Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiba Khan | -- | 6419 | 2024-01-12 19:18:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 6419 | 2024-01-15 02:17:18 | | | | |
| 3 | Lindsay Dong | -5 word(s) | 6414 | 2024-01-22 02:49:43 | | | | |
| 4 | Lindsay Dong | Meta information modification | 6414 | 2024-03-01 09:26:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, H.; Singh, N.; Leyva, L.Y.; Malawana, J.; Shah, N.M. Precision-Based Medicine for the management of Preterm Birth. Encyclopedia. Available online: https://encyclopedia.pub/entry/53787 (accessed on 07 February 2026).
Khan H, Singh N, Leyva LY, Malawana J, Shah NM. Precision-Based Medicine for the management of Preterm Birth. Encyclopedia. Available at: https://encyclopedia.pub/entry/53787. Accessed February 07, 2026.
Khan, Hiba, Natasha Singh, Luis Y. Leyva, Johann Malawana, Nishel M. Shah. "Precision-Based Medicine for the management of Preterm Birth" Encyclopedia, https://encyclopedia.pub/entry/53787 (accessed February 07, 2026).
Khan, H., Singh, N., Leyva, L.Y., Malawana, J., & Shah, N.M. (2024, January 12). Precision-Based Medicine for the management of Preterm Birth. In Encyclopedia. https://encyclopedia.pub/entry/53787
Khan, Hiba, et al. "Precision-Based Medicine for the management of Preterm Birth." Encyclopedia. Web. 12 January, 2024.
Copy Citation
Preterm birth (PTB) is a leading cause of childhood disability, and it has become a key public health priority recognized by the World Health Organization and the United Nations. Studies have summarised the use of precision based medicine in the management of spontaneous PTB. In particular, the advent of novel approaches for the use of biomarkers in prediction, diagnostics, and the emerging use of artificial intelligence and computational modelling in this important field of research.
pregnancy
preterm birth
preterm labor
personalized medicine
1. Introduction
Preterm birth (PTB), defined as neonates born before the 37th week of gestation, is a leading cause of death and disability in children under five years worldwide. Globally, there are 15 million PTBs per year [1]. As such, PTB is a key public health priority that is recognized and closely monitored by the World Health Organization (WHO). In the UK, 52,000 babies a year are born preterm, of which 8000 are born before 32 weeks [2]. These babies are disproportionately affected by significant long-term morbidity when compared to births at later gestations. For women identified as high-risk in early pregnancy, prevention strategies can be initiated to modify their risk. However, in more than 50% of women who deliver preterm, there are no identifiable risk factors to target [3]. Worse still, when these women present to hospital with early signs of preterm labor (PTL), there is no effective treatment to delay their progression to birth. Furthermore, over the last decade, there has been no measurable improvement in PTB rates despite changes in national and international guidance and improvements in the education of healthcare professionals [4]. Therefore, clinical care is aimed at preparing the neonate for PTB using interventions such as maternal steroids for fetal lung maturity and magnesium sulphate to reduce the risk of cerebral palsy [5]. In the United Kingdom (UK), the Government’s ‘Safer Maternity Care’ action plan set a target of reducing the PTB rate from 8% to 6% by 2025 and included subsequent tributary initiatives such as the ‘Saving Babies Lives Bundle 2′, PERIprem (Perinatal Excellence to Reduce Injury in Premature Birth) initiative and ‘Better Births’ vision to equip healthcare professionals with the tools they need to predict, suspect, diagnose and delay PTB as quickly as possible [6]. However, the PTB rate remains at 8% in the UK, and one of the key recommendations from the ‘Safer Maternity Care’ Progress report was the enabling of innovation in local clinical practice [7]. However, it has been recognized by stakeholders that PTL is a complex problem with several different etiologies, meaning that for any approach to the management of PTL to be effective, the first step must be to identify the cause in each individual woman [8]. Personalized medicine, often referred to as precision-based medicine, has been developing in many areas of medicine, most recently in the fields and research related to respiratory disease, hematology and cancer care. Contrary to the population approach, it offers an individualized targeted approach that could be ideal in the context of the early identification and delay of PTB [9].
2. Preterm Birth
PTB is divided into the following sub-categories: extremely preterm (less than 28 weeks), very preterm (28 to 32 weeks) and moderate to late preterm (32 to 37 weeks). The majority of PTBs are moderate to late, which account for 85%, and the remainder are split between extreme and very preterm, with rates of 4% and 11%, respectively [1]. The morbidity and mortality associated with PTB are considerable. Moreover, the earlier the delivery, the higher the risk of disability or death [10]. Neonatal complications of PTB include chronic lung disease, developmental delay, growth reduction, hearing impairment, intraventricular hemorrhage, necrotizing enterocolitis, nosocomial infections, patent ductus arteriosus, periventricular leukomalacia, respiratory distress syndrome, retinopathy of prematurity and pulmonary barotrauma [11].
2.1. Risk Factors and Pathophysiology of Preterm Birth
There are several risk factors for PTB that can be identified preconception and in early pregnancy (Table 1). These can be divided into modifiable (such as smoking) and non-modifiable risk factors (such as previous cervical surgery or PTB) to direct preventative interventions or methods of surveillance.
Table 1. Table to show the recognized risk factors for preterm birth, categorized as past medical history, lifestyle, pregnancy complications and other. (Royal College of Obstetricians and Gynecologists).
| Past Medical History | Pregnancy Complications |
| Previous preterm birth Short cervix < 25 mm Early cervical dilatation Past procedures on the cervix (LLETZ) Injury during a past delivery |
Carrying more than one fetus Vaginal bleeding during pregnancy Infections during pregnancy |
| Lifestyle | Other |
| Low pre-pregnancy weight Smoking during pregnancy Dietary deficiencies Injury during a past delivery |
Younger than 17 or older than 35 years |
Though the exact pathophysiology of PTB remains unclear, most researchers agree that it is likely to be multifactorial (Figure 1). However, the most prevalently recognized mechanism is an inflammatory process occurring within the amniotic cavity due to maternal infection [12]. This may occur in around 40% of cases of PTL before 34 weeks, as a result of a microbial invasion of the amniotic cavity (MIAC) that precedes labor [13]. Other potential causes of PTB include a disruption of the vaginal microbiome that is linked with infection- and inflammation-mediated PTB, modified glycan-binding protein circuits that cause a disruption in maternal immune tolerance of the semi-allogenic fetus and maternal psychological stress manifesting through a neuroendocrine response [14][15][16][17].
