
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alejandro K. Samhan-Arias | -- | 2971 | 2024-01-15 14:06:40 | | | |
| 2 | Peter Tang | + 2 word(s) | 2973 | 2024-01-16 03:22:18 | | |
Video Upload Options
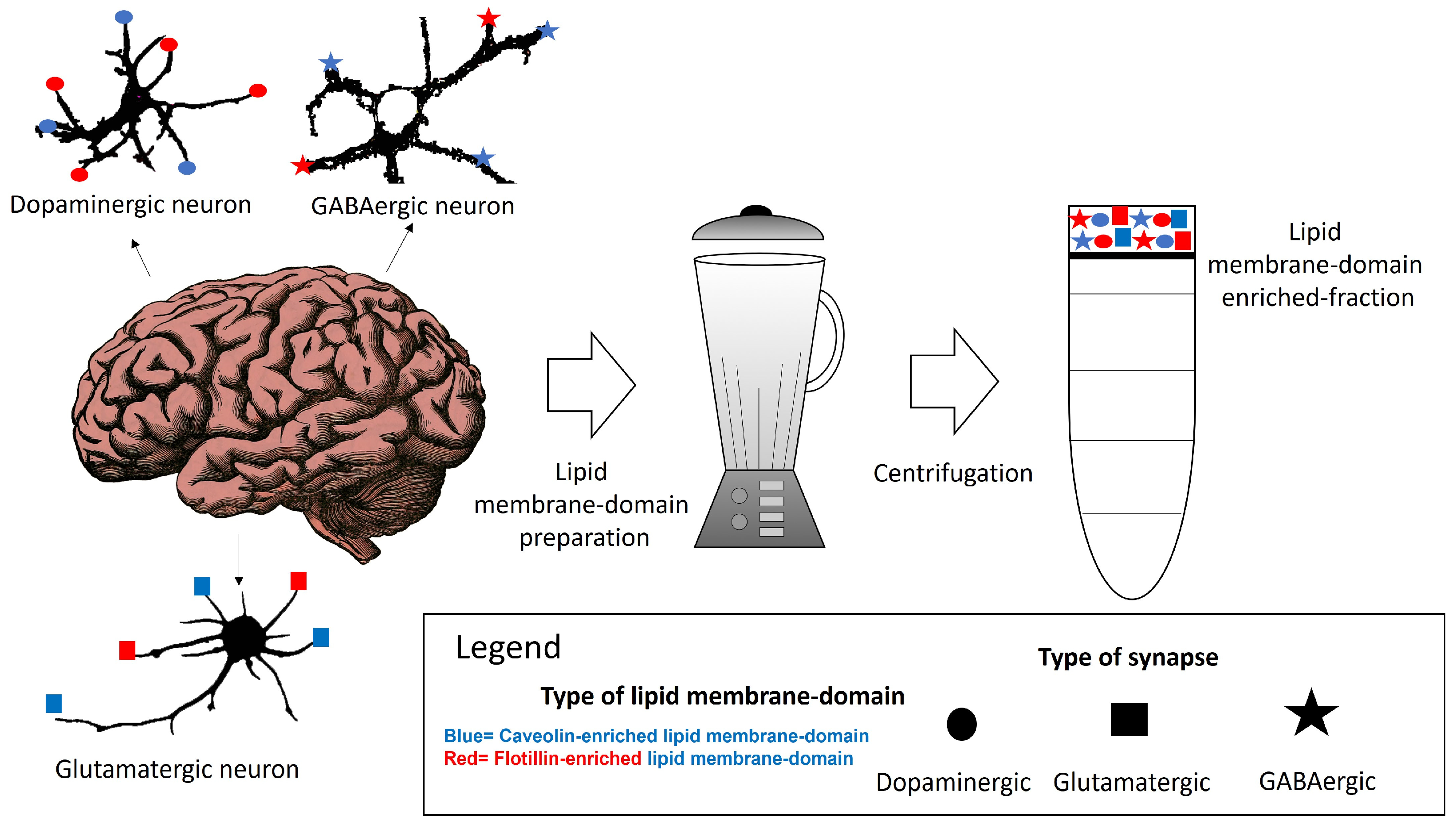
Lipid membrane nanodomains are membrane areas enriched on proteins that can form oligomers and cluster in the membranes. The formation of these oligomers is favored by cholesterol and other lipid species. The described size of these domains is diverse, from 10–200 nm diameter, and their characteristics are sometimes associated with the lipid microenvironment ruling the interaction between cholesterol- and sphingolipids and proteins enriched in these domains, gathering different proteins with different roles, in the same domain. Isolation and characterization of plasma membrane proteins by differential centrifugation and proteomic studies have revealed a remarkable diversity of proteins in these domains. The limited size of the lipid membrane nanodomain challenges the simple possibility that all of them can coexist within the same lipid membrane domain.
