Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoichiro Tamori | -- | 2064 | 2024-01-17 06:29:46 | | | |
| 2 | Jessie Wu | Meta information modification | 2064 | 2024-01-17 06:46:13 | | | | |
| 3 | Jessie Wu | Meta information modification | 2064 | 2024-01-17 06:50:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, Y.; Tamori, Y. Advantages of Drosophila Model in Polyploid Cell Investigation. Encyclopedia. Available online: https://encyclopedia.pub/entry/53934 (accessed on 08 February 2026).
Wang Y, Tamori Y. Advantages of Drosophila Model in Polyploid Cell Investigation. Encyclopedia. Available at: https://encyclopedia.pub/entry/53934. Accessed February 08, 2026.
Wang, Yuqing, Yoichiro Tamori. "Advantages of Drosophila Model in Polyploid Cell Investigation" Encyclopedia, https://encyclopedia.pub/entry/53934 (accessed February 08, 2026).
Wang, Y., & Tamori, Y. (2024, January 17). Advantages of Drosophila Model in Polyploid Cell Investigation. In Encyclopedia. https://encyclopedia.pub/entry/53934
Wang, Yuqing and Yoichiro Tamori. "Advantages of Drosophila Model in Polyploid Cell Investigation." Encyclopedia. Web. 17 January, 2024.
Copy Citation
Polyploid cells are frequently observed in advanced cancer, particularly after standard cancer treatment such as anticancer drugs and radiation therapy. This suggests that polyploid cells lurking in a cancer tissue possess a superior ability to withstand environmental stress, making them more likely to survive anticancer therapies. The polyploid cells in cancer tissues, commonly termed polyploid giant cancer cells (PGCCs), but also referred to as blastomere-like cancer cells, osteoclast-like cancer cells, pleomorphic cancer cells, large cancer stem cells, and polyaneuploid cancer cells (PACCs), are thought to play an important role in tumor progression.
polyploidy
polyploid cancer cells
tumorigenesis
stress resistance
Drosophila
1. Polyploid Tumor Model—Larval Salivary Gland
The imaginal ring of Drosophila larval salivary glands is composed of adult progenitor cells and undergoes proliferation during the late larval stages to replace the neighboring larval tissues for adult organ formation [1]. It has been shown that the cells in the narrow transition zone that reside at the border between the posterior end of the imaginal ring and the secretary polytene salivary gland cells endogenously undergo endoreplication to become polyploid [2]. These polyploid cells in the transition zone have an endogenously high activation level of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling, c-Jun N-terminal kinase (JNK) signaling, and JNK-induced matrix metalloproteinase 1 (MMP1) expression all of which are involved in oncogenesis. These tissue characteristics confer the cells in this area oncogenic potential, which is called “tumor hotspots” [3][4] (Figure 1).