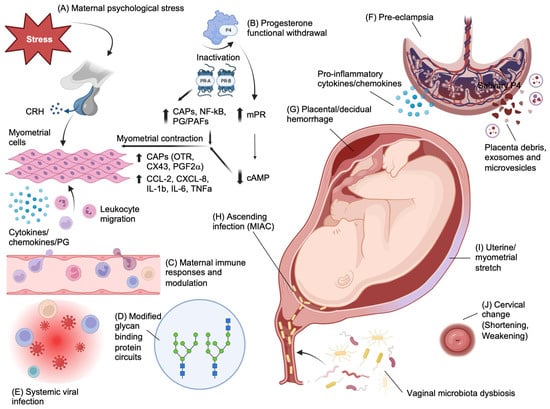
Figure 1. Pathophysiological mechanisms of preterm birth. (A) In contrast to the inhibitory effects of cortisol on hypothalamic CRH secretion, maternal stress causing cortisol release from the zona fasciculata of the adrenal cortex can increase CRH production by the placenta and may provide a fetal trigger for labor by upregulating cortisol and dehydroepiandrosterone sulphate (DHEAS) release by the fetal adrenals. DHEAS is metabolized in the placenta to estrogens, which results in upregulation of proinflammatory cytokines and chemokines that mediate myometrial cell contractility. (B) Functional progesterone withdrawal due to the differential expression of nuclear progesterone receptor (PR) isoforms PR-A and PR-B in myometrial smooth muscle cells, favoring a greater PR-A/PR-B ratio prior to labor onset that upregulates pro-contractile gene expression. The overall effect is an upregulation of contractile-associated proteins (CAPs), including OTR, COX-2, Cx43 and prostaglandin F2α (PGF2α), and upregulation of pro-inflammatory cytokines. In addition to this, membrane PRs (mPRs) mediate progesterone intracellular signaling through activation of G-proteins and subsequent upregulation of adenylyl cyclase activity that decreases cAMP levels, which causes an increase in myosin phosphorylation. All this is further enhanced by a background of functional progesterone withdrawal and a reduction in PR-B transcriptional activity. (C) Dysregulated maternal immune responses due to (D) modified glycan-binding protein circuits that are responsible for several immune-modulatory functions, such as those mediated by progesterone as well as antimicrobial activity, and dendritic cell and NK interactions with trophoblast during placentation; and to (E) chronic viral infections (such as HIV) causing pro-inflammatory immune responses. Together, this disruption in immune regulation causes inflammatory triggers that can induce myometrial contractility via NFκB. (F) Pre-eclampsia is a known risk factor for iatrogenic and spontaneous PTB through a number of mechanisms that include abnormal trophoblast invasion during placentation (e.g., via the PI3K-AKT pathway) and release of syncytiotrophoblast debris and placental exosomes and microvesicles that predispose to maternal endothelial dysfunction caused by circulating placental-derived sFlt and an altered local and systemic maternal immune response that favors TH1 cytokines. (G) Decidual hemorrhage presenting as vaginal bleeding or retroplacental hematoma results in formation of thrombin that can stimulate myometrial contractility but also inhibits progesterone and mediates infiltration of neutrophils to the area. (H) MIAC through ascending organisms, including those associated with vaginal dysbiosis, causes intra-amniotic infection that can induce inflammation of feto-maternal tissues and immune dysregulation and myometrial contractility. (I) Uterine distension due to polyhydramnios, multiple pregnancies and large-for-gestational-age fetuses result in a myometrial stretch response with upregulated IL-8, as well as COX-1/2 activity. (J) Cervical pathology includes congenital factors and conditions such as Ehlers Danlos, Marfan’s syndrome and cervical agenesis or dysgenesis that develop as a consequence of congenital collagenopathies. Acquired conditions include cervicitis caused by sexually transmitted infections or non-sexually acquired infections that ascend from the vagina to the cervix. The cervical epithelium can also be weakened by mechanical or chemical trauma and previous surgeries such as cone and excision biopsies to treat cervical intraepithelial neoplasia (CIN) and prevent cancer of the cervix. Created with Biorender.com—accessed on 1 November 2023.
A common neuroendocrine mechanism associated with spontaneous PTB is the elevation of placental corticotrophin-releasing hormone (CRH) levels due to a fall in CRH-binding protein (CRHBP), leading to presumed cervical ripening and a myometrial contraction response [18]. The theory of functional progesterone withdrawal reasons that the effect of continued high levels of progesterone throughout pregnancy and labor on myometrial tissue is attenuated due to a regulated metabolic progesterone withdrawal. This occurs through the differential expression of progesterone receptor isoforms, reduced activation and expression of progesterone co-activators, binding of progesterone to receptors that activate alternative cellular pathways, direct progesterone withdrawal mediated by the upregulation of cytokines and chemokines, and catabolism of progesterone from an active to an inactive compound in the uterus [19][20].
A common cause of iatrogenic PTB includes placenta previa, accreta, vasa previa and velamentous insertion of the cord [21]. These abnormalities of placental development are associated with significant maternal-fetal morbidity and mortality that, in the cases of previa, accreta and vasa previa, are not compatible with vaginal birth and so are delivered before 37 weeks of gestation.
Another iatrogenic cause is uteroplacental insufficiency, which is very often discussed in relation to pre-eclampsia (PET). The risk of PTB associated with PET is based on disease severity and often co-relates with the extent of uteroplacental insufficiency and consequent fetal growth restriction. The extent of placental insufficiency is believed to be due to reduced placental perfusion mediated through the vasoconstricting effects of the renin-angiotensin pathway, leading to sub-optimal trophoblast invasion [22]. It has been recognized that 30% of patients with PTL have been found to have placental lesions [23]. The mechanism behind vaginal bleeding or retroplacental hematoma formation is believed to be due to a defect in decidual hemostasis, leading to decidual hemorrhage or placental abruption. This triggers thrombin-stimulated myometrial contractility through protease-activated receptors PAR1 and PAR3 as well as degradation of the extracellular matrix, causing chorioamniotic membrane compromise [24].
Cervical insufficiency is also a recognized cause of PTL and is either congenital or acquired. Acquired causes of cervical insufficiency include cervical shortening through a procedure involving large loop excision of the transformation zone (LLETZ), cone biopsy, cervical lacerations from childbirth or weakening of the epithelium through laser ablation. The composition of the cervix comprises 60% type I fibrillar collagen, 30% type III fibrillar collagen and the proteoglycans hyaluronic acid, chondroitin sulphate, keratan sulphate and dermatan sulphate.
