shown that intercalation only occurs for certain polymer/solvent or monomer/solvent pairs [76]. Nanocomposites based on high-density polyethylene [77], and polyimide [78] can be synthesized by this method.
-
Melt intercalation method, mixing the clay (usually organoclay) with the polymer matrix above its softening point in either static or flow conditions. The polymer chains spread from the molten mass into the silicate galleries to form either intercalated or delaminated hybrids according to the degree of penetration [55]. This process was first reported by Vaia et al. [79] in 1993. This method is relatively easy and allows for the use of current processing equipment for nanocomposite technology. Traditional processing techniques could be used for melt intercalation, like a two-roll mill, twin-screw extruder (PA, PP, PE, and PVC), injection molding, blow molding, and thermal spraying [2].
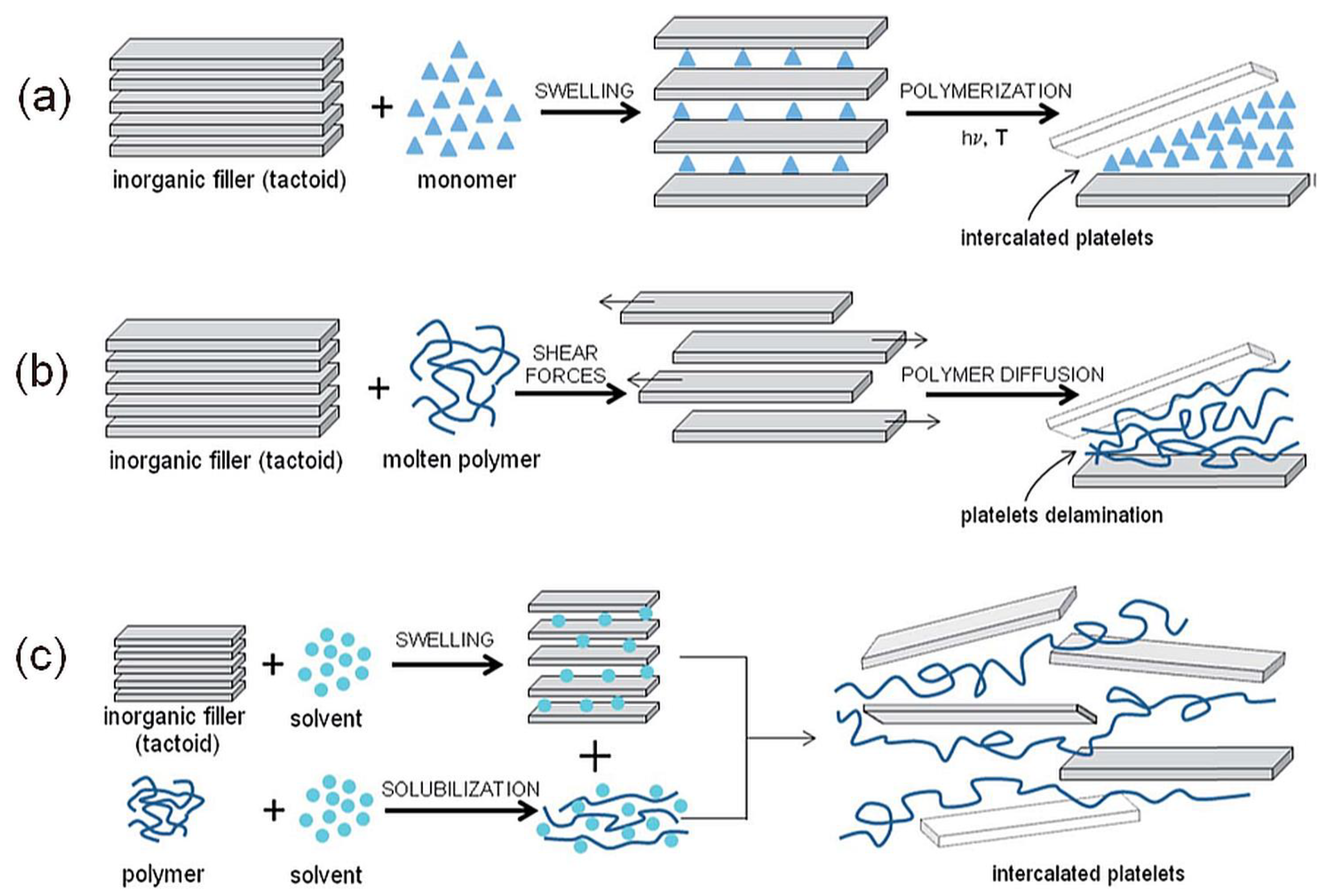
Figure 7. Illustration of (a) in situ polymerization, (b) melt intercalation, and (c) solution intercalation.