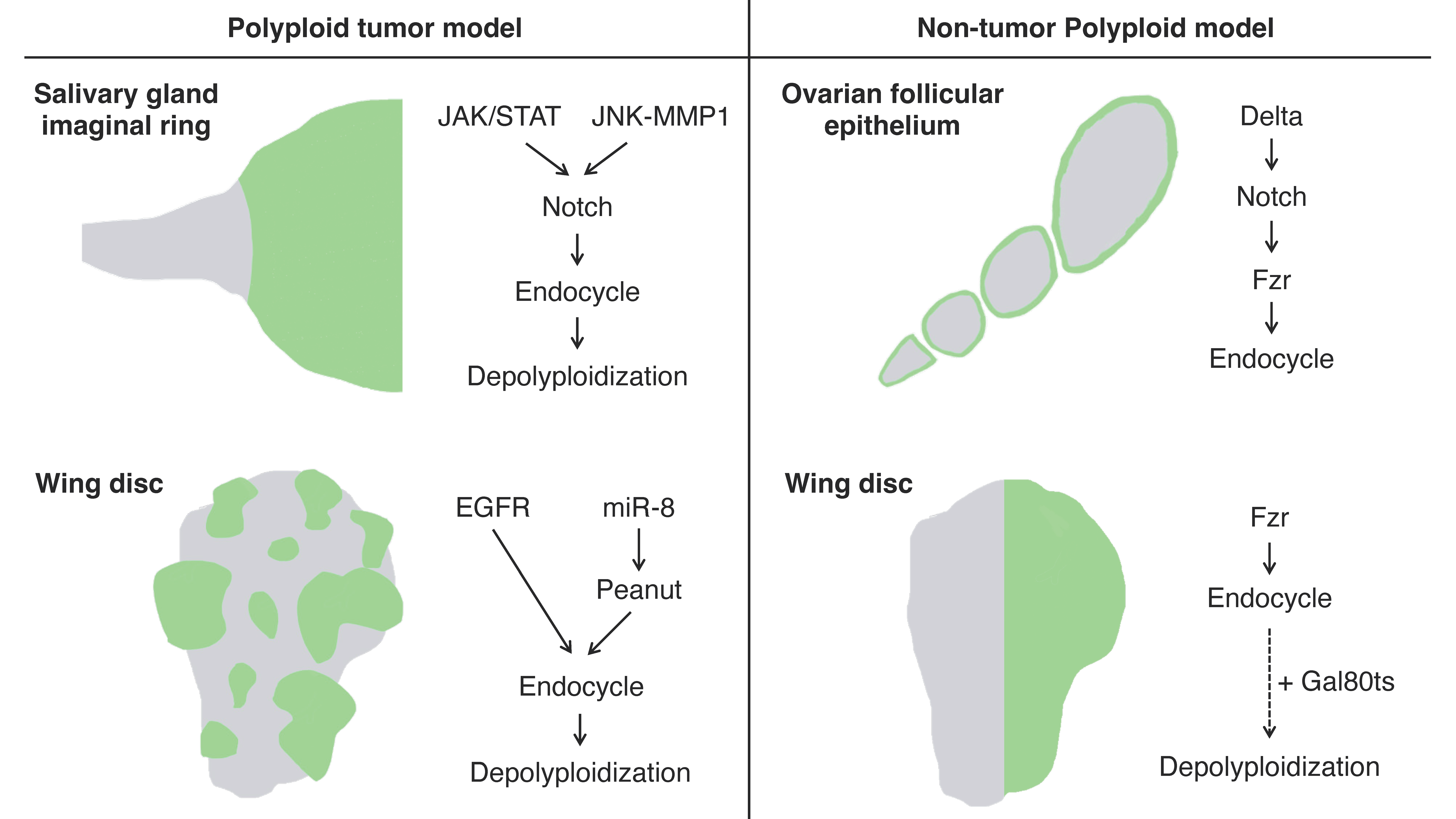
Figure 1. Schematics of the experimental models for polyploidy in Drosophila: Left panel: the artificially induced tumor models featuring polyploidization and depolyploidization during the tumor progression induced in the larval salivary glands (top) and wing imaginal discs (bottom) using the Gal4-UAS system. The green areas indicate polyploid tumors induced by the Gal4-UAS system. Right panel: non-tumor polyploid cell models in the ovarian follicular epithelium (endogenously programmed endocycle) and larval wing imaginal discs (artificially induced endocycle). The green areas indicate the regions where cells are going to become polyploid in these models.
Misexpression of an active form of Notch, the Notch intracellular domain (NICD), in salivary glands during the third instar larval stage induces tumorigenesis. In the tumorigenic salivary glands, a hyperplastic overgrowth is induced in the diploid imaginal ring cells, but neoplastic tumor phenotypes including multilayer growth and polarity loss are induced in the polyploid transition zone [2]. The re-entry to mitosis followed by continuous cell division of the polyploid transition-zone cells in response to the Notch signaling overactivation is key to tumor initiation and progression [5]. These tumor cells display a high degree of variations in ploidy levels and show a marked increase in chromosomal instabilities and aneuploidy as the tumors continue to grow. Interestingly, some genes involved in the DNA damage response (DDR) pathway are necessary for tumorigenesis, like Meiotic recombination 11 (MRE11), Replication protein A1 (RPA1), Structural maintenance of chromosomes protein 5 (SMC5), and DDR kinase—meiotic 41 (mei-41, the Drosophila homolog of ATR), which imply the important function of DNA repair factors in this tumorigenesis [5].