Chronic systemic infections such as human immunodeficiency virus (HIV) that cause altered maternal immune responses are also associated with PTB. Globally, it is estimated that there are over 19.3 million women of reproductive age with HIV [25]. Pregnant women with HIV have a three-fold increased risk of spontaneous PTB versus the background population risk [26][27]. The mechanism behind this is thought to be due to the dysregulation of CD4 and CD8 cell immune responses. This is in part mediated by a reduction in CD4 T cells and an altered phenotype of monocytes following viral infection. This results in an upregulation of a subset of suppressive Tregs (T-follicular regulatory cells), increased expression of the co-inhibitory receptor TIGIT on natural killer (NK) cells that attenuates their functional capacity and disturbances in the Th17/Treg ratio [28]. Even with treatment and subsequent immune reconstitution of CD4 T cells, the effects of chronic viral infection potentiate the proinflammatory environment, and some elements of immune dysregulation persist.
In addition to immune dysregulation, several studies have implicated protease inhibitors used to treat HIV infection as a cause of PTB [29]. In 1998, Lorenzi et al. investigated the hypothesis that pregnant women taking reverse transcriptase and protease inhibitors were at higher risk of PTB to evaluate the safety of this HIV treatment for this demographic group and discovered a high incidence of prematurity (spontaneous PTB) in these women [30]. Two years later, a larger prospective study of 3920 European women was published that also found an association between protease inhibitors and PTB (17% PTB rate) [31]. However, no clear mechanism for this has been identified, and further work refutes this assertion [32].
The interplay and complexity of these causes are indicative of the multifactorial etiology of PTB. The National Institute for Health and Care Excellence (NICE) based in the UK and the WHO have published guidelines to support clinical staff to better identify risk factors for PTB in early pregnancy and offer evidence-based interventions [33][34]. In addition to recommending methods to modify risk, as well as methods of surveillance of high-risk groups, they also provide guidance on the nature of obstetric and neonatal support that should be available to patients in the acute setting where PTB is inevitable.
2.2. Current Clinical Tools to Predict Preterm Birth
There are currently several aids available in clinical practice to predict PTB in high-risk groups. These include ultrasound, serum biomarkers, vaginal biomarkers and a clinical decision-making app (Figure 2). The Placental Growth Factor (PLGF) biomarker is also approved for use by the NHS and is available in a few units as a point-of-care test, primarily to be used to exclude pre-eclampsia. However, it is also a reliable predictor of disease progression and time to delivery [35]. As a result, it is used to predict the need for delivery within 14 days, with a sensitivity (the ability of the test to identify true positives) and specificity (the ability of the test to identify true negatives) of 80% for both and positive and negative predictive values (the proportion of individuals with a positive test result who will deliver preterm or with a negative test result who will not deliver preterm) of 0.41 and 0.95, respectively (using the Triage MeterPro point-of-care analyzer that uses a fluorescence immunoassay) [36].
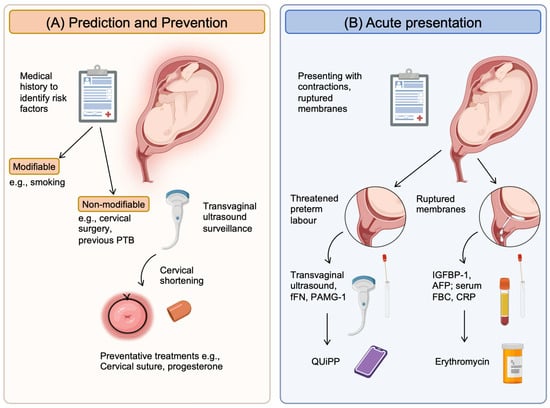
Figure 2. Current clinical tools available to help clinicians predict and manage preterm birth. (A) Prediction and prevention rely on identifying high-risk patients using their medical and obstetric history to determine risk factors. These can then be categorized into modifiable and non-modifiable groups. Those that are modifiable can be managed to reduce the individual’s risk, such as smoking with smoking cessation. For those risks that are non-modifiable, surveillance strategies such as serial transvaginal ultrasound can be initiated. When cervical shortening occurs, preventative treatments can be started (e.g., cervical suture, progesterone supplements). (B) Acute presentations will be in the form of pregnant women with contractions and/or ruptured membranes. Confirmation will be with clinical examination, but diagnostic tools that will help include: (1) for threatened PTL, ultrasound (intact membranes) to assess cervical shortening and a vaginal swab to measure fFN or PAMG-1, which is released in labor from the cervix; (2) for ruptured membranes, the presence of proteins from the amniotic fluid (AFP and IGFBP-1) released into the vagina and serum markers of infection (full blood counts/FBC and CRP). New tools that combine methods to improve accuracy of these clinical tools include the QUiPP app, which combines cervical length with quantitative fFN concentrations. Created with BioRender.com—accessed on 13 December 2023.
Specifically for spontaneous PTB, ultrasound and fetal fibronectin (FFN) are used in clinical practice. The International Society of Ultrasound in Obstetrics and Gynecology Standards Committee (ISUOG) has published good practice recommendations with regards to the standardized and accurate measurement of cervical length, including measurement cut-offs (25 mm at <24 weeks has a 30% likelihood of spontaneous preterm delivery), best practice, operator qualifications and screening. Supportive indicators such as amniotic sludge were also recently reported by the ISUOG as an ultrasound finding that demonstrated increased risk of PTB [37]. Though transvaginal ultrasound to measure the cervix is primarily used for surveillance in high-risk groups, it can be used in the acute setting to determine the risk of PTB within 2 weeks. In addition to ultrasound, a quantitative point-of-care test to measure cervicovaginal FFN provides an additional method to predict spontaneous PTB in symptomatic pregnant women. In a metanalysis of 64 studies with a total of 26,876 participating pregnant women, Honest et al. reported a likelihood ratio of >6 in four of the highest-quality studies for predicting PTB within 7–10 days [38].
3. Precision-Based Medicine
3.1. Biomarkers
There are a number of biomarkers (Figure 3) associated with the pathophysiology of PTB that have the potential to be used for clinical diagnostics (Figure 4).