Highly polar polymers such as Nylon [80][81][82] or polyimides [83][84] are more easily intercalated than non-polar polymers such as polypropylene because polar polymers have a higher affinity for the polar clay galleries. In situ polymerization monomer intercalates directly into the organically modified clay galleries and the monomer either can adsorb onto the layer surface or can be anchored by free radical techniques. Melt intercalation involves mixing the clay and a polymer melt with or without the shear. The success of melt intercalation is surprising, given that the gallery spacing is only about 2 nm and the radius of gyration of the polymer is significantly larger than this. Even more surprising is that the speed of melt intercalation is faster than that of the self-diffusion of polymers and scales with the inverse of the molecular weight. The results of molecular dynamics and experimental studies indicate that the stronger the clay/polymer interaction, the lower the intercalation rate. In addition, layer flexibility seems to control the mechanism of intercalation [2].
2.3.2. Polymer Matrix
Many different polymers have already been used to produce polymer/clay nanocomposites. Both thermoplastics and thermosets can be successfully utilized for the preparation of nanocomposites. Intensive research is carried out in rubber mixtures too. The first and the most studied thermoplastics for the synthesis of polymer-clay nanocomposites has been polyamide 6 [85][86][87][88][89][90]. Polyamide 6/clay hybrids were discovered by Toyota researchers in the early nineties [91][92][93][94] and nowadays they are used in automotive parts. The teams dealing with these materials were concentrated around Usuki A., Kojima Y., Okada A. [91][92][93][94][95], Azuma H. [96], Fukusima Y. [97], Wu T. [98], Devaux, E. [99], Tanaka G. [100], and Utracki L. A. [86][88][89].
Polyvinylchloride [8][101][102] nanocomposite processing was studied, in addition. Polyvinylchloride (PVC) is an important commercial polymer. It is one of the most versatile and oldest thermoplastics. It is a material that offers several positive aspects like low cost, recoverability, facile processing, and excellent electrical and chemical resistance. Commonly, PVC is available in two broad categories: flexible PVC (plasticized one), and rigid PVC (unplasticized one). The PVC products range from piping and siding, door and window profiles, blood bags, and tubing, to wire and cable insulation and more. Therefore, alkylamines with different alkyl chain lengths were tested as the organic compatibilizer. Especially shorter alkyl chains were tested as suitable chemical modifiers of MMT. Firstly, Na+ montmorillonite was ion-dipole intercalated with dodecylamine (DDA, 12 C) and octylamine (OA, 8C) molecules. The structure of octadecylamine (ODA, 18 C) ion-dipole intercalated into MMT was described by Pospisil et al. previously [103]. The material structure was determined based on X-ray diffraction and molecular simulation results. Molecular mechanics and classical molecular dynamics were carried out in the Cerius2 modeling environment. Based on calculated values of interaction energies between two guest layers in the interlayer space of montmorillonite and XRD patterns, a probable intercalant molecules arrangement in the MMT interlayer was designed. It was confirmed by Pospisil [103], Figure 8, that ODA ion-dipole intercalated into MMT allows the existence of supramolecular structure which is presented by bilayer arrangement alkyl chains perpendicular or slightly oblique to the aluminosilicate layer. The results are consistent with the studies carried out by Lagaly [43][104][105][106] in the field of ion-exchange intercalation. In addition, the Octylamine (OA) ion-dipole intercalated was also not able to provide the bilayer arrangement in the MMT interlayer space. From the obtained data it follows that montmorillonite intercalated with short alkylamine chains suggests a disadvantage for polymer/MMT nanocomposite production.
