
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Darina Bačenková | -- | 2631 | 2024-01-16 12:52:16 | | | |
| 2 | Lindsay Dong | Meta information modification | 2631 | 2024-01-17 02:16:39 | | |
Video Upload Options
Articular cartilage is a load-bearing connective tissue that has a low self-repair potential. There are high demands placed on articular hyaline cartilage in the organism, mainly mechanical flexibility, load-bearing capacity, and the ability to reduce friction. The function of the cartilage in joints is to ensure low friction and the ability to distribute the weight load acting in the joint. An articular cartilage defect can persist without healing, or if it extends into the blood-filled subchondrium, then it is replaced by cartilage tissue that does not have suitable strength properties.
1. Introduction
Hyaline cartilage is composed of a complex structured extracellular matrix (ECM) that facilitates friction on the surface of the articular cartilage and is adapted to repeated compression during movement. Synovial fluid has an important function in the nutrition of articular chondrocytes, as this process is ensured by diffusion and improving the lubrication of cartilage joint surfaces [1]. The joint capsule with its cell lining on the surface of the synovial membrane contains cells of the synovial intima called synoviocytes. Fibroblast-like cells are involved in the production of synovial fluid, and the macrophage-like type can be considered resident macrophage cell types [2]. In the organism, articular cartilage is located in the articular surfaces at the end of the epiphyses of long bones and consists of several interconnected zones, namely superficial, transitional, deep, and calcified areas separated from the underlying bone. Parts of the pelvis and the bones of the long limbs are replaced by bone through the process of ossification [3].
2. Articular Cartilage
2.1. Extracellular Matrix
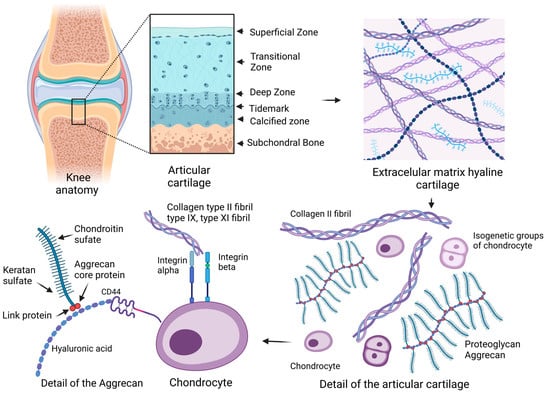
2.2. Chondrocyte
2.2.1. Progenitor Cells of Cartilage
2.2.2. Characteristic Phenotype of Chondrocytes
2.2.3. Specific Growth Factors during the Expansion of Articular Chondrocytes
2.2.4. Biomechanical Principles of Articular Cartilage and Chondrocytes
References
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: Structure and potential functions. Microsc. Res. Tech. 1994, 28, 385–397.
- Iwanaga, T.; Shikichi, M. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31.
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths &misconceptions—Progress & prospects. Osteoarth Cart. 2015, 23, 334–350.
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P. Strategies to convert cells into hyaline cartilage: Magic spells for adult stem cells. Int. J. Mol. Sci. 2022, 23, 11169.
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. Transl. Integr. 1996, 81, 535–545.
- Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell. Mol. Life Sci. 2005, 62, 2241–2256.
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84.
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57.
- Kheir, E.; Shaw, D. Hyaline articular cartilage. Orthop. Trauma 2009, 23, 450–455.
- Hayes, A.J.; Melrose, J. Glycosaminoglycan and proteoglycan biotherapeutics in articular cartilage protection and repair strategies: Novel approaches to visco-supplementation in orthobiologics. Adv. Ther. 2019, 2, 1900034.
- Pomin, V.H. Keratan sulfate: An up-to-date review. Int. J. Biol. Macromol. 2015, 72, 282–289.
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71, 51–69.
- Smith, L.R.; Trindade, M.C.D.; Ikenoue, T. Effects of shear stress on articular chondrocyte metabolism. Biorheology 2000, 37, 95–107.
- Fox, S.; Bedi, A.J.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468.
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.H. The chondrocyte: Biology and clinical application. Tissue Eng. 2006, 12, 1971–1984.
- Cancedda, R.; Cancedda, F.D.; Castagnola, P. Chondrocyte differentiation. Int. Rev. Cytol. 1995, 159, 265–358.
- Responte, D.J.; Lee, J.K.; Hu, J.C.; Athanasiou, K.A. Biomechanics-driven chondrogenesis: From embryo to adult. FASEB J. 2012, 26, 3614.
- Zhao, Q.; Eberspaecher, H.; Lefebvre, V.; de Crombrugghe, B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1997, 209, 377–386.
- Loeser, R.F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16.
- Heinegård, D. Fell-Muir Lecture: Proteoglycans and more–from molecules to biology. Int. J. Exp. Pathol. 2009, 90, 575–586.
- Tseng, C.C.; Chen, Y.J.; Chang, W.A.; Tsai, W.C. Dual role of chondrocytes in rheumatoid arthritis: The chicken and the egg. Int. J. Mol. Sci. 2020, 21, 1071.