1. Lipid Membrane Nanodomains Organization in the Neuronal Plasma Membrane
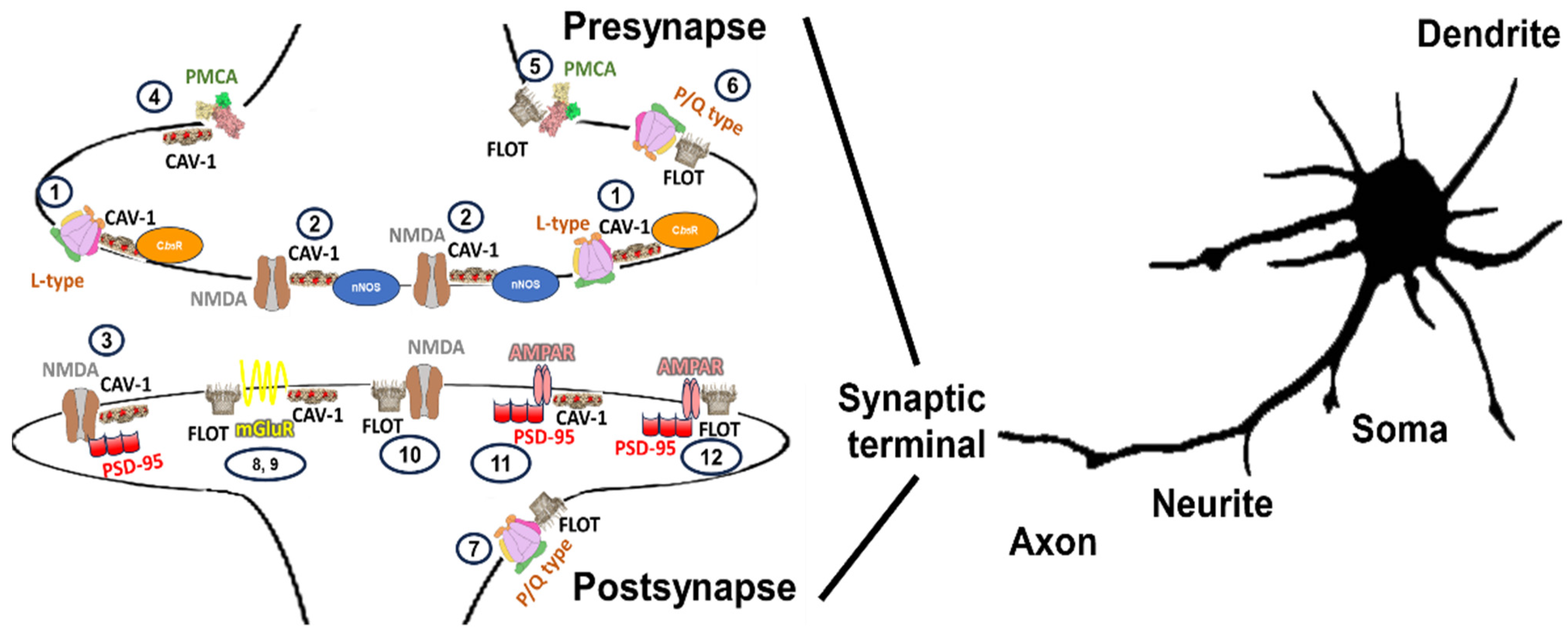
2. Properties of Caveolin-, Flotillin- or Ganglioside-Containing Lipid Membrane Domains
3. The Summary of the Distribution Map
|
Type |
Subunit |
Neuronal Type |
Associated with Raft Component |
Main Distribution in Brain and Subcellular Location |
Function |
|---|---|---|---|---|---|
|
L-type |
Cav1.2 |
Primary culture of cerebellar granule neurons and Purkinje cells [30][77] |
Neuronal calcium transients in cell bodies and dendrites, regulation of enzyme activity, regulation of transcription [78] |
||
|
P/Q-type |
Cav2.1 |
Cerebellar Purkinje neurons (tissue [70]; primary culture [79]; brain synaptosomal fraction [71]) |
Hippocampus [80], dorsal root ganglion neurons [81], presynaptic areas [71][81] |
Neurotransmitter release, dendritic calcium transients [78] |
|
|
L/P/Q/N-type |
α2δ-2, α2δ-3 [72] |
Hippocampal neurons (raft isolation and microscopy) [72] |
Flot-1 [72] |
GPI-enriched areas [72] |
|
|
NMDA |
NR1 |
Primary cultures of hippocampal neurons [73]; ganglion cells in rat retina (tissue) [82][83]; ventral part of lamina III and in laminae III and IV [84] |
Small uniform puncta throughout the neuron, pre and postsynapse [73][84]; ganglion cell dendrites [82], extrasynaptic plasma membrane [83] |
Signaling complexes in the postsynaptic density [85], glutamatergic signaling, synaptic plasticity, excitotoxicity, and memory [65], neurite outgrowth and axonal growth cone motility [86][87] |
|
|
NR2B |
Anterior cingulate cortex neurons in tissue and cultured (microscopy and immunoprecipitation) [88]; neurons from normal rat cerebral cortex (raft isolation, microscopy and immunoprecipitation) [89]; primary culture of cortical neurons (microscopy and raft isolation) [65]; ganglion cells in rat retina (tissue) [82][83] |
Soma and postsynapses [88][89]; ganglion cell dendrites extrasynapses peri-synapses [82][83] |
|||
|
NR2A [90] |
Cultured hippocampal neurons (microscopy and raft isolation) [90] |
Flot-1 and -2 [90] |
Small uniform puncta throughout the neuron [90] |
||
|
AMPAR |
GluA2 [91] |
Primary culture of hippocampal neurons (microscopy, immunoprecipitation and raft preparation) [91] |
Cav-1 [91], |
Cell body and as puncta localized to areas of cellular outgrowth [91] |
Postsynaptic currents mediated by the AMPA subtype of glutamate receptors in LTP [92]; long-term potentiation (LTP) induced GluA1 surface exposure [93] |
|
Primary culture of hippocampal neurons (microscopy and raft isolation) [94][95] |
Flot-1 and -2 [95], Cav-1 [96], GM1 [94] |
||||
|
GluR2/3 [96] |
Primary culture of hippocampal neurons (microscopy) [96], synaptosomes [97]; ganglion cells in rat retina (tissue) [82] |
Synapses and dendritic spines [96]; dendrites and somata [82] |
|||
|
GluR4 |
Ganglion cells in rat retina (tissue) [82] |
GM1 [82] |
Dendrites and somata [82] |
||
|
mGluR |
mGluR1/5 |
Primary hippocampal neurons (microscopy and immunoprecipitation) [74] |
Cav-1 [74] |
Soma and dendrites [74]; postsynaptic density late in development [98] |
Synapse formation and plasticity [75] |
|
mGluR1a |
Hippocampus, arcuate nucleus, hypothalamus [99] |
Cav-1 [99] |
Caveolin proteins act to functionally isolate distinct estrogen receptors and mGluRs, leading to activation of specific second messenger signaling cascades [99] |
||
|
mGluR1α |
Synaptosomes from pig cerebellum |
By application of MβCD, interaction of phosphorylated caveolin with the receptor decreased, and finally, internalization of the receptor was blocked [100] |