Nonetheless, predisposition to degradation, poor thermal resistance, and unsuitable mechanical and barrier properties limit industrial application for long-term performance products in automotive and electronic industries. For items such as these, resistance to degradation is required, unlike for disposable applications [107]. Further, high price is also an obstacle to greater expansion of PLA on the market. To address one of these limitations, nanoscale structured layered clay particles can be incorporated into PLA conferring strength, increasing its gas barrier properties [108][109][110], and ultimately allowing the nanocomposite to compete with conventional plastics in a wide range of applications [111][112][113]. A very important role of PLA is in short-term food packaging (bags). For long-term food storage, PLA offers insufficient barrier properties. Commercial products of multilayer packaging films consist of several layers created from different polymers. From the viewpoint of circular economy, separation and recycling of such multilayered material is impossible. An alternative to the traditional approach could be multilayered films from one material type, where the barrier layer could be based on the polymer/clay nanocomposites. Such films should offer uncomplicated recyclability. Kalendova et al. [108] describe the role of different modified motnmorillonites in the barrier properties of PLA/clay nanocomposites. The air, CO2, and water vapor permeability, along with morphology were investigated. The mixtures with Cloisite 10A and 30B give similar results with enhancement of more than 70% VWT after 24 h. The best barrier properties were exhibited by the sample with Cloisite 10A and Cloisite 30B. The barrier properties improvement is connected probably with the nanofiller arrangement in the polymer matrix, Figure 8, and filler aspect ratio. A large aspect ratio of nanoplatelets creates a more dramatic reduction in permeability, which was explained by Choudalakis G. and Lu C. [114][115].
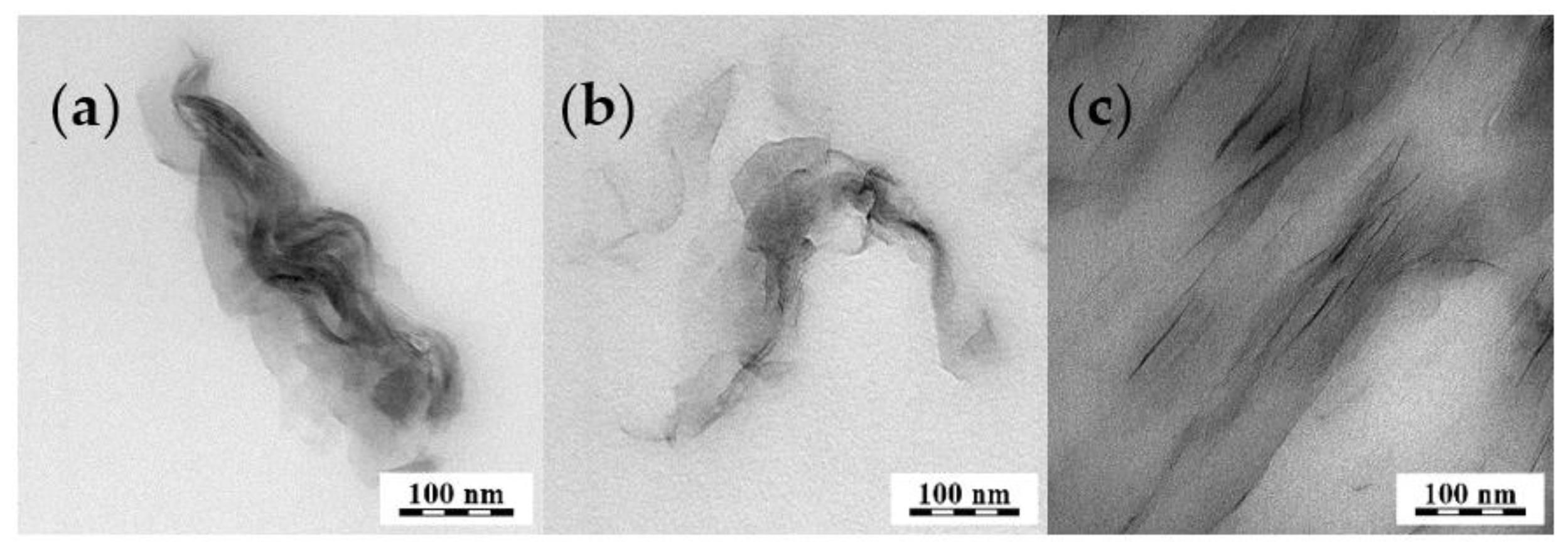
Figure 8. TEM: (a) PLA/Na+ 5%, (b) PLA/10A 5%, and (c) PLA/30B 5%.
References
- History of Nanotechnology. Available online: https://trynano.org/about-nanotechnology/history-of-nanotechnology/ (accessed on 28 November 2023).
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; Wiley—WCH: Weinheim, Germany, 2003; ISBN 3-527-30359-6.
- What Is Nanotechnology? Available online: https://www.nanowerk.com/nanotechnology/introduction/introduction_to_nanotechnology_1.php (accessed on 19 November 2023).