- Ecke, A.; Lutter, A.H.; Scholka, J.; Hansch, A. Tissue specific differentiation of human chondrocytes depends on cell microenvironment and serum selection. Cells 2019, 8, 934.
- Camper, L.; Hellman, U.; Lundgren-Åkerlund, E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998, 273, 20383–20389.
- Lundgren-Åkerlund, E.; Aszòdi, A. Integrin α10β1: A collagen receptor critical in skeletal development. Adv. Exp. Med. Biol. 2014, 819, 61–71.
- Bengtsson, T.; Aszodi, A.; Nicolae, C.; Hunziker, E.B.; Lundgren-Åkerlund, E. Loss of α10β1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J. Cell Sci. 2005, 118, 929–936.
- Knudson, W.; Casey, B.; Nishida, Y.; Eger, W. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2000, 43, 1165–1174.
- Salter, D.M.; Hughes, D.E.; Simpson, R. Integrin expression by human articular chondrocytes. Rheumatology 1992, 31, 231–234.
- Enomoto, M.; Leboy, P.S.; Menko, A.S. β1 integrins mediate chondrocyte interaction with type I collagen, type II collagen, and fibronectin. Exp. Cell Res. 1993, 205, 276–285.
- Aguiar, D.J.; Knudson, W.; Knudson, C.B. Internalization of the hyaluronan receptor CD44 by chondrocytes. Exp. Cell Res. 1999, 252, 292–302.
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Miner. Res. 1997, 12, 1657–1663.
- Hu, X.; Zhang, W.; Li, X.; Zhong, D.; Li, Y.; Li, J. Strategies to modulate the redifferentiation of chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 764193.
- Jakob, M.; Demarteau, O.; Schäfer, D.; Hintermann, B.; Dick, W. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001, 81, 368–377.
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224.
- Burt, D.W.; Law, A.S. Evolution of the transforming growth factor-beta superfamily. Prog. Growth Factor Res. 1994, 5, 99–118.
- De Caestecker, M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 2004, 15, 1–11.
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y. Role of transforming growth factor-β1 in regulating fetal-maternal immune tolerance in normal and pathological pregnancy. Front. Immunol. 2021, 12, 689181.
- Mantel, P.Y.; Schmidt-Weber, C.B. Transforming growth factor-beta: Recent advances on its role in immune tolerance. Methods Mol. Biol. 2011, 677, 303–338.
- Van Osch, G.J.; Van Der Veen, S.W.; Buma, P.; Verwoerd-Verhoef, H.L. Effect of transforming growth factor-β on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998, 17, 413–424.
- Luo, K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137.
- Duan, D.; Derynck, R. Transforming growth factor–β (TGF-β)–induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J. Biol. Chem. 2019, 294, 8490–8504.
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63, 515–524.
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145.
- Bailey, K.N.; Nguyen, J.; Yee, C.S.; Dole, N.S.; Dang, A. Mechanosensitive control of articular cartilage and subchondral bone homeostasis in mice requires osteocytic transforming growth factor β signaling. Arthritis Rheumatol. 2021, 73, 414–425.
- Li, T.F.; O’Keefe, R.J.; Chen, D. TGF-β signaling in chondrocytes. Front. Biosci. 2005, 10, 681.
- Puolakkainen, P.A.; Twardzik, D.R.; Ranchalis, J.E. The enhancement in wound healing by transforming growth factor-β1 (TGF-β1) depends on the topical delivery system. J. Surg. Res. 1995, 58, 321–329.
- Critchlow, M.A.; Bland, Y.S.; Ashhurst, D.E. The effect of exogenous transforming growth factor-β2 on healing fractures in the rabbit. Bone 1995, 16, 521–527.
- Wells, R.G. TGF-β signaling pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, 845–850.
- Duan, M.; Wang, Q.; Liu, Y.; Xie, J. The role of TGF-β2 in cartilage development and diseases. Bone Jt. Res. 2021, 10, 474–487.
- Das, R.; Timur, U.T.; Edip, S.; Haak, E.; Wruck, C.; Weinans, H. TGF-β2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann. Anat. Anat. Anz. 2015, 198, 1–10.
- Park, J.H.; Ushida, T.; Akimoto, T. Control of cell differentiation by mechanical stress. J. Phys. Fit. Sport. Med. 2013, 2, 49–62.
- Guilak, F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology 2000, 37, 27–44.
- Carter, D.R.; Beaupré, G.S.; Wong, M.; Smith, R.L. The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. Relat. Res. 2004, 427, 69–77.
- Franzen, A.; Inerot, S.; Hejderup, S. Variations in the composition of bovine hip articular cartilage with distance from the articular surface. Biochem. J. 1981, 195, 535–543.
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A. The biomechanics of cartilage—An overview. Life 2021, 11, 302.
- Lu, X.L.; Mow, V.C. Biomechanics of articular cartilage and determination of material properties. Med. Sci. Sport. Exerc. 2008, 40, 193–199.