|||
|
Pumps |
PMCA isoform 4 |
Synaptosomes from pig cerebellum (Brij96 extracts) [102] |
ganglioside GM1 [102] |
Discrete functional positions on the synaptic nerve terminals [102] |
|
|
Purinergic receptors |
P2X3 |
Rat brain, cerebellar granule neurons in culture (microscopy, immunoprecipitation and raft preparation), dorsal root ganglion neurons in culture |
Flot-2, Cav-1 |
P2X3 subunit is expressed in cell bodies as well as in peripheral and central terminals of sensory neurons in dorsal root ganglia (DRG) [103][104] |
Well-defined role in pain perception [105][106]. Cav-1 is required for basal and ligand-induced membrane delivery of the P2X3 receptor [107] |
Note: The reason for no data regarding some of the calcium components and the main distribution in brain and subcellular location is the description of these calcium components in experiments performed in vitro in culture. Although some of these cultures were prepared from tissue, the researchers thought this should be differentiated from histochemical studies reporting calcium transported elements in rafts directly visualized on tissue slices or directly prepared or isolated from those tissues.
References
- Pike, L.J. Rafts Defined: A Report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598.
- Goñi, F.M. “Rafts”: A Nickname for Putative Transient Nanodomains. Chem. Phys. Lipids 2019, 218, 34–39.
- Eggeling, C.; Ringemann, C.; Medda, R.; Schwarzmann, G.; Sandhoff, K.; Polyakova, S.; Belov, V.N.; Hein, B.; von Middendorff, C.; Schönle, A.; et al. Direct Observation of the Nanoscale Dynamics of Membrane Lipids in a Living Cell. Nature 2009, 457, 1159–1162.
- Klotzsch, E.; Schütz, G.J. A Critical Survey of Methods to Detect Plasma Membrane Rafts. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120033.
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.-A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining Raft Domains in the Plasma Membrane. Traffic 2020, 21, 106–137.
- Hernández-Adame, P.L.; Meza, U.; Rodríguez-Menchaca, A.A.; Sánchez-Armass, S.; Ruiz-García, J.; Gomez, E. Determination of the Size of Lipid Rafts Studied through Single-Molecule FRET Simulations. Biophys. J. 2021, 120, 2287–2295.
- Pralle, A.; Keller, P.; Florin, E.L.; Simons, K.; Hörber, J.K. Sphingolipid-Cholesterol Rafts Diffuse as Small Entities in the Plasma Membrane of Mammalian Cells. J. Cell Biol. 2000, 148, 997–1008.
- Yethiraj, A.; Weisshaar, J.C. Why Are Lipid Rafts Not Observed In Vivo? Biophys. J. 2007, 93, 3113–3119.
- Sharma, P.; Varma, R.; Sarasij, R.C.; Ira; Gousset, K.; Krishnamoorthy, G.; Rao, M.; Mayor, S. Nanoscale Organization of Multiple GPI-Anchored Proteins in Living Cell Membranes. Cell 2004, 116, 577–589.
- Martosella, J.; Zolotarjova, N.; Liu, H.; Moyer, S.C.; Perkins, P.D.; Boyes, B.E. High Recovery HPLC Separation of Lipid Rafts for Membrane Proteome Analysis. J. Proteome Res. 2006, 5, 1301–1312.
- Yu, H.; Wakim, B.; Li, M.; Halligan, B.; Tint, G.S.; Patel, S.B. Quantifying Raft Proteins in Neonatal Mouse Brain by “tube-Gel” Protein Digestion Label-Free Shotgun Proteomics. Proteome Sci. 2007, 5, 17.
- Kalinowska, M.; Castillo, C.; Francesconi, A. Quantitative Profiling of Brain Lipid Raft Proteome in a Mouse Model of Fragile X Syndrome. PLoS ONE 2015, 10, e0121464.
- Ledesma, M.D.; Da Silva, J.S.; Schevchenko, A.; Wilm, M.; Dotti, C.G. Proteomic Characterisation of Neuronal Sphingolipid-Cholesterol Microdomains: Role in Plasminogen Activation. Brain Res. 2003, 987, 107–116.