- Nanocomposites Market, Global Indurstry Analysis, Size, Share, Growth & Forecast 2022–2031. Available online: https://growthmarketreports.com/report/nanocomposites-market-global-industryanalysis#:~:text=The%20global%20nanocomposites%20market%20size%20was%20USD%205.6,use%20of%20nanocomposites%20in%20biomedical%20applications%20and%20packaging (accessed on 29 November 2023).
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Chapter 1: General introduction: Clays, clay minerals and clay science. In Handbook of Clay Science, Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006.
- Harvey, C.C.; Lagaly, G. Chapter 10.1: Conventional Applications. In Handbook of Clay Science, Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006.
- Konta, J. Clay and man: Clay raw materialsin the service of man. Appl. Clay Sci. 1995, 10, 275–335.
- Valášková, M. Clays, Clay minerals and cordierite ceramics—A review. Ceram. Silik. 2015, 59, 331–340.
- Bergaya, F.; Jaber, M.; Lambert, J.-F. Chapter 1: Clays and Clay Mineral. In Rubber-Clay Nanocomposites: Science, Technology, and Applications; Galimberti, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011.
- Pospisil, M.; Capkova, P.; Merinska, D.; Malac, Z.; Simonik, J. Sructure analysis of montmorillonite intercalated with cetylpyridinium and cetyltrimethylammonium: Molecular simulations and XRD analysis. J. Colloid Interface Sci. 2001, 236, 127–131.
- Murray, H.H. Overview, clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395.
- Glebova, A.A.; Skovorodnikova, M.S.; Pavlova, I.A.; Farafontova, E.P. Research on the Ceramicc Properties of Orenburg Oblast Clay. Glass Ceram. 2023, 79, 11–12.
- Yang, X.; Yang, W.; Hu, J. Preparation of Low-Dielectric-Constant Kaolin Clay Ceramics by Chemical Cleaning Method. Front. Mater. 2021, 8, 692759.
- Petronela, N. The Influence of Drying Conditions of Clay-Based Polymer Coatings on Coated Paper Properties. Coatings 2021, 11, 12.
- Devisetti, S.; Lempsink, G.; Malla, P.B. Use of kaolin clay in aqueous barrier coating applications. Tappi J. 2023, 22, 685–697.
- Granetto, M.; Serpella, L.; Fogliatto, S.; Re, L.; Bianco, C.; Vidotto, F.; Tosco, T. Natural clay and biopolymer-based nanopesticides to control the environ-mental spread of a soluble herbicide. Sci. Total Environ. 2022, 806 Pt 3, 151199.
- Mahmoodi, A.; Dadras, A.; Jiryaei, Z.; Khorasani, M.; Shi, X.M. A Yellow Lead-Free Pavement Marking Paint Based on Hybrid Dye-Clay Nanopigment: Morphological, Thermome-chemical, and Photophysical Properties. ACS Sustain. Chem. Eng. 2021, 9, 16466–16473.
- Vakhitova, L.; Kalafat, K.; Vakhitov, R.; Drizhd, V.; Taran, N.; Bessarabov, V. Nano-clays as rheology modifiers in intumescent coatings for steel building structures. Chem. Eng. J. Adv. 2023, 16, 100544.
- Choi, G.; Piao, H.Y.; Eom, S.; Choy, J.H. Vectorized Clay Nanoparticles in Therapy and Diagnosis. Clay Clays Miner. 2019, 67, 25–43.
- Nomicisio, C.; Ruggeri, M.; Bianchi, E.; Vigani, B.; Valentino, C.; Aguzzi, C.; Viseras, C.; Rossi, S.; Sandri, G. Natural and Synthetic Clay Minerals in the Pharmaceutical and Biomedical Fields. Pharmaceutics 2023, 15, 1368.
- Torbert, H.A.; Harmel, R.D.; Potter, K.N.; Dozier, M. Evaluation of some phosphorus index criteria in cultivated agriculture in clay soils. J. Soil Water Conserv. 2005, 60, 21–29.
- Kubo, K.; Hirayama, T.; Fujimura, S.; Eguchi, T.; Nihei, N.; Hamamoto, S.; Takeuchi, M.; Saito, T.; Ota, T.; Shinano, T. Potassium behavior and clay mineral composition in the soil with low effectiveness of potassium application. Soil Sci. Plant Nutr. 2018, 64, 265–271.
- Degrave-Lemeurs, M.; Glé, P.; de Menibus, A.H. Acoustical properties of hemp concretes for buildings thermal insulation: Application to clay and lime binders. Constr. Build. Mater. 2018, 160, 462–474.