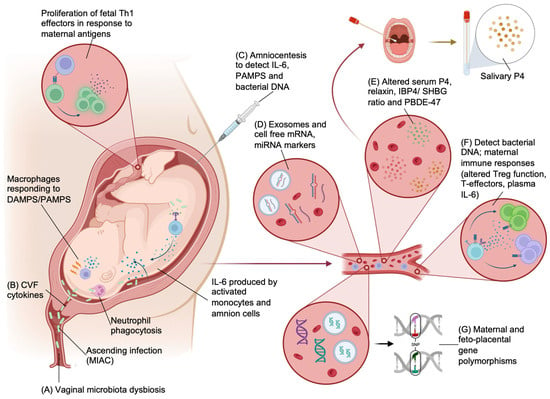
Figure 3. Biomarkers that can be used for the prediction, detection and guidance of the treatment of preterm birth. (A,B) Vaginal microbiome and cervicovaginal fluid (CVF) can be used to detect dysbiosis and cervical cytokines that predispose to PTB. (C) Amniocentesis can be used to sample amniotic fluid to detect both intra-amniotic bacteria and products of the maternal immune response (IL-6 and PAMPs/DAMPs). Ascending organisms (MIAC) into the amniotic cavity may induce an immune response including phagocytosis by neutrophils and PAMP and DAMP generation that will activate macrophages, causing pro-inflammatory cytokine release (IL-6). Activated intracavity monocytes and amnion cells will also produce IL-6. Infective PTB is also associated with greater fetal exposure to maternal antigens and proliferation of proinflammatory fetal T cells. (D) Several candidate cell-free RNA signatures (mRNA and miRNA) have been discovered in the plasma of pregnant women that are associated with PTB. (E) Measurable hormonal biomarkers that have been shown to have immunomodulatory functions and myometrial quiescence include progesterone (P4), relaxin, IBP4/SHBG ratio and PBDE-47. These can be detected in maternal plasma and in some instances, in maternal saliva. (F) Systemic infection and immune responses associated with infection and PTB can be detected from peripheral blood. Methods to detect infections include traditional NAATs that are rapid and highly sensitive but lack isolation of AMR and 16S rRNA. Whole-genome metagenomics give better coverage, and new methods such as multiplex PCR are rapid and suitable as a point-of-care test. Maternal immune response can be detected with fluorescence-based immune profiling to detect a shift in immune-suppressive phenotypes and plasma cytokines. (G) Maternal and fetal polymorphisms in genes associated with components of potential etiological pathways of PTB have been explored and are detectable in maternal blood. Created with BioRender.com—accessed 13 December 2023.
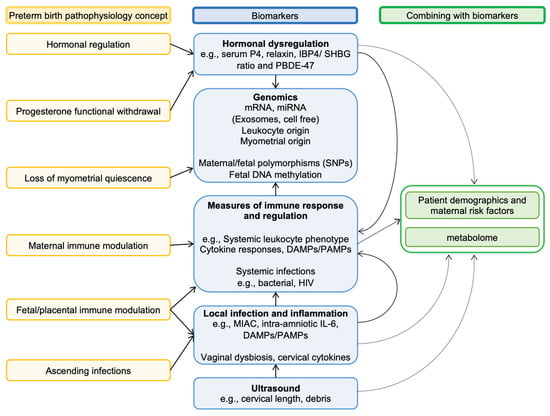
Figure 4. Linking key pathophysiological concepts of preterm birth with biomarkers. Biomarkers are linked to key concepts of the pathologies thought to be causes of preterm birth. Most pathologies are multifactorial and can often progress to affect other systems/compartments. An example is vaginal bacteria, which can invade the amniotic cavity (i.e., MIAC), leading to systemic infection and disrupting maternal immune modulation of the feto-placental unit. This is illustrated in the diagram by how various biomarkers are connected. Several researchers have further refined the utility of many biomarkers by combining these with maternal/fetal risk factors as well as associated metabolites (metabolome).
3.1.1. Bacterial Biomarkers
Targeting the vaginal microbiome
Infections related to the reproductive tract have been investigated as an etiology of PTB. However, a causal link between sexually transmitted infections (STI) and PTB is uncertain. Fetal and neonatal concerns regarding common STIs (such as chlamydia and gonorrhea) and less common infections (such as syphilis, HIV) are mostly aimed at vertical transmission. However, HIV infection is associated with an increased risk of PTB, and as discussed earlier, immune dysregulation associated with infection is likely to contribute to this finding [39]. A large population-based retrospective cohort study of 14,373,023 pregnant women concluded that the odds ratio (measure of association between exposure and outcome) for PTB relating to chlamydia was 1.11, relating to gonorrhea 1.17 and relating to syphilis 1.06 compared with pregnant women without infection [40]. This study formed the basis of targeted prevention prior to and screening during the antenatal period. In the UK, HIV and syphilis tests are offered as part of the antenatal booking appointment through the NHS [41][42]. However, other sexually transmissible diseases such as chlamydia and gonorrhea are not routinely screened. Nevertheless, screening is recommended by NICE for 15- to 24-year-old men and women who are sexually active [43]. Following this important work, there has been much speculation around the potential for targeted interventions around STI screening, resulting in personalized treatment, though critics have identified a lack of data linking the impact of treatment to the prevention of PTB [44]. One of the techniques in development is a novel multiplex real-time PCR melting curve assay method for the simultaneous detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and herpes simplex virus [45].
-
Targeting amniotic fluid microbial colonization
Though maternal systemic infections are not a major cause of PTB in the post-antibiotic era [46], almost 60% of cases of spontaneous PTL are thought to be due to a microbial invasion of the amniotic cavity (MIAC)—with a likely source being the vagina. Invading organisms will often include bacteria that are known to be associated with PTB in the literature, such as Mycoplasma and Ureaplasma species [47][48]. The seminal work by the ORACLE collaborative in the 2000s investigated the use of erythromycin or co-amoxiclav for PTL. The antibiotics were given to pregnant women presenting with PTL with intact membranes and/or preterm ruptured membranes before 37 weeks. They showed a reduction in maternal infection and short-term improvement in neonatal morbidity, but not in perinatal mortality [49][50][51]. Importantly, in the intact membrane group, antibiotics did not improve neonatal outcomes or delay birth, and the use of co-amoxiclav was associated with necrotizing enterocolitis in the neonate. The mixed effectiveness in this study may be due to the non-specific nature of the antibiotics used. Consequently, clinical practice is to give erythromycin for preterm ruptured membranes only.
-
Targeting maternal infection
A less-invasive method than amniocentesis to detect maternal-fetal infections, and perhaps even intra-amniotic organisms would be ideal. However, this would require detection of organisms without sampling the compartment with the highest concentration of organisms to detect. Currently, PCR-based technology that uses multiplex is already available in clinical practice, that provides rapid detection of organisms and antimicrobial genes from a predefined panel tailored to the sample source [52][53]. For more comprehensive characterization, novel methods for next-generation sequencing have been developed that can be used to detect organisms, their resistance genes and enables profiling of host immune responses [54]. Techniques such as real-time nanopore sequencing as a method of rapid identification of bacteria, antibiotic resistance genes and plasmids in blood cultures have been shown to enable correct use of antibiotics within 4 h of a positive blood culture [55]. Other methods include cell-free metagenomic sequencing, which provides a way to identify organisms and their resistance genes without the issue of host DNA contamination but also enables the profiling of host tissue injury as a measure of host response [56].