2. Polyploid Tumor Model—Wing Imaginal Disc
Imaginal discs are larval epithelial tissues developing into particular parts of adult insect bodies. Among several imaginal discs of Drosophila larvae, wing discs have been commonly used as an experimental model for organ growth, pattern formation, and tumorigenesis [6]. Recently, a genetic experimental study has shown that an overexpression of EGFR combined with microRNAs (bantam or miR-8) causes cancerous phenotypes in the Drosophila wing discs including epithelial polarity defect and invasive migration, which provides researchers with a polyploid tumor model in this epithelial tissue [7] (Figure 1). Specifically, the tumor is made of polyploid cancer cells. Although overexpression of EGFR alone causes benign tissue hyperplasia in the epithelial tissues, a combinatorial overexpression of miR-8 and EGFR induces polyploid tumors. In this model, miR-8 expression causes genome instability by downregulating the expression of the Septin family protein Peanut. Peanut is similar to mammalian Septin7, and its depletion induces binuclear cell formation through cytokinesis failure [8]. The cytokinesis failure combined with EGFR overactivation leads to the formation of neoplasia with polyploid cancer cells (Figure 1).
One disadvantage of using wing imaginal discs as a tumor model is the limited time available to observe cancer progression during larval stages (~7 days). Recently, however, a continuous culture system that can allow researchers to observe the tumor progression over a much longer period has been developed [9]. This experimental system is to perform generational allotransplantation of imaginal disc tumors into the abdomen of adult hosts. This technique will not only allow researchers to observe the transition of cancer tissues over time but will also be a very powerful tool for studying the relationship between cancer and its host body.
3. Artificially Induced Polyploid Cells—Wing Imaginal Disc
The artificially induced polyploid cell models in Drosophila are based on the Gal4-UAS binary expression system, which facilitates the expression of a specific cell-cycle regulatory gene such as fizzy-related (fzr, mammalian homolog of CDH1). In the Gal4-UAS system, Gal4 is a transcriptional activator that binds to the UAS enhancer sequences, thereby driving the expression of a gene of interest linked to UAS. Gal4 is typically fused to an enhancer sequence of a gene that is spatiotemporally active in specific organ regions, thereby controlling Gal4 expression in the specific region [10]. For instance, the engrailed-Gal4 (en-Gal4) is associated with the engrailed (en, a homeodomain selector gene) enhancer sequence, which is specifically expressed in the posterior compartment of the organ, enabling the induction of a UAS-gene expression in the posterior side of wing imaginal discs [11] (Figure 2).