- Galimzyanov, T.R.; Lyushnyak, A.S.; Aleksandrova, V.V.; Shilova, L.A.; Mikhalyov, I.I.; Molotkovskaya, I.M.; Akimov, S.A.; Batishchev, O.V. Line Activity of Ganglioside GM1 Regulates the Raft Size Distribution in a Cholesterol-Dependent Manner. Langmuir 2017, 33, 3517–3524.
- Porta, J.C.; Han, B.; Gulsevin, A.; Chung, J.M.; Peskova, Y.; Connolly, S.; Mchaourab, H.S.; Meiler, J.; Karakas, E.; Kenworthy, A.K.; et al. Molecular Architecture of the Human Caveolin-1 Complex. Sci. Adv. 2022, 8, eabn7232.
- Yokoyama, H.; Matsui, I. Higher-Order Structure Formation Using Refined Monomer Structures of Lipid Raft Markers, Stomatin, Prohibitin, Flotillin, and HflK/C-Related Proteins. FEBS Open Bio 2023, 13, 926–937.
- Ayuyan, A.G.; Cohen, F.S. Raft Composition at Physiological Temperature and pH in the Absence of Detergents. Biophys. J. 2008, 94, 2654–2666.
- Lamaze, C.; Tardif, N.; Dewulf, M.; Vassilopoulos, S.; Blouin, C.M. The Caveolae Dress Code: Structure and Signaling. Curr. Opin. Cell Biol. 2017, 47, 117–125.
- Stoeber, M.; Schellenberger, P.; Siebert, C.A.; Leyrat, C.; Helenius, A.; Grünewald, K. Model for the Architecture of Caveolae Based on a Flexible, Net-like Assembly of Cavin1 and Caveolin Discs. Proc. Natl. Acad. Sci. USA 2016, 113, E8069–E8078.
- Matthaeus, C.; Sochacki, K.A.; Dickey, A.M.; Puchkov, D.; Haucke, V.; Lehmann, M.; Taraska, J.W. The Molecular Organization of Differentially Curved Caveolae Indicates Bendable Structural Units at the Plasma Membrane. Nat. Commun. 2022, 13, 7234.
- Lee, J.; Glover, K.J. The Transmembrane Domain of Caveolin-1 Exhibits a Helix-Break-Helix Structure. Biochim. Biophys. Acta 2012, 1818, 1158–1164.
- Parton, R.G.; Tillu, V.; McMahon, K.-A.; Collins, B.M. Key Phases in the Formation of Caveolae. Curr. Opin. Cell Biol. 2021, 71, 7–14.
- Jarsch, I.K.; Daste, F.; Gallop, J.L. Membrane Curvature in Cell Biology: An Integration of Molecular Mechanisms. J. Cell Biol. 2016, 214, 375–387.
- Has, C.; Das, S.L. Recent Developments in Membrane Curvature Sensing and Induction by Proteins. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129971.
- Gambin, Y.; Ariotti, N.; McMahon, K.-A.; Bastiani, M.; Sierecki, E.; Kovtun, O.; Polinkovsky, M.E.; Magenau, A.; Jung, W.; Okano, S.; et al. Single-Molecule Analysis Reveals Self Assembly and Nanoscale Segregation of Two Distinct Cavin Subcomplexes on Caveolae. Elife 2013, 3, e01434.
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells Respond to Mechanical Stress by Rapid Disassembly of Caveolae. Cell 2011, 144, 402–413.
- Parton, R.G.; McMahon, K.-A.; Wu, Y. Caveolae: Formation, Dynamics, and Function. Curr. Opin. Cell Biol. 2020, 65, 8–16.
- Samhan-Arias, A.K.; García-Bereguiaín, M.A.; Martín-Romero, F.J.; Gutiérrez-Merino, C. Regionalization of Plasma Membrane-Bound Flavoproteins of Cerebellar Granule Neurons in Culture by Fluorescence Energy Transfer Imaging. J. Fluoresc. 2006, 16, 393–401.
- Samhan-Arias, A.K.; Garcia-Bereguiain, M.A.; Martin-Romero, F.J.; Gutierrez-Merino, C. Clustering of Plasma Membrane-Bound Cytochrome B5 Reductase within “lipid Raft” Microdomains of the Neuronal Plasma Membrane. Mol. Cell Neurosci. 2009, 40, 14–26.
- Marques-da-Silva, D.; Samhan-Arias, A.K.; Tiago, T.; Gutierrez-Merino, C. L-Type Calcium Channels and Cytochrome B5 Reductase Are Components of Protein Complexes Tightly Associated with Lipid Rafts Microdomains of the Neuronal Plasma Membrane. J. Proteom. 2010, 73, 1502–1510.
- Samhan-Arias, A.K.; Marques-da-Silva, D.; Yanamala, N.; Gutierrez-Merino, C. Stimulation and Clustering of Cytochrome B5 Reductase in Caveolin-Rich Lipid Microdomains Is an Early Event in Oxidative Stress-Mediated Apoptosis of Cerebellar Granule Neurons. J. Proteom. 2012, 75, 2934–2949.
- Marques-da-Silva, D.; Gutierrez-Merino, C. L-Type Voltage-Operated Calcium Channels, N-Methyl-d-Aspartate Receptors and Neuronal Nitric-Oxide Synthase Form a Calcium/Redox Nano-Transducer within Lipid Rafts. Biochem. Biophys. Res. Commun. 2012, 420, 257–262.