-
Targeting indicators of maternal immune response
The phenotype of circulating T cells has promise as a measurable biomarker or series of biomarkers for the prediction of PTB. The mechanism underpinning this work is twofold, representing the concept of the loss of immune tolerance to the semi-allogenic fetus by the host immune system prior to labor, as well as the maternal immune response to intra-amniotic infection driving PTL. For example, Frascoli et al. found fetal T helper 1 (Th1) cells from preterm infants exhibiting a proinflammatory response to maternal antigens within the maternal circulation, which were not found in term infants [57].
Interleukin-6 (IL-6) has been linked to risk of PTB [58][59][60]. Automated electrochemiluminescence immunoassay methods have been developed to detect and confirm the presence of IL-6 in intra-amniotic and cervical fluid samples of pregnant women who are delivering prematurely [61]. Park et al. went on to investigate plasma IL-6 through a retrospective cohort study but found that although less sensitive and specific than amniotic fluid IL-6, plasma IL-6 levels are equivalent in sensitivity to C-reactive protein (CRP) for detecting intra-amniotic infection predictive of imminent PTB [62]. Early work is being conducted to better identify possible proteins that reduce IL-6-mediated immune response as a precursor to pharmacological solutions to delay preterm birth [63]. Though traditionally processed through enzyme-linked immunosorbent assay (ELISA), Chaemsaithong et al. investigated the performance of a commercially available lateral flow-based immunoassay using amniotic fluid that can deliver results within twenty minutes, and they showed that this had a specificity of 96% and a sensitivity of 97% to detect intra-amniotic infection with a concentration of IL-6 of 745 pg/mL or more [64].
3.1.2. Hormonal Markers
Abuelghar et al. conducted a prospective observational study to compare serial salivary progesterone concentrations with serial cervical length ultrasound measurements to predict spontaneous PTL after 24 weeks [65]. For all participants (134 women), these measurements were taken at 26 weeks and repeated 3–4 weeks later and grouped based on the gestation of birth for analysis (early PTB defined as 28–34 weeks, late PTB as 34–37 weeks and >37 weeks as term births). Interestingly, the initial measurement and the rate of reduction of both salivary progesterone and cervical lengths were greater in women who delivered preterm. As a tool to predict early PTB, they found that low salivary progesterone levels were comparable to cervical length. The area under the curve (AUC) for the first visit cervical length and salivary progesterone was 0.937 and 0.984, respectively, and for the second visit, the values were 0.908 and 0.976. Serial differences in only cervical length or salivary progesterone were not statistically significant, but when combined, the performance was much better. Similarly, elevated serum relaxin has been associated with PTB. This is believed to be mediated by the effect of relaxin to increase leukocyte numbers, stimulate their functions (including adhesion and migration) during pregnancy.
3.1.3. Genomic Markers
There is increasing work being conducted to consider the use of precision-based medicine through genomics. Genomics can be used to predict PTB through the recognition of DNA/RNA and gene polymorphisms and through the identification of fetoplacental genes.
Targeting cell-free RNA
As a biomarker, cell-free RNA may represent disease activity and are extremely stable in the circulation, which makes them particularly suitable for clinical use. Zhou et al. at Michigan State University have been building on the concept of predicting PTB through the identification of maternal serum biomarkers in their nested case-control study. They used the bioinformatic tools miRWalk and STarMirDB to search for miRNA transcripts that target the EBF1 gene, which is a transcription factor that is important during lymphopoiesis, and to regulate B and T cell lineage [66]. This was chosen based on a previous work by the same group in which they screened six genes (EBF1, EEFSEC, AGTR2, WNT4, ADCY5 and RAP2C) and their variants that have been linked to gestational duration and/or spontaneous PTB in the literature [67]. Patient samples were taken at 17–23 weeks and 27–33 weeks and subsequently grouped into PTB (<37 weeks) or term births (>37 weeks) based on the gestation of birth. They found that four potential maternal blood EBF1-based miRNA transcripts (MIR4266, MIR1251, MIR601, MIR3612) were significantly associated with spontaneous PTB. For samples taken in the third trimester (27–33 weeks), when combined these had a specificity of 72% and sensitivity of 81% for predicting PTL (AUC 0.82; Yoden’s index 0.53, 95% CI 0.26–0.69). When combined, the performance of the four transcripts was significantly more accurate in predicting PTB when compared with single transcripts (p < 0.0034) [68].
-
Targeting maternal gene polymorphisms
Gupta et al. identified different metabolite signatures in the sera of second-trimester women at risk of PTL in comparison with term controls using 1H nuclear magnetic resonance (NMR) metabolomics and genome-wide-screening microarray techniques [69]. More recently, Bhattacharjee et al. conducted a genome-wide association study of spontaneous PTB on 6211 women from India, and following subgroup analysis, they found five single-nucleotide polymorphisms (SNPs; rs4798499, rs2689089, rs7645913, rs10026052 and rs16238) that could help to identify women with an increased risk of early spontaneous PTB (<33 weeks of gestation) [70].
The research team at the Liverpool Women’s Hospital in the UK conducted a cohort study using blood samples from women in their second trimester to test through the UK Biobank Axiom Array for genome data and the Clariom D Human assay for transcriptome analysis. They found that a genome-wide significant SNP rs14675645 (ASTN1) was associated with spontaneous PTB whereas the microRNA-142 transcript and PPARG1-FOXP3 gene set were associated with PPROM at a gestation of 20 weeks. This work has demonstrated the potential for multi-omic biomarkers as predictive tools for PTB (133).
-
Targeting fetoplacental genes
Li et al. from Stanford University analyzed a dataset of de novo mutations in whole blood samples obtained from 816 parent-offspring trios using whole-genome sequencing to show that PTB is associated with a significant increase in de novo mutation burden in fetal genomes [71]. Chien et al. took this further in 2019, when they studied the molecular expressions of mesenchymal stem cells in preterm infants. They used an integrative analysis of dual-omics data to investigate 5615 commonly identified genes/proteins and found 29 genes/proteins that showed a consistent pattern of up- or downregulation in PTB. These methods could be used to identify markers that could be early predictors of PTB [72]. One of the more recent breakthroughs in this area includes the development of a transcriptomic signature-based model by Ran et al in China which is validated to identify women at risk of PTB within the following seven days. Using peripheral blood mRNA expression data, the team performed weighted correlation network analysis and a gene set enrichment analysis to pinpoint the key genes involved in preterm delivery. They determined that the following four core genes impacted the development of PTB: JOSD1, IDNK, ZMYM3, and IL1B. In combination, they conferred a sensitivity and specificity of 83.9% and 87.0% respectively (with a PPV 86.6%, NPV 84.4%). This work promises a novel strategy for clinical risk assessment and management in the acute setting [73].