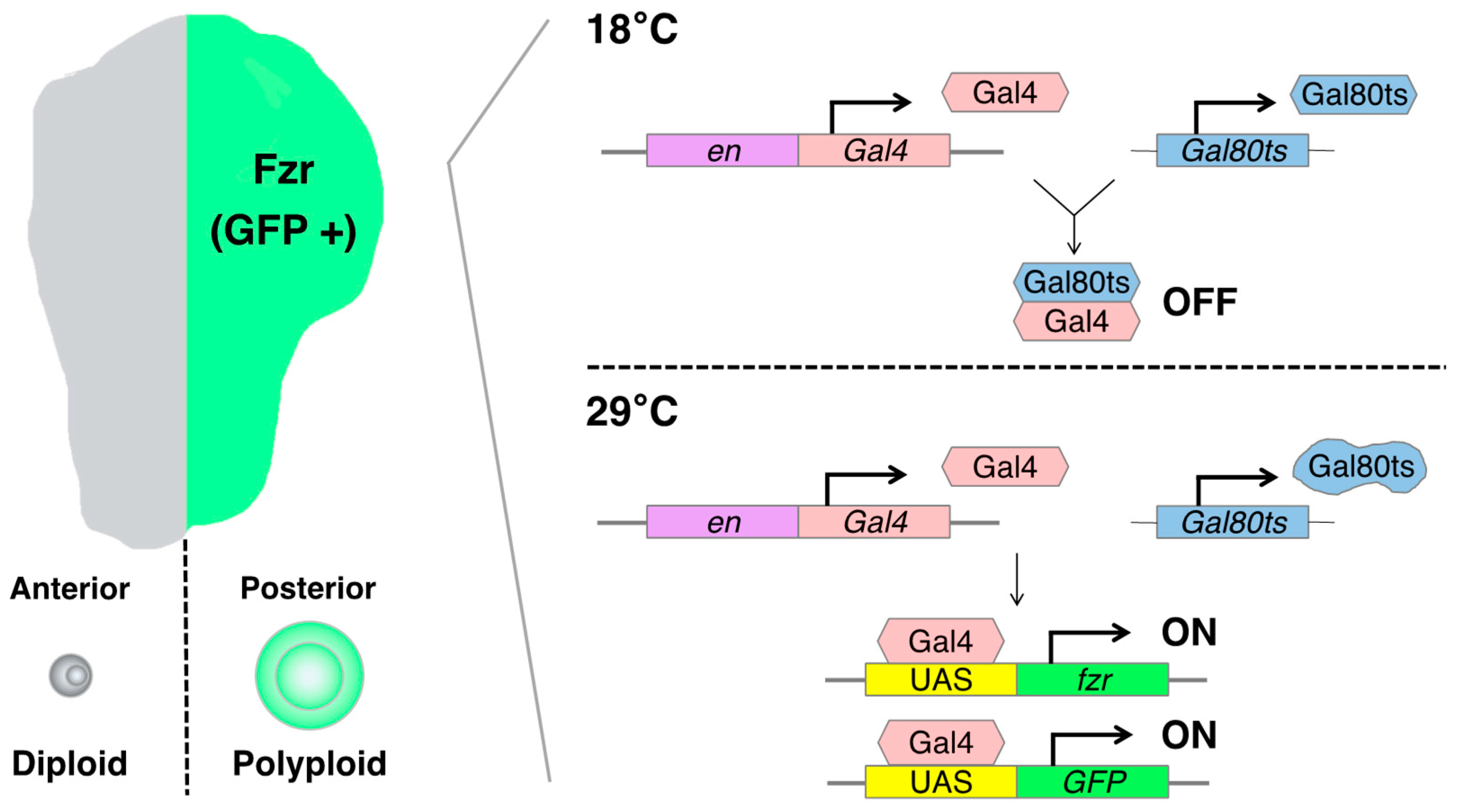
Figure 2. Schematics of the artificially induced polyploidy model using the Gal4-UAS binary expression system in Drosophila wing imaginal discs: The engrailed-Gal4 (en-Gal4) transcriptional activator drives expression of the UAS-linked fizzy-related (fzr) specifically in the posterior compartment of wing imaginal discs, enabling the induction of endoreplication-mediated polyploidization in the posterior half of the tissue. The Gal80 blocks Gal4-mediated transcriptional activation by binding to its transcriptional activation domain. The Gal80ts is functional to inhibit Gal4 activation at 18 °C but ceases to function at 29 °C.
fzr is a regulatory gene involved in endoreplication, and its overexpression can induce the endocycle-mediated polyploidization [12][13]. Mechanistically, Fzr interacts with the APC/C (anaphase-promoting complex/cyclosome) and induces a transition from mitotic to endocycle by degrading mitotic cyclins and skipping the M phase [12][14][15]. Researchers can employ the en-Gal4 to induce the misexpression of fzr in the posterior compartment of the wing discs, resulting in the generation of polyploid cells. This approach allowed researchers to obtain diploid cells in the anterior side and polyploid cells in the posterior side of the same wing disc, facilitating a convenient comparison of phenotypes and molecular regulation simultaneously.