- Marques-da-Silva, D.; Gutierrez-Merino, C. Caveolin-Rich Lipid Rafts of the Plasma Membrane of Mature Cerebellar Granule Neurons Are Microcompartments for Calcium/Reactive Oxygen and Nitrogen Species Cross-Talk Signaling. Cell Calcium 2014, 56, 108–123.
- Samhan-Arias, A.K.; López-Sánchez, C.; Marques-da-Silva, D.; Lagoa, R.; Garcia-Lopez, V.; García-Martínez, V.; Gutierrez-Merino, C. High Expression of Cytochrome B5 Reductase Isoform 3/Cytochrome B5 System in the Cerebellum and Pyramidal Neurons of Adult Rat Brain. Brain Struct. Funct. 2016, 221, 2147–2162.
- Poejo, J.; Salazar, J.; Mata, A.M.; Gutierrez-Merino, C. Binding of Amyloid β(1-42)-Calmodulin Complexes to Plasma Membrane Lipid Rafts in Cerebellar Granule Neurons Alters Resting Cytosolic Calcium Homeostasis. Int. J. Mol. Sci. 2021, 22, 1984.
- Poejo, J.; Orantos-Aguilera, Y.; Martin-Romero, F.J.; Mata, A.M.; Gutierrez-Merino, C. Internalized Amyloid-β (1–42) Peptide Inhibits the Store-Operated Calcium Entry in HT-22 Cells. Int. J. Mol. Sci. 2022, 23, 12678.
- Bonini, I.C.; Antollini, S.S.; Gutiérrez-Merino, C.; Barrantes, F.J. Sphingomyelin Composition and Physical Asymmetries in Native Acetylcholine Receptor-Rich Membranes. Eur. Biophys. J. 2002, 31, 417–427.
- Antollini, S.S.; Soto, M.A.; Bonini de Romanelli, I.; Gutiérrez-Merino, C.; Sotomayor, P.; Barrantes, F.J. Physical State of Bulk and Protein-Associated Lipid in Nicotinic Acetylcholine Receptor-Rich Membrane Studied by Laurdan Generalized Polarization and Fluorescence Energy Transfer. Biophys. J. 1996, 70, 1275–1284.
- Gutiérrez-Merino, C.; Bonini de Romanelli, I.C.; Pietrasanta, L.I.; Barrantes, F.J. Preferential Distribution of the Fluorescent Phospholipid Probes NBD-Phosphatidylcholine and Rhodamine-Phosphatidylethanolamine in the Exofacial Leaflet of Acetylcholine Receptor-Rich Membranes from Torpedo Marmorata. Biochemistry 1995, 34, 4846–4855.
- Parekh, A.B. Ca2+ Microdomains near Plasma Membrane Ca2+ Channels: Impact on Cell Function. J. Physiol. 2008, 586, 3043–3054.
- Mironov, S.L. Rethinking Calcium Profiles around Single Channels: The Exponential and Periodic Calcium Nanodomains. Sci. Rep. 2019, 9, 17196.
- Wu, Y.-L.; Tschanz, A.; Krupnik, L.; Ries, J. Quantitative Data Analysis in Single-Molecule Localization Microscopy. Trends Cell Biol. 2020, 30, 837–851.
- Wang, L.-Y.; Augustine, G.J. Presynaptic Nanodomains: A Tale of Two Synapses. Front. Cell. Neurosci. 2015, 8, 455.
- Chen, Y.; Matveev, V. Stationary Ca2+ Nanodomains in the Presence of Buffers with Two Binding Sites. Biophys. J. 2021, 120, 1942–1956.
- Kwon, D. The Quest to Map the Mouse Brain. Nature 2023, 620, 685–687.
- Pol, A.; Morales-Paytuví, F.; Bosch, M.; Parton, R.G. Non-Caveolar Caveolins—Duties Outside the Caves. J. Cell Sci. 2020, 133, jcs241562.
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Lang, D.M.; Lommel, S.; Jung, M.; Ankerhold, R.; Petrausch, B.; Laessing, U.; Wiechers, M.F.; Plattner, H.; Stuermer, C.A. Identification of Reggie-1 and Reggie-2 as Plasmamembrane-Associated Proteins Which Cocluster with Activated GPI-Anchored Cell Adhesion Molecules in Non-Caveolar Micropatches in Neurons. J. Neurobiol. 1998, 37, 502–523.
- Lipid Raft Microdomains and Neurotransmitter Signalling|Nature Reviews Neuroscience. Available online: https://www.nature.com/articles/nrn2059 (accessed on 26 August 2023).
- Ge, L.; Qi, W.; Wang, L.-J.; Miao, H.-H.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. Flotillins Play an Essential Role in Niemann-Pick C1-like 1-Mediated Cholesterol Uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 551–556.
- Roitbak, T.; Surviladze, Z.; Tikkanen, R.; Wandinger-Ness, A. A Polycystin Multiprotein Complex Constitutes a Cholesterol-Containing Signalling Microdomain in Human Kidney Epithelia. Biochem. J. 2005, 392, 29–38.
- Volonte, D.; Galbiati, F.; Li, S.; Nishiyama, K.; Okamoto, T.; Lisanti, M.P. Flotillins/Cavatellins Are Differentially Expressed in Cells and Tissues and Form a Hetero-Oligomeric Complex with Caveolins in Vivo. Characterization and Epitope-Mapping of a Novel Flotillin-1 Monoclonal Antibody Probe. J. Biol. Chem. 1999, 274, 12702–12709.