- Zhou, S.Q.; Niu, Y.Q.; Liu, J.H.; Chen, X.X.; Li, C.S.; Gates, W.P.; Zhou, C.H. Functional Montmorillonite/Polymer Coatings. Clays Clay Miner. 2022, 70, 209–232.
- Moradihamedani, P. Recent development in polymer/montmorillonite clay mixed matrix membranes for gas separation: A short review. Polym. Bull. 2023, 80, 4663–4687.
- Zhu, Y.; Iroh, J.O.; Rajagopolan, R.; Aykanat, A.; Vaia, R. Optimizing the Synthesis and Thermal Properties of Conducting Polymer-Montmorillonite Clay Nanocomposites. Energies 2022, 15, 1291.
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Miner. 2022, 69, 561–575.
- Suzuki, A.; Shikinaka, K.; Ishii, R.; Yoshida, H.; Ebina, T.; Ishida, T.; Nge, T.T.; Yamada, T. Heat-resistant insulation film containing clay and wood components. Appl. Clay Sci. 2019, 180, 105189.
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301.
- Das, P.; Manna, S.; Behera, A.K.; Shee, M.; Basak, P.; Sharma, A.K. Current synthesis and characterization techniques for clay-based polymer nano-composites and its biomedical applications: A review. Environ. Res. 2022, 212, 113534.
- Barry Carter, C.; Grant Norton, M. Ceramic Materials: Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2007.
- Kagonbé, B.P.; Tsozué, D.; Nzeukou, A.N.; Ngos III, S. Mineralogical, physico-chemical and ceramic properties of clay materials from Sekandé and Gashiga (North, Cameroon) and their suitability in earthenware production. Heliyon 2021, 7, e07608.
- Global Nanoceramics Market—Industry Trends and Forecast to 2030. Available online: https://www.databridgemarketresearch.com/reports/global-nanoceramics-market (accessed on 29 November 2023).
- Krishnamoorti, R.; Vaia, A.R. Polymer Nanocomposites: Synthesis, Characterization, and Modeling; American Chemical Society: Washington, DC, USA, 2002; ISBN 0-8412-3768-9.
- Lai, Y.-H.; Chiu, C.-W.; Chen, J.-G.; Wang, C.-C.; Ho, K.-C. Enhancing the performance of dye-sensitized solar cells by incorporating nanosilicate platelets in gel electrolyte. Sol. Energy Mater. Sol. Cells 2009, 93, 1860–1864.
- Pethrick Richard, A. Polymer Science and Technology for Scientists and Engineers; Whittles Publishing: Caithnes, UK, 2010; p. 354. ISBN 978-1904445-40-1.
- Bee, S.-L.; Abdullah, M.A.A.; Lee Bee, S.-T.; Sin, T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82.
- Sapalidis, A.A.; Katsaros, F.K.; Kanellopoulos, N.K. PVA/montmorillonite nanocomposites: Development and properties. In Nanocomposites and Polymers with Analytical Methods; Cappoletti, J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 29–50.
- Zulfiqar, S.; Kausar, A.; Rizwan, M.; Sarwar, M.I. Probing the role of surface treated montmorillonite on the properties of semiaromatic polyamide/clay nanocomposites. Appl. Surf. Sci. 2008, 255, 2080–2086.
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51.
- Kádár, F.; Százdi, L.; Fekete, E.; Pukánszky, B. Surface characteristics of layered silicates: Influence on the properties of clay/polymer nanocomposites. Langmuir 2006, 22, 7848–7854.
- Fudala, A.; Palinko, I.; Kiricsi, I. Preparation and characterization of hybrid organic-inorganic composite materials using the amphoteric property of amino acids: Amino acid intercalated layered double hydroxide and montmorillonite. Inorg. Chem. 1999, 38, 4653–4658.
- Zhu, J.; Zhanga, P.; Qinga, Y.; Wena, K.; Sua, X.; Ma, L.; Jingming Wei, J.; Liu, H.; He, H.; Xi, Y. Novel intercalation mechanism of zwitterionic surfactant modified montmorillonites. Appl. Clay Sci. 2017, 141, 265–271.
- Lagaly, G. Interaction of Alkylamines with different types of layered compounds. Solid State Ion. 1986, 22, 43–51.
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 1994, 6, 1017–1022.
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic-Table Force-field for Molecular Mechanics and Molecular-Dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035.
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular-Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690.