To control the duration of fzr expression, researchers can utilize a temperature-sensitive form of Gal80 (Gal80ts). Gal80 blocks Gal4-mediated transcriptional activation by binding to its transcriptional activation domain. The Gal80ts is functional to inhibit Gal4 activation at 18 °C but ceases to function at 29 °C [16]. In the experimental setting, the fly larvae bearing en-Gal4, UAS-fzr, and ubiquitous Gal80ts (tub-Gal80ts) are kept at 18 °C for 6 days while waiting the eggs to develop into first instar larvae. Then, researchers shift the temperature to 29 °C to start Gal4-mediated fzr expression and keep them at 29 °C for 2 days before the dissection of wing discs from third instar larvae (Figure 2).
Prolonged misexpression of fzr leads to developmental defects in the wing disc. For example, when researchers keep them at 29 °C for 3 days after the temperature shift, the growth of the posterior compartment is not enough to develop into its normal size probably because the polyploid cells show apoptosis and do not proliferate. As a result, diploid cells in the anterior compartment proliferate to compensate for the undergrown posterior side of the wing disc. This is an interesting phenotype because the mechanism by which long-time fzr causes polyploid cell apoptosis is unknown. It is quite likely that the apoptosis of those polyploid cells resulted from chromosomal instability and mitotic catastrophe.
There is one more thing to take into consideration when researchers use this experimental model. The ploidy of those polyploid cells induced in the posterior compartment of wing discs is not uniform. The nuclear size of polyploid cells in the posterior compartment 2 days after temperature shifting is larger in the wing pouch than in the proximal hinge regions. This difference in ploidy level between these regions might be attributable to the differential regulation of Cyclin E in this tissue [17].
Using this experimental model, researchers can also investigate the mitotic cell division of polyploid cells by canceling the misexpression of fzr. When researchers shift back the temperature from 29 °C to 18 °C, the Gal4-driven fzr expression ceases and the polyploid cells go back to the mitotic cycle. When polyploid cells undergo mitotic cell division, there is a high probability of chromosome missegregation that could produce aneuploid daughter cells. There are many apoptotic cells observed in the posterior compartment after canceling the fzr expression, probably because aneuploid cells produced through the chromosome missegregation cannot gain survival advantages. Therefore, this experimental model also provides a tool to study how polyploid cells undergo depolyploidization that produces stable diploid or unstable aneuploid daughter cells.
4. Endogenously Developed Polyploid Cells—Ovarian Follicular Epithelia
In Drosophila, there are many organs endogenously bearing polyploid cells. One such well-studied example is, as already mentioned, the ovarian follicular epithelium composed of the monolayer of epithelial cells covering germline cells in an egg chamber [18]. The follicle cells endogenously undergo the cell-cycle transition from mitotic to endoreplication cycle and generate polyploid cells during the mid-stage of the oogenesis (stages 7 to 10) [19]. This natural process allows researchers to observe both diploid and polyploid cells within the same ovariole [6]. The mitotic-to-endocycle transition is triggered by the Delta-Notch signaling pathway activated at stage 7, and Fzr plays a crucial role in this cell-cycle switch as a downstream event of Notch signaling activation [12] (Figure 1).
In this experimental model, researchers can use a pan-follicle cell driver traffic-jam-Gal4 (tj-Gal4) that can induce UAS-gene expression in every follicle cell of egg chambers in all oogenesis stages [20][21]. Although the tj-Gal4 is expressed in ovarian follicle cells, its expression can be observed also in several other organs during different larval stages [22]. Therefore, the combination of Gal80ts and Gal4-UAS system with the temperature shifting can be utilized to activate the tj-Gal4 expression in the adult stage. To bypass the larval stages, the flies bearing tj-Gal4 and UAS-gene should be maintained at 18 °C after fertilization to the adult stage. Two days before dissection, researchers transfer the adult female and male flies to the same fooded vial to stimulate ovary maturation of the female flies and keep them at 29 °C.