- Yang, G.; Xu, H.; Li, Z.; Li, F. Interactions of Caveolin-1 Scaffolding and Intramembrane Regions Containing a CRAC Motif with Cholesterol in Lipid Bilayers. Biochim. Biophys. Acta 2014, 1838, 2588–2599.
- Hanafusa, K.; Hayashi, N. The Flot2 Component of the Lipid Raft Changes Localization during Neural Differentiation of P19C6 Cells. BMC Mol. Cell Biol. 2019, 20, 38.
- Wåhlén, E.; Olsson, F.; Söderberg, O.; Lennartsson, J.; Heldin, J. Differential Impact of Lipid Raft Depletion on Platelet-Derived Growth Factor (PDGF)-Induced ERK1/2 MAP-Kinase, SRC and AKT Signaling. Cell. Signal. 2022, 96, 110356.
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The Ins and Outs of Lipid Rafts: Functions in Intracellular Cholesterol Homeostasis, Microparticles, and Cell Membranes. J. Lipid Res. 2020, 61, 676–686.
- Davidović, D.; Kukulka, M.; Sarmento, M.J.; Mikhalyov, I.; Gretskaya, N.; Chmelová, B.; Ricardo, J.C.; Hof, M.; Cwiklik, L.; Šachl, R. Which Moiety Drives Gangliosides to Form Nanodomains? J. Phys. Chem. Lett. 2023, 14, 5791–5797.
- Matsubara, T.; IIjima, K.; Kojima, T.; Hirai, M.; Miyamoto, E.; Sato, T. Heterogeneous Ganglioside-Enriched Nanoclusters with Different Densities in Membrane Rafts Detected by a Peptidyl Molecular Probe. Langmuir 2021, 37, 646–654.
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965.
- Berg, L.K.; Larsson, M.; Morland, C.; Gundersen, V. Pre- and Postsynaptic Localization of NMDA Receptor Subunits at Hippocampal Mossy Fibre Synapses. Neuroscience 2013, 230, 139–150.
- Carter, B.C.; Jahr, C.E. Postsynaptic, Not Presynaptic NMDA Receptors Are Required for Spike-Timing-Dependent LTD Induction. Nat. Neurosci. 2016, 19, 1218–1224.
- Nosov, G.; Kahms, M.; Klingauf, J. The Decade of Super-Resolution Microscopy of the Presynapse. Front. Synaptic Neurosci. 2020, 12, 32.
- Dawson, T.M.; Snyder, S.H. Gases as Biological Messengers: Nitric Oxide and Carbon Monoxide in the Brain. J. Neurosci. 1994, 14, 5147–5159.
- Sato, Y.; Sagami, I.; Shimizu, T. Identification of Caveolin-1-Interacting Sites in Neuronal Nitric-Oxide Synthase: Molecular Mechanism for Inhibition of No Formation. J. Biol. Chem. 2004, 279, 8827–8836.
- Head, B.P.; Patel, H.H.; Tsutsumi, Y.M.; Hu, Y.; Mejia, T.; Mora, R.C.; Insel, P.A.; Roth, D.M.; Drummond, J.C.; Patel, P.M. Caveolin-1 Expression Is Essential for N-Methyl-D-Aspartate Receptor-Mediated Src and Extracellular Signal-Regulated Kinase 1/2 Activation and Protection of Primary Neurons from Ischemic Cell Death. FASEB J. 2008, 22, 828–840.
- Volonté, D.; Galbiati, F.; Pestell, R.G.; Lisanti, M.P. Cellular Stress Induces the Tyrosine Phosphorylation of Caveolin-1 (Tyr(14)) via Activation of P38 Mitogen-Activated Protein Kinase and c-Src Kinase. Evidence for Caveolae, the Actin Cytoskeleton, and Focal Adhesions as Mechanical Sensors of Osmotic Stress. J. Biol. Chem. 2001, 276, 8094–8103.
- Jiang, L.; Bechtel, M.D.; Bean, J.L.; Winefield, R.; Williams, T.D.; Zaidi, A.; Michaelis, E.K.; Michaelis, M.L. Effects of Gangliosides on the Activity of the Plasma Membrane Ca2+-ATPase. Biochim. Biophys. Acta 2014, 1838, 1255–1265.
- Zhao, Y.; Fan, X.; Yang, F.; Zhang, X. Gangliosides Modulate the Activity of the Plasma Membrane Ca2+-ATPase from Porcine Brain Synaptosomes. Arch. Biochem. Biophys. 2004, 427, 204–212.
- Jiang, L.; Fernandes, D.; Mehta, N.; Bean, J.L.; Michaelis, M.L.; Zaidi, A. Partitioning of the Plasma Membrane Ca2+-ATPase into Lipid Rafts in Primary Neurons: Effects of Cholesterol Depletion. J. Neurochem. 2007, 102, 378–388.
- Davies, A.; Douglas, L.; Hendrich, J.; Wratten, J.; Tran Van Minh, A.; Foucault, I.; Koch, D.; Pratt, W.S.; Saibil, H.R.; Dolphin, A.C. The Calcium Channel A2δ-2 Subunit Partitions with CaV2.1 into Lipid Rafts in Cerebellum: Implications for Localization and Function. J. Neurosci. 2006, 26, 8748–8757.