3.2. Artificial Intelligence and Technology
3.2.1. Computational Modeling
There are several examples of the use of computational modeling to investigate uterine smooth muscle cell activation and contraction. Aslanidi et al. developed a computational model that recognized uterine cell activation [74]. Tong et al. developed a computational model that took 105 differential equations into account to understand uterine smooth muscle cell excitation [75]. Bastos et al built a computational model to simulate intrauterine pressure in women in labor which has been used as a delivery simulator to support the training of healthcare professionals and which Lobo et al later used to simulate the effect of oxytocin on a laboring uterus [75][76].
3.2.2. Machine Learning
This has been used to identify novel associations between risk factors, serum markers and clinical pathology with PTB. The Vanderbilt Genetics Institute led a retrospective study of the electronic health records related to 35,282 deliveries in the US and employed a machine-learning framework and boosted decision tree models (non-parametric supervised learning algorithm) to predict spontaneous PTB [77]. This model used billing codes up to 28 weeks of gestation and was able to predict spontaneous PTB with a sensitivity of 48%, which was significantly higher than a risk factor-only prediction model (sensitivity of 35%). The machine-learning model was also able to risk-stratify deliveries based on co-morbidities and risk factors.
3.2.3. Artificial Intelligence
With the advent of artificial intelligence accelerating at pace across all industries, research groups have investigated its use to analyze datasets to develop new predictive tools for PTB. For example, several groups have used neural networks to classify uterine electrical activity into different labor phenotypes [78][79][80]. Chen and Xu built deep neural networks for the semi-automatic classification of preterm versus term uterine electrical activity [80]. They fed data from tocograms (instrument that records uterine muscle contraction activity) into wave and sample entropies, which extracted features and submitted them to the deep neural network. This enabled the team to classify the uterine recordings and predict PTB with a sensitivity of 98% and a specificity of 97.7%. Tomialowicz et al investigated bioelectric activity measured by electrohysterography rather than mechanical activity to predict PTB. This approach was preferred since bioelectric activity precedes mechanical activity in the initiation of labor. In this study, measurement of bioelectric uterine activity has been found to enable personalized tocolytic treatment for pregnant women [81] The application of artificial intelligence for predicting and managing PTB has a great deal of promise. However, it is important to appreciate the current limitations of using artificial intelligence in this context.
3.3. Combining Methods
So far, this paper has explored the effect of individual markers and techniques in the identification, diagnosis and management of PTL. More recently, researchers have been combining different techniques to further improve their predictive accuracy for PTL and PTB. An example of this is the use of multiple biomarkers rather than a single one. Goldenberg et al. proposed using a combination of serum biomarkers (defensins, α-fetoprotein, granulocyte colony-stimulating factor), cervical length ultrasound and vaginal FFN with a history of previous spontaneous PTB. Low levels of defensins have been linked with PTB, chorioamnionitis and neonatal sepsis [82], α-fetoprotein is recognized as a protein released after the rupture of membranes and is a part of the ROM-plus point-of-care diagnostic test [33], and higher levels of serum granulocyte colony-stimulating factor have been associated with PTB through possible leucocyte-mediated interference with placental function [83]. Two positive test results provided a sensitivity and specificity of 78.8% and 62%, respectively, with an odds ratio of 6.0 for predicting PTB [84].
4. Precision-Based Medicine to Direct Treatments
4.1. Prevention Treatment
There are many ways in which precision-based medicine can improve clinical care related to PTB. For example, the current gold standard for Group B Streptococcus (GBS) identification is the culture of vaginal swab samples, which take 48 h to yield results that can influence patient care. More recently, lateral flow assays for detection of GBS have become available with good specificity, and they provide a point-of-care test [85]. These use a nucleic acid amplification process (NAAT) that is highly specific and sensitive, but they do not provide additional information that can be clinically useful such as antimicrobial resistance or sensitivity [86]. The mechanism by which NAATs are used in lateral flow assays is the identification of small amounts of DNA or RNA samples—in this case, the GBS cAMP factor gene. This is unlike 16S rRNA, metagenomics and culture methods. However, new NAATs and next-generation sequencing (including multiplex-based PCR) provide a method of point-of-care testing that can augment the traditional diagnostic NAAT or a lateral flow. These methods do not require time-consuming enrichment cultures and isolation of pure cultures but are able to use non-purified polymicrobial clinical samples [87][88]. With results available within 30 min or less, these point-of-care assays enable accurate identification of GBS in the outpatient, acute or intrapartum clinical setting, enabling prompt management to improve perinatal outcomes. There are several advantages of using new NAAT and next-generation sequencing methods over traditional 16S rRNA, metagenomics and culture methods for diagnostic testing. These include the rapidity of results to enable prompt management. These tests have a high sensitivity and specificity and so can be relied upon to make decisions. They aid in quantitative analysis and have an ease of use, requiring less clinical time and having improved antibiotic stewardship. The disadvantages of these point-of-care tests are their cost (more costly than cultures), availability and the technical limitation of not having the sensitivity information that cultures provide [89].
An example of current point-of-care testing is the GBS3 trial, where researchers are using a NAAT system to diagnose GBS and then provide prophylactic antibiotics for laboring women [90]. However, the use of a rapid point-of-care test can also help to direct vaccines. Globally, an estimated 20 million pregnant women were thought to have been colonized with GBS in 2020 (230,000 neonatal infections), and approximately 3.5% of all PTBs in the same year were likely associated with GBS (518,000 births) [91]. Immunization against GBS would have a significant impact in preventing both neonatal sepsis and PTB. However, a GBS vaccine needs to not only provide immunity against GBS colonization but also induce a sufficient maternal humoral immune response to protect the mother and neonate from infection. Robust and sustained vaccine immunogenicity will protect against ascending infection and transfer seroprotective antibodies across the placenta to protect the neonate.
4.2. Acute Treatment
Very little clinical options exist for pregnant women in this setting. With a high prevalence of microbial invasion of the amniotic cavity driving spontaneous PTL, novel methods to identify infections (as discussed earlier), profile maternal immune responses and provide targeted treatment may be beneficial. In a non-human primate model of PTL using intrauterine-administered Escherichia coli (E. coli), Cappelletti et al. have shown that giving systemic antibiotics 24 h after injecting E. coli is effective at eradicating bacteria but does not resolve choriodecidual or amnion inflammation. In their model, PTL was only prevented in in 25% of cases [92]. During infective PTB, the IL-1 signaling pathway drives intrauterine inflammation and chorio-decidua neutrophil recruitment [93]. However, it is important to appreciate that in this model of PTB, the E. coli used was a highly uropathogenic strain (UTI89), injected at a concentration of 106 CFU/mL. This is known to induce an exaggerated proinflammatory response in vitro, with raised expression of cytokines IL-1b (21-fold) and IL-8 (6-fold) by choriodecidual and amniotic tissue, respectively [94][95]. For the purposes of establishing an animal model of infective PTB, this will have the desired effect of inducing tissue inflammation and pro-contractile myometrial responses. In humans, commonly identified strains of E. coli are usually from phylogroups B2 and D, which are less uropathogenic than UTI89 and are more likely to colonize in the genital or intestinal tract [96].