- Kalendova, A.; Pospisil, M.; Kovarova, L.; Merinska, D.; Valaskova, M.; Vlkova, H.; Simonik, J.; Capkova, P. Influence of chain length on inter-calation process of polyvinylchloride/clay nanocomposites based on alkyl-amine. Plast. Rubber Compos. 2004, 33, 279–286.
- Meier, L.P.; Nuesch, R. The Lower Cation Exchange Capacity Limit of Montmorillonite. J. Colloid Interface Sci. 1999, 217, 77–85.
- Rappé, A.K.; Goddard, W.A. Charge Equilibration for Molecular-Dynamics Simulations. J. Phys. Chem. 1991, 95, 3358–3363.
- Karasawa, A.; Goddard, W.A. Acceleration of convergence for Lattice Sums. J. Phys. Chem. 1989, 93, 7320–7327.
- Dabbaghianamiria, D.M.; Beall, G.W. Self-assembling nanostructured intercalates via ion–dipole bonding. Dalton Trans. 2018, 47, 3178–3184.
- Merinska, D.; Chmielova, M.; Kalendova, A.; Weiss, Z.; Capkova, P.; Simonik, J. Montmorillonite co-intercalated with octadecylamine and stearic acid by low temperature melting and its influence on PP nanocomposites. Int. Polym. Process. 2003, 18, 133–137.
- Pospisil, M.; Kalendova, A.; Capkova, P.; Simonik, J.; Valaskova, M. Structure analysis of intercalated layer silicates: Combination of molecular simulations and experiment. J. Colloid Interface Sci. 2004, 277, 154–161.
- Bergaya, F.; Jaber, M.; Lambert, J.F. Organophilic clay minerals. In Rubber-Clay Nanocomposites: Science, Technology, and Applications; Galimberti, M., Ed.; John Wiley & Sons: New York, NY, USA, 2011; pp. 45–86.
- Shen, W.; He, H.; Zhu, J.; Yuan, P.; Frost, R.L. Grafting of montmorillonite with different functional silanes via two different reaction systems. J. Colloid Interface Sci. 2007, 313, 268–273.
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 1–511. ISBN 9780444533548.
- Albdiry, M.T.; Yousif, B.F.; Ku, H.; Lau, K.T. A critical review on the manufacturing processes in relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2013, 47, 1093–1115.
- Buruga, K.; Song, H.; Shang, J.; Bolan, N.; Kim, K.-H. A review on functional polymer-clay based nanocomposite membranes for treatment of water. J. Hazard. Mater. 2019, 379, 120584.
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66.
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476.
- Du, J.H.; Cheng, H.M. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077.
- Li, X.Q.; Wang, C.Y.; Cao, Y.; Wang, G.X. Functional MXene materials: Progress of their applications. Chem. Asian J. 2018, 13, 2742–2757.
- Tu, S.B.; Jiang, Q.; Zhang, X.X.; Alshareef, H.N. Large dielectric constant enhancement in MXene percolative polymer composites. ACS Nano 2018, 12, 3369–3377.
- Calvert, P. Nanotube composites—A recipe for strength. Nature 1999, 399, 210–211.
- Liu, M.X.; Jia, Z.X.; Jia, D.M.; Zhou, C.R. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525.
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174.
- Albdiry, M.T.; Yousif, B.F. Toughening of brittle polyester with functionalized halloysite nanocomposites. Compos. Part B Eng. 2019, 160, 94–109.
- Gao, D.; Chang, R.; Lyu, B.; Ma, J. Growth from spherical to rod-like SiO2: Impact on microstructure and performance of nanocomposite. J. Alloys Compd. 2019, 810, 151814.
- Mark, J.E. Ceramic-reinforced polymers and polymer-modified ceramics. Polym. Eng. Sci. 1996, 36, 2905–2920.
- Herron, N.; Thorn, D.L. Nanoparticles: Uses and relationships to molecular cluster compounds. Adv. Mater. 1998, 10, 1173–1184.
- Huang, P.; Shi, H.Q.; Fu, S.Y.; Xiao, H.M.; Hu, N.; Li, Y.Q. Greatly decreased redshift and largely enhanced refractive index of mono-dispersed ZnO-QD/silicone nanocomposites. J. Mater. Chem. 2016, 4, 8663–8669.
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30.
- Information Resources Management Association. Materials Science and Engineering—Concepts, Methodologies, Tools, and Application; IGI Global: Hershey, PA, USA, 2017; Volume 1, ISBN 9781522517986.
- Unalan, I.U.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite films and coatings using inorganic nanobuilding blocks (NBB): Current applications and future opportunities in the food packaging sector. RSC Adv. 2014, 4, 29393–29428.