- Taverna, E.; Saba, E.; Rowe, J.; Francolini, M.; Clementi, F.; Rosa, P. Role of Lipid Microdomains in P/Q-Type Calcium Channel (Cav2.1) Clustering and Function in Presynaptic Membranes. J. Biol. Chem. 2004, 279, 5127–5134.
- Davies, A.; Kadurin, I.; Alvarez-Laviada, A.; Douglas, L.; Nieto-Rostro, M.; Bauer, C.S.; Pratt, W.S.; Dolphin, A.C. The A2δ Subunits of Voltage-Gated Calcium Channels Form GPI-Anchored Proteins, a Posttranslational Modification Essential for Function. Proc. Natl. Acad. Sci. USA 2010, 107, 1654–1659.
- Swanwick, C.C.; Shapiro, M.E.; Vicini, S.; Wenthold, R.J. Flotillin-1 Promotes Formation of Glutamatergic Synapses in Hippocampal Neurons. Dev. Neurobiol. 2010, 70, 875–883.
- Roh, S.-E.; Hong, Y.H.; Jang, D.C.; Kim, J.; Kim, S.J. Lipid Rafts Serve as Signaling Platforms for mGlu1 Receptor-Mediated Calcium Signaling in Association with Caveolin. Mol. Brain 2014, 7, 9.
- Francesconi, A.; Kumari, R.; Zukin, R.S. Regulation of Group I Metabotropic Glutamate Receptor Trafficking and Signaling by the Caveolar/Lipid Raft Pathway. J. Neurosci. 2009, 29, 3590–3602.
- Bodin, S.; Planchon, D.; Rios Morris, E.; Comunale, F.; Gauthier-Rouvière, C. Flotillins in Intercellular Adhesion-from Cellular Physiology to Human Diseases. J. Cell Sci. 2014, 127, 5139–5147.
- Wu, G.; Lu, Z.-H.; Nakamura, K.; Spray, D.C.; Ledeen, R.W. Trophic Effect of Cholera Toxin B Subunit in Cultured Cerebellar Granule Neurons: Modulation of Intracellular Calcium by GM1 Ganglioside. J. Neurosci. Res. 1996, 44, 243–254.
- Atlas, D. Voltage-Gated Calcium Channels Function as Ca2+-Activated Signaling Receptors. Trends Biochem. Sci. 2014, 39, 45–52.
- Nakatani, Y.; Hotta, S.; Utsunomiya, I.; Tanaka, K.; Hoshi, K.; Ariga, T.; Yu, R.K.; Miyatake, T.; Taguchi, K. Cav2.1 Voltage-Dependent Ca2+ Channel Current Is Inhibited by Serum from Select Patients with Guillain-Barré Syndrome. Neurochem. Res. 2009, 34, 149–157.
- Taylor, C.P.; Garrido, R. Immunostaining of Rat Brain, Spinal Cord, Sensory Neurons and Skeletal Muscle for Calcium Channel Alpha2-Delta (Alpha2-Delta) Type 1 Protein. Neuroscience 2008, 155, 510–521.
- Bauer, C.S.; Nieto-Rostro, M.; Rahman, W.; Tran-Van-Minh, A.; Ferron, L.; Douglas, L.; Kadurin, I.; Sri Ranjan, Y.; Fernandez-Alacid, L.; Millar, N.S.; et al. The Increased Trafficking of the Calcium Channel Subunit Alpha2delta-1 to Presynaptic Terminals in Neuropathic Pain Is Inhibited by the Alpha2delta Ligand Pregabalin. J. Neurosci. 2009, 29, 4076–4088.
- Zhang, J.; Diamond, J.S. Distinct Perisynaptic and Synaptic Localization of NMDA and AMPA Receptors on Ganglion Cells in Rat Retina. J. Comp. Neurol. 2006, 498, 810–820.
- Zhang, J.; Diamond, J.S. Subunit- and Pathway-Specific Localization of NMDA Receptors and Scaffolding Proteins at Ganglion Cell Synapses in Rat Retina. J. Neurosci. 2009, 29, 4274–4286.
- Lu, C.-R.; Hwang, S.J.; Phend, K.D.; Rustioni, A.; Valtschanoff, J.G. Primary Afferent Terminals That Express Presynaptic NR1 in Rats Are Mainly from Myelinated, Mechanosensitive Fibers. J. Comp. Neurol. 2003, 460, 191–202.
- Grant, S.G.N. Synapse Signalling Complexes and Networks: Machines Underlying Cognition. Bioessays 2003, 25, 1229–1235.
- Henley, J.; Poo, M. Guiding Neuronal Growth Cones Using Ca2+ Signals. Trends Cell Biol. 2004, 14, 320–330.
- Zheng, J.Q.; Poo, M.-M. Calcium Signaling in Neuronal Motility. Annu. Rev. Cell Dev. Biol. 2007, 23, 375–404.
- Yang, J.-X.; Hua, L.; Li, Y.-Q.; Jiang, Y.-Y.; Han, D.; Liu, H.; Tang, Q.-Q.; Yang, X.-N.; Yin, C.; Hao, L.-Y.; et al. Caveolin-1 in the Anterior Cingulate Cortex Modulates Chronic Neuropathic Pain via Regulation of NMDA Receptor 2B Subunit. J. Neurosci. 2015, 35, 36–52.
- Bigford, G.E.; Alonso, O.F.; Dietrich, W.D.; Keane, R.W. A Novel Protein Complex in Membrane Rafts Linking the NR2B Glutamate Receptor and Autophagy Is Disrupted Following Traumatic Brain Injury. J. Neurotrauma 2009, 26, 703–720.