Unlike the imaginal-disc experiment, researchers do not need to misexpress an additional gene such as fzr to induce polyploidy in follicle cells because they endogenously undergo endocycle-mediated polyploidization. This makes the genetic cross scheme to have another genetic manipulation in the polyploid cells simpler and allows researchers to examine easily what genetic backgrounds affect the nature of polyploidy. At the same time, it is important to note that egg chamber development is so quick (the oogenesis completes within 3 days) that researchers need to be careful with precise control of Gal4 expression and that the spatiotemporally varying developmental signals according to oogenesis stages may affect the comparison between diploid and polyploid follicle cells. Nevertheless, the advantages of using endogenously programmed polyploidization system like ovarian follicle cells is that the ploidy is controlled (16C in the stage 10 egg chamber) and the number of polyploid cells remains relatively consistent within the same oogenesis stage (approximately 650 follicle cells in the stage 10 egg chamber) [19].
Drosophila ovarian follicular epithelia offer researchers yet another polyploid tumor model. Using tj-Gal4 with the Gal80ts system to control the expression of UAS-RNAi for a neoplastic tumor suppressor gene lethal giant larvae (lgl), researchers can induce epithelial polarity defect and multilayer formation in the follicular epithelia during midoogenesis [6][23]. The basally located cells in the multilayered epithelium cannot stop proliferating and become tumorigenic because they lose contact with the inner germ-line cells which are the source of the Notch signaling ligand Delta and thus the blockade of Notch signaling prevents the mitotic-to-endocycle transition [6]. These tumorigenic cells in the multilayer show a distinguishable variability in their ploidy that is enhanced when Notch signaling activation is ectopically induced (via co-expression of NICD) in the lgl-knockdown cells [23][24]. At the same time, ectopic expression of Snail-family transcriptional factor Escargot promoted in the tumorigenic lgl-knockdown cells enhances ploidy heterogeneity, likely via depolyploidization [23].
References
- Madhavan, M.M.; Schneiderman, H.A. Histological Analysis of the Dynamics of Growth of Imaginal Discs and Histoblast Nests during the Larval Development of Drosophila melanogaster. Wilhelm Roux’s Arch. Dev. Biol. 1977, 183, 269–305.
- Yang, S.-A.; Portilla, J.-M.; Mihailovic, S.; Huang, Y.-C.; Deng, W.-M. Oncogenic Notch Triggers Neoplastic Tumorigenesis in a Transition-Zone-like Tissue Microenvironment. Dev. Cell 2019, 49, 461–472.e5.
- Tamori, Y.; Suzuki, E.; Deng, W.-M. Epithelial Tumors Originate in Tumor Hotspots, a Tissue-Intrinsic Microenvironment. PLoS Biol. 2016, 14, e1002537-23.
- Tamori, Y.; Deng, W.-M. Tissue-Intrinsic Tumor Hotspots: Terroir for Tumorigenesis. Trends Cancer 2017, 3, 259–268.
- Wang, X.-F.; Yang, S.-A.; Gong, S.; Chang, C.-H.; Portilla, J.M.; Chatterjee, D.; Irianto, J.; Bao, H.; Huang, Y.-C.; Deng, W.-M. Polyploid Mitosis and Depolyploidization Promote Chromosomal Instability and Tumor Progression in a Notch-Induced Tumor Model. Dev. Cell 2021, 56, 1976–1988.e4.
- Tamori, Y. The Initial Stage of Tumorigenesis in Drosophila Epithelial Tissues. Adv. Exp. Med. Biol. 2019, 1167, 87–103.
- Eichenlaub, T.; Cohen, S.M.; Herranz, H. Cell Competition Drives the Formation of Metastatic Tumors in a Drosophila Model of Epithelial Tumor Formation. Curr. Biol. 2016, 26, 419–427.