- Okamota, M. Recent Advances in Polymer/Layered Silicate Nanocomposites: An Overview from Science to Technology. Mater. Sci. Technol. 2006, 22, 756–779.
- Dadfar, S.M.R.; Ramazani, S.A.A.; Dadfar, S.M.A. Investigation of Oxygen Barrier Properties of Organoclay/HDPE/EVA Nanocomposite Films Prepared Using a Two-Step Solution Method. Polym. Compos. 2009, 30, 812–819.
- Yu, Y.-Y.; Chiu, C.-T.; Chueh, C.-C. Solution-Processable, Transparent Polyimide for High-Performance High-k Nanocomposite: Synthesis, Characterization, and Dielectric Applications in Transistors. Asian J. Org. Chem. 2018, 7, 2263–3370.
- Vaia, R.A.; Ishii, H.; Giannelis, E.P. Synthesis and Properties of 2-Dimensional Nanostructures by Direct Intercalation of Polymer Melts in Layered Silicates. Chem. Mater. 1993, 5, 1694–1696.
- Tiwari, S.K.; Sarang Pande, S.; Bobade, S.M.; Kumar, S. A Targeted Functional Value Based Nanoclay/PA12 Composite, Material Development for Selective Laser Sintering Process. Procedia Manuf. 2017, 21, 630–637.
- Hasani, Z.; Youssefi, M.; Borhani, S.; Mallakpour, S. Structure and properties of nylon-6/amino acid modified nanoclay composite fibers. J. Text. Inst. 2019, 110, 1336–1342.
- Liu, X.C.; Liu, Y.J.; Shi, P.; Lai, D.W.; Yi, Z.W.; Mao, L.; Yang, J.; Zheng, W. Thermal, Rheological and Mechanical Properties of PA6-66 Nanocomposites Co-Incorporated with Montmorillonite and Nanosilica. Nanosci. Nanotechnol. Lett. 2018, 10, 177–184.
- Zhang, Q.; Li, D.; Lai, D.; You, Y.; Ou, B. Preparation, microstructure, mechanical, and thermal properties of in situ polymerized polyimide/organically modified sericite mica composites. Polym. Compos. 2016, 37, 2243–2251.
- Kwon, K.; Chang, J.-H. Comparison of the properties of polyimide nanocomposites containing three different nanofillers: Organoclay, functionalized graphene, and organoclay/functionalized graphene complex. J. Compos. Mater. 2015, 49, 3031–3044.
- Dabrowski, F.; Bourbigot, S.; Delobel, R.; Le Bras, M. Kinetic modelling of the thermal degradation of polyamide-6 nanocomposite. Eur. Polym. J. 2000, 36, 273–284.
- Utracki, L.A.; Lyngaae-Jorgensen, J. Dynamic melt flow of nanocomposites based on poly-epsilon-caprolactam. Rheol. Acta 2002, 41, 394–407.
- Bourbigot, S.; Devaux, E.; Flambard, X. Flammability of polyamide-6/clay hybrid nanocomposite textiles. Polym. Degrad. Stab. 2002, 75, 397–402.
- Utracki, L.A. Equations of State for Polyamide-6 and Its Nanocomposites. II. Effects of Clay. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 966–980.
- Utracki, L.A. Equations of State for Polyamide-6 and Its Nanocomposites. 1. Fundamentals and the Matrix. J. Polym. Sci. Part B-Polym. Phys. 2009, 47, 299–313.
- dos Santos Filho, E.A.; de Medeiros, K.M.; Araujo, E.M.; Ferreira, R.D.B.; Oliveira, S.S.L.; Medeiros, V.D. Membranes of polyamide 6/clay/salt for water/oil separation. Mater. Res. Express 2019, 6, 105313.
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis of Nylon-6-Clay Hybrid by Montmorillonite Intercalated with Epsilon-Caprolactam. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 983–986.
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical-Properties of Nylon 6-Clay Hybrid. J. Mater. Res. 1993, 8, 1185–1189.
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O. One-Pot Synthesis of Nylon-6 Clay Hybrid. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1755–1758.
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of Nylon 6-Clay Hybrid. J. Mater. Res. 1993, 8, 1179–1184.
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O.; Kaji, K. Fine-Structure of Nylon-6-Clay Hybrid. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 625–630.