- Swanwick, C.C.; Shapiro, M.E.; Yi, Z.; Chang, K.; Wenthold, R.J. NMDA Receptors Interact with Flotillin-1 and -2, Lipid Raft-Associated Proteins. FEBS Lett. 2009, 583, 1226–1230.
- Gaudreault, S.B.; Chabot, C.; Gratton, J.-P.; Poirier, J. The Caveolin Scaffolding Domain Modifies 2-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionate Receptor Binding Properties by Inhibiting Phospholipase A2 Activity. J. Biol. Chem. 2004, 279, 356–362.
- Davies, S.N.; Lester, R.A.; Reymann, K.G.; Collingridge, G.L. Temporally Distinct Pre- and Post-Synaptic Mechanisms Maintain Long-Term Potentiation. Nature 1989, 338, 500–503.
- Brown, T.C.; Correia, S.S.; Petrok, C.N.; Esteban, J.A. Functional Compartmentalization of Endosomal Trafficking for the Synaptic Delivery of AMPA Receptors during Long-Term Potentiation. J. Neurosci. 2007, 27, 13311–13315.
- Hou, Q.; Huang, Y.; Amato, S.; Snyder, S.H.; Huganir, R.L.; Man, H.-Y. Regulation of AMPA Receptor Localization in Lipid Rafts. Mol. Cell Neurosci. 2008, 38, 213–223.
- Bodrikov, V.; Pauschert, A.; Kochlamazashvili, G.; Stuermer, C.A.O. Reggie-1 and Reggie-2 (Flotillins) Participate in Rab11a-Dependent Cargo Trafficking, Spine Synapse Formation and LTP-Related AMPA Receptor (GluA1) Surface Exposure in Mouse Hippocampal Neurons. Exp. Neurol. 2017, 289, 31–45.
- Hering, H.; Lin, C.-C.; Sheng, M. Lipid Rafts in the Maintenance of Synapses, Dendritic Spines, and Surface AMPA Receptor Stability. J. Neurosci. 2003, 23, 3262–3271.
- Cole, A.A.; Dosemeci, A.; Reese, T.S. Co-Segregation of AMPA Receptors with GM1 Ganglioside in Synaptosomal Membrane Sub-Fractions. Biochem. J. 2010, 427, 535–540.
- Petralia, R.S.; Wang, Y.-X.; Wenthold, R.J. Internalization at Glutamatergic Synapses during Development. Eur. J. Neurosci. 2003, 18, 3207–3217.
- Grove-Strawser, D.; Boulware, M.I.; Mermelstein, P.G. Membrane Estrogen Receptors Activate the Metabotropic Glutamate Receptors mGluR5 and mGluR3 to Bidirectionally Regulate CREB Phosphorylation in Female Rat Striatal Neurons. Neuroscience 2010, 170, 1045–1055.
- Hong, Y.H.; Kim, J.Y.; Lee, J.H.; Chae, H.G.; Jang, S.S.; Jeon, J.H.; Kim, C.H.; Kim, J.; Kim, S.J. Agonist-Induced Internalization of mGluR1α Is Mediated by Caveolin. J. Neurochem. 2009, 111, 61–71.
- Díaz, M.; de Pablo, D.P.; Valdés-Baizabal, C.; Santos, G.; Marin, R. Molecular and Biophysical Features of Hippocampal “Lipid Rafts Aging” Are Modified by Dietary N-3 Long-chain Polyunsaturated Fatty Acids. Aging Cell 2023, 22, e13867.
- Sepúlveda, M.R.; Berrocal-Carrillo, M.; Gasset, M.; Mata, A.M. The Plasma Membrane Ca2+-ATPase Isoform 4 Is Localized in Lipid Rafts of Cerebellum Synaptic Plasma Membranes. J. Biol. Chem. 2006, 281, 447–453.
- Vulchanova, L.; Riedl, M.S.; Shuster, S.J.; Buell, G.; Surprenant, A.; North, R.A.; Elde, R. Immunohistochemical Study of the P2X2 and P2X3 Receptor Subunits in Rat and Monkey Sensory Neurons and Their Central Terminals. Neuropharmacology 1997, 36, 1229–1242.
- Llewellyn-Smith, I.J.; Burnstock, G. Ultrastructural Localization of P2X3 Receptors in Rat Sensory Neurons. Neuroreport 1998, 9, 2545–2550.
- Cook, S.P.; Vulchanova, L.; Hargreaves, K.M.; Elde, R.; McCleskey, E.W. Distinct ATP Receptors on Pain-Sensing and Stretch-Sensing Neurons. Nature 1997, 387, 505–508.
- Souslova, V.; Cesare, P.; Ding, Y.; Akopian, A.N.; Stanfa, L.; Suzuki, R.; Carpenter, K.; Dickenson, A.; Boyce, S.; Hill, R.; et al. Warm-Coding Deficits and Aberrant Inflammatory Pain in Mice Lacking P2X3 Receptors. Nature 2000, 407, 1015–1017.
- Chen, X.-Q.; Zhu, J.-X.; Wang, Y.; Zhang, X.; Bao, L. CaMKIIα and Caveolin-1 Cooperate to Drive ATP-Induced Membrane Delivery of the P2X3 Receptor. J. Mol. Cell Biol. 2014, 6, 140–153.