Other groups have had some success in human studies. Lee et al. used amniocentesis to identify intraamniotic infection in 314 women with tPTL. They showed that a combination of ceftriaxone, clarithromycin and metronidazole was able to double the median antibiotics-to-delivery interval, with lower rates of histologic chorioamnionitis (23 versus 12 days, p < 0.05) [97]. In contrast, women treated in the absence of infection and/or inflammation did not see a difference in latency to birth, with many delivering preterm. Other research groups have since directly investigated amniotic fluid infection in women with PTL using cytokine ELISA and 16S rDNA. Combs et al. conducted a prospective study, conducting amniocentesis on 305 pregnant women with PTL. They measured IL-6 levels in the amniotic fluid and stratified groups based on IL-6 quantities into negative, mild, moderate and severe inflammation and cultured the fluid for bacterial growth. Their results showed that the degree of inflammation with or without the presence of bacteria directly correlated with PTB and poor neonatal outcomes [40].
5. Conclusions
In conclusion, there are certainly many different avenues in development with regards to the prediction, acute diagnosis and treatment of PTB. Work on the identification of infective agents is most developed, with bacterial infections and host immune responses being identified and assessed using serum, amniotic fluid and vaginal fluid samples. However, researchers have identified a number of techniques to assess a range of biomarkers that have the potential to further personalize the early detection and prediction spontaneous PTB as well as develop point-of-care diagnosis and targeted treatment. Genomics is currently still in its infancy within this context. Further work involving the validation of genes and transcripts and integration with other omics techniques needs to be conducted. Work incorporating artificial intelligence and the use of technology is also in its early stages and requires further development, validation and clinical modelling before application to any clinical setting. Though these techniques exist, there will always be a cohort that requires acute management in the clinical setting, and this remains the most challenging group of patients. It is highly likely that combining methods to develop precise diagnostic tools will enable the best form of personalized care for the individual.
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46.
- Birth Characteristics in England and Wales: 2021. Released 19 January 2023, ONS Website, Statistical Bulletin. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2021 (accessed on 1 November 2023).
- Moutquin, J.M. Classification and heterogeneity of preterm birth. BJOG 2003, 110 (Suppl. S20), 30–33.
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271.
- Vogel, J.P.; Oladapo, O.T.; Manu, A.; Gülmezoglu, A.M.; Bahl, R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob. Health 2015, 3, e589–e590.
- Department of Health and Social Care (Ed.) Safer Maternity Care; Department of Health and Social Care: London, UK, 2016.
- Department of Health and Social Care (Ed.) Safer Maternity Care—NHS England, Progress Report 2021: Safer Maternity Care; Department of Health and Social Care: London, UK, 2021.
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J.; the Born Too Soon Preterm Birth Action Group. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2.
- Brew-Sam, N.; Parkinson, A.; Lueck, C.; Brown, E.; Brown, K.; Bruestle, A.; Chisholm, K.; Collins, S.; Cook, M.; Daskalaki, E.; et al. The current understanding of precision medicine and personalised medicine in selected research disciplines: Study protocol of a systematic concept analysis. BMJ Open 2022, 12, e060326.
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obs. Gynecol. 2016, 215, 103.e101–103.e114.
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110 (Suppl. S20), 8–16.
- Menon, R.; Behnia, F.; Polettini, J.; Richardson, L.S. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin. Immunopathol. 2020, 42, 431–450.
- Cobo, T.; Vives, I.; Rodríguez-Trujillo, A.; Murillo, C.; Ángeles, M.A.; Bosch, J.; Vergara, A.; Gratacós, E.; Palacio, M. Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet. Gynecol. Scand. 2017, 96, 570–579.
- Diemert, A.; Arck, P.C. Preterm birth: Pathogenesis and clinical consequences revisited. Semin. Immunopathol. 2020, 42, 375–376.
- Wadhwa, P.D.; Culhane, J.F.; Rauh, V.; Barve, S.S.; Hogan, V.; Sandman, C.A.; Hobel, C.J.; Chicz-DeMet, A.; Dunkel-Schetter, C.; Garite, T.J.; et al. Stress, infection and preterm birth: A biobehavioural perspective. Paediatr. Perinat. Epidemiol. 2001, 15 (Suppl. S2), 17–29.
- Blois, S.M.; Verlohren, S.; Wu, G.; Clark, G.; Dell, A.; Haslam, S.M.; Barrientos, G. Role of galectin-glycan circuits in reproduction: From healthy pregnancy to preterm birth (PTB). Semin. Immunopathol. 2020, 42, 469–486.
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975.
- Vrachnis, N.; Malamas, F.M.; Sifakis, S.; Tsikouras, P.; Iliodromiti, Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clin. Dev. Immunol. 2012, 2012, 937618.
- Brown, A.G.; Leite, R.S.; Strauss, J.F., 3rd. Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann. N. Y. Acad. Sci. 2004, 1034, 36–49.
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565.
- Vahanian, S.A.; Lavery, J.A.; Ananth, C.V.; Vintzileos, A. Placental implantation abnormalities and risk of preterm delivery: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2015, 213, S78–S90.
- Yart, L.; Roset Bahmanyar, E.; Cohen, M.; Martinez de Tejada, B. Role of the Uteroplacental Renin-Angiotensin System in Placental Development and Function, and Its Implication in the Preeclampsia Pathogenesis. Biomedicines 2021, 9, 1332.
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765.
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best Pr. Res. Clin. Obs. Gynaecol. 2018, 52, 13–22.
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021.
- Elenga, N.; Djossou FÉ, L.; Nacher, M. Association between maternal human immunodeficiency virus infection and preterm birth: A matched case-control study from a pregnancy outcome registry. Medicine 2021, 100, e22670.
- Albert, A.Y.K.; Elwood, C.; Wagner, E.C.; Pakzad, Z.; Chaworth-Musters, T.; Berg, K.; Van Schalkwyk, J.; Maan, E.J.; Azampanah, A.; McClymont, E.; et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS 2020, 34, 719–727.