- Azuma, H.; Takeichi, A.; Noda, S. Structure-Analysis of Nylon6-Clay Hybrid by Spectral Reflectance of Laser-Plasma Soft X Rays. Jpn. J. Appl. Phys. Part 1–Regul. Pap. Short Notes Rev. Pap. 1993, 32, 5558–5563.
- Fukusima, Y. Development of clay minerals/organic polymer nano-composit materials. Nippon. Kagaku Kaishi 2000, 9, 605–611.
- Wu, T.M.; Liao, C.S. Crystalline transitions in nylon/clay nanocomposites. In Proceedings of the 58th Annual Technical Conference of the Society-of-Plastics-Engineers ANTEC 2000—Proceedings, Orlando, FL, USA, 2–4 April 2000; Volume I–III, pp. 1514–1517.
- Devaux, E.; Bourbigot, S.; El Achari, A. Crystallization behavior of PA-6 clay nanocomposite hybrid. J. Appl. Polym. Sci. 2002, 86, 2416–2423.
- Tanaka, G.; Goettler, L.A. Predicting the binding energy for nylon 6,6/clay nanocomposites by molecular modeling. Polymer 2002, 43, 541–553.
- Silva, T.F.; Soares, B.G.; Ferreira, S.C.; Livi, S. Silylated montmorillonite as nanofillers for plasticized PVC nanocomposites: Effect of the plasticizer. Appl. Clay Sci. 2014, 99, 93–99.
- Thabet, A.; Ebnalwaled, A.A. Improvement of surface energy properties of PVC nanocomposites for enhancing electrical applications. Measurement 2017, 110, 78–83.
- Pospíšil, M.; Čapková, P.; Weiss, Z.; Maláč, Z.; Šimoník, J. Intercalation of Octadecylamine into Montmorillonite: Molecular Simulations and XRD Analysis. J. Colloid Interface Sci. 2002, 245, 126–132.
- Lagaly, G.; Weiss, A. Arrangement and orientation of tensides on silicate surfaces II. Paraffin-like structure in alkylammonium layer silicate with a high layer charge (mica). Kolloid Z. Z. Fur Polym. 1970, 237, 364–368.
- Lagaly, G.; Weiss, A. Arrangement and orientation of cationic tensides on silicate surfaces IV. Arrangement of alkylammonium ions in low-charged silicates of films. Kolloid Z. Z. Fur Polym. 1971, 243, 48.
- Lagaly, G.; Weiss, A. Layer intercalation coumpouns as models for structure and structural conversions of monomolecular and bimolecular films of long-chain compounds II. Phase changes in alkylammonium layer silicate-alkanol complexes. Kolloid Z. Z. Fur Polym. 1971, 248, 979.
- Harris, A.M.; Lee, E.C. Heat and humidity performance of injection molded PLA for durable applications. J. Appl. Polym. Sci. 2010, 115, 1380–1389.
- Kalendova, A.; Smotek, J.; Stloukal, P.; Kracalik, K.M.; Slouf, M.; Laske, S. Transport Properties of PLA/Clay Nanocomposites. Polym. Eng. Sci. 2019, 59, 2498–2501.
- Prakalathan, K.; Mohanty, S.; Nayak, S.K. Polylactide/modified layered silicates nanocomposites: A critical analysis of morphological, mechanical and thermalproperties. J. Reinf. Plast. Compos. 2012, 31, 1300–1310.
- Darie, R.N.; Paslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiatǎ, A.; Baklavaridis, A.; Vasile, C. Effect of nanoclay hydrophilicity on the poly(lactic acid)/clay nanocomposites properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890.
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46.
- Connolly, M.; Zhang, Y.; Brown, D.M.; Ortuno, N.; Jorda-Beneyto, M.; Stone, V.; Fernandes, T.F.; Johnston, H.J. Novel polylactic acid (PLA)-organoclay nanocomposite bio-packaging for the cosmetic industry; migration studies and in vitro assessment of the dermal toxicity of migration extracts. Polym. Degrad. Stab. 2019, 168, 108938.
- Moldovan, A.; Cuc, S.; Prodan, D.; Rusu, M.; Popa, D.; Taut, A.C.; Petean, I.; Bombos, D.D.R.; Nemes, O. Development and Characterization of Polylactic Acid (PLA)-Based Nanocomposites Used for Food Packaging. Polymers 2023, 15, 2855.
- Choudalakis, G.; Gotsis, A. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984.
- Lu, C.; Mai, Y.W. Influence of aspect ratio on barrier properties of polymer-clay nanocomposites. Phys. Rev. Lett. 2005, 95, 088303.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