- Founounou, N.; Loyer, N.; Le Borgne, R. Septins Regulate the Contractility of the Actomyosin Ring to Enable Adherens Junction Remodeling during Cytokinesis of Epithelial Cells. Dev. Cell 2013, 24, 242–255.
- Gong, S.; Zhang, Y.; Bao, H.; Wang, X.; Chang, C.-H.; Huang, Y.-C.; Deng, W.-M. Tumor Allotransplantation in Drosophila melanogaster with a Programmable Auto-Nanoliter Injector. J. Vis. Exp. 2021, 168, e62229.
- Brand, A.H.; Perrimon, N. Targeted Gene Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development 1993, 118, 401–415.
- Johnson, R.L.; Grenier, J.K.; Scott, M.P. Patched Overexpression Alters Wing Disc Size and Pattern: Transcriptional and Post-Transcriptional Effects on Hedgehog Targets. Development 1995, 121, 4161–4170.
- Schaeffer, V.; Althauser, C.; Shcherbata, H.R.; Deng, W.-M.; Ruohola-Baker, H. Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr. Biol. 2004, 14, 630–636.
- Stormo, B.M.; Fox, D.T. Distinct Responses to Reduplicated Chromosomes Require Distinct Mad2 Responses. eLife 2016, 5, e15204.
- Sigrist, S.J.; Lehner, C.F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 1997, 90, 671–681.
- García-Higuera, I.; Manchado, E.; Dubus, P.; Cañamero, M.; Méndez, J.; Moreno, S.; Malumbres, M. Genomic Stability and Tumour Suppression by the APC/C Cofactor Cdh1. Nat. Cell Biol. 2008, 10, 802–811.
- McGuire, S.E.; Le, P.T.; Osborn, A.J.; Matsumoto, K.; Davis, R.L. Spatiotemporal Rescue of Memory Dysfunction in Drosophila. Science 2003, 302, 1765–1768.
- Shu, Z.; Deng, W.-M. Differential Regulation of Cyclin E by Yorkie-Scalloped Signaling in Organ Development. G3 Genes Genomes Genet. 2017, 7, 307–315.
- Bastock, R.; Johnston, D.S. Drosophila Oogenesis. Curr. Biol. 2008, 18, R1082–R1087.
- Klusza, S.; Deng, W.-M. At the crossroads of differentiation and proliferation: Precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. BioEssays 2011, 33, 124–134.
- Sahai-Hernandez, P.; Nystul, T.G. A Dynamic Population of Stromal Cells Contributes to the Follicle Stem Cell Niche in the Drosophila Ovary. Development 2013, 140, 4490–4498.
- Weaver, L.N.; Ma, T.; Drummond-Barbosa, D. Analysis of Gal4 Expression Patterns in Adult Drosophila Females. G3 Genes Genomes Genet. 2020, 10, 4147–4158.
- Babski, H.; Jovanic, T.; Surel, C.; Yoshikawa, S.; Zwart, M.F.; Valmier, J.; Thomas, J.B.; Enriquez, J.; Carroll, P.; Garcès, A. A GABAergic Maf-Expressing Interneuron Subset Regulates the Speed of Locomotion in Drosophila. Nat. Commun. 2019, 10, 4796.
- Chatterjee, D.; Cong, F.; Wang, X.-F.; Costa, C.A.M.; Huang, Y.-C.; Deng, W.-M. Cell Polarity Opposes Jak/STAT-Mediated Escargot Activation That Drives Intratumor Heterogeneity in a Drosophila Tumor Model. Cell Rep. 2023, 42, 112061.
- Jevitt, A.; Huang, Y.-C.; Zhang, S.-M.; Chatterjee, D.; Wang, X.-F.; Xie, G.-Q.; Deng, W.-M. Modeling Notch-Induced Tumor Cell Survival in the Drosophila Ovary Identifies Cellular and Transcriptional Response to Nuclear NICD Accumulation. Cells 2021, 10, 2222.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
578
Revisions:
3 times
(View History)
Update Date:
17 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