- Boyd, M.A.A.; van Bockel, D.; Munier, C.M.L.; Kelleher, A.D. Navigating the complexity of chronic HIV-1 associated immune dysregulation. Curr. Opin. Immunol. 2022, 76, 102186.
- Mesfin, Y.M.; Kibret, K.T.; Taye, A. Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod. Health 2016, 13, 30.
- Lorenzi, P.; Spicher, V.M.; Laubereau, B.; Hirschel, B.; Kind, C.; Rudin, C.; Irion, O.; Kaiser, L. Antiretroviral therapies in pregnancy: Maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS 1998, 12, F241–F247.
- Combination antiretroviral therapy and duration of pregnancy. AIDS 2000, 14, 2913–2920.
- Cowdell, I.; Beck, K.; Portwood, C.; Sexton, H.; Kumarendran, M.; Brandon, Z.; Kirtley, S.; Hemelaar, J. Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: A systematic review and meta-analysis. EClinicalMedicine 2022, 46, 101368.
- National Institute for Health and Care Excellence. Preterm Labour and Birth: Guidance; NICE: London, UK, 2022.
- Darmstadt, G.L.; Al Jaifi, N.H.; Arif, S.; Bahl, R.; Blennow, M.; Cavallera, V.; Chou, D.; Chou, R.; Comrie-Thomson, L.; Edmond, K.; et al. New World Health Organization recommendations for care of preterm or low birth weight infants: Health policy. eClinicalMedicine 2023, 63, 102155.
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N., Jr.; Kupfer, K.; Sibai, B.M. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obs. Gynecol. 2020, 222, 259.e1–259.e11.
- McCarthy, F.P.; Gill, C.; Seed, P.T.; Bramham, K.; Chappell, L.C.; Shennan, A.H. Comparison of three commercially available placental growth factor-based tests in women with suspected preterm pre-eclampsia: The COMPARE study. Ultrasound Obs. Gynecol. 2019, 53, 62–67.
- Coutinho, C.M.; Sotiriadis, A.; Odibo, A.; Khalil, A.; D’Antonio, F.; Feltovich, H.; Salomon, L.J.; Sheehan, P.; Napolitano, R.; Berghella, V.; et al. ISUOG Practice Guidelines: Role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obs. Gynecol. 2022, 60, 435–456.
- Honest, H.; Bachmann, L.M.; Gupta, J.K.; Kleijnen, J.; Khan, K.S. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: Systematic review. BMJ 2002, 325, 301.
- Xiao, P.-L.; Zhou, Y.-B.; Chen, Y.; Yang, M.-X.; Song, X.-X.; Shi, Y.; Jiang, Q.-W. Association between maternal HIV infection and low birth weight and prematurity: A meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015, 15, 246.
- Gao, R.; Liu, B.; Yang, W.; Wu, Y.; Wang, B.; Santillan, M.K.; Ryckman, K.; Santillan, D.A.; Bao, W. Association of Maternal Sexually Transmitted Infections with Risk of Preterm Birth in the United States. JAMA Netw. Open 2021, 4, e2133413.
- Screening for Hepatitis B, HIV and Syphilis. Available online: https://www.nhs.uk/pregnancy/your-pregnancy-care/screening-for-hepatitis-b-hiv-and-syphilis/ (accessed on 30 August 2023).
- Public Health England (Ed.) Infectious Diseases in Pregnancy Screening (IDPS): Programme Overview; PHE: London, UK, 2021.
- National Institute for Health and Care Excellence. When Should I Screen for Chlamydia in Primary Care; NICE: London, UK, 2022.
- Adhikari, E.H.; Roberts, S. Sexually Transmitted Infections and Preterm Birth-Attempting to Pin Down Targets for Intervention from Population-Level Observational Data. JAMA Netw. Open 2021, 4, e2134459.
- Hu, X.M.; Xu, J.X.; Jiang, L.X.; Deng, L.R.; Gu, Z.M.; Xie, X.Y.; Ji, H.C.; Wang, W.H.; Li, L.M.; Tian, C.N.; et al. Design and Evaluation of a Novel Multiplex Real-Time PCR Melting Curve Assay for the Simultaneous Detection of Nine Sexually Transmitted Disease Pathogens in Genitourinary Secretions. Front. Cell. Infect. Microbiol. 2019, 9, 382.
- Stinson, L.; Hallingström, M.; Barman, M.; Viklund, F.; Keelan, J.; Kacerovsky, M.; Payne, M.; Jacobsson, B. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples from Preterm and Term Deliveries. Front. Microbiol. 2020, 11, 415.
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15.
- Urushiyama, D.; Suda, W.; Ohnishi, E.; Araki, R.; Kiyoshima, C.; Kurakazu, M.; Sanui, A.; Yotsumoto, F.; Murata, M.; Nabeshima, K.; et al. Microbiome profile of the amniotic fluid as a predictive biomarker of perinatal outcome. Sci. Rep. 2017, 7, 12171.
- Kenyon, S.L.; Taylor, D.J.; Tarnow-Mordi, W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: The ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001, 357, 979–988.
- Kenyon, S.L.; Taylor, D.J.; Tarnow-Mordi, W. Broad-spectrum antibiotics for spontaneous preterm labour: The ORACLE II randomised trial. Lancet 2001, 357, 989–994.
- Kenyon, S.; Taylor, D.J.; Tarnow-Mordi, W.O. ORACLE—Antibiotics for preterm prelabour rupture of the membranes: Short-term and long-term outcomes. Acta Paediatr. Suppl. 2002, 91, 12–15.
- Yoo, I.Y.; Huh, K.; Shim, H.J.; Yun, S.A.; Chung, Y.N.; Kang, O.K.; Huh, H.J.; Lee, N.Y. Evaluation of the BioFire FilmArray Pneumonia Panel for rapid detection of respiratory bacterial pathogens and antibiotic resistance genes in sputum and endotracheal aspirate specimens. Int. J. Infect. Dis. 2020, 95, 326–331.
- Peri, A.M.; Bauer, M.J.; Bergh, H.; Butkiewicz, D.; Paterson, D.L.; Harris, P.N. Performance of the BioFire Blood Culture Identification 2 panel for the diagnosis of bloodstream infections. Heliyon 2022, 8, e09983.
- Trotter, A.J.; Aydin, A.; Strinden, M.J.; O’Grady, J. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance. Curr. Opin. Microbiol. 2019, 51, 39–45.
- Taxt, A.M.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622.
- Cheng, A.P.; Burnham, P.; Lee, J.R.; Cheng, M.P.; Suthanthiran, M.; Dadhania, D.; De Vlaminck, I. A cell-free DNA metagenomic sequencing assay that integrates the host injury response to infection. Proc. Natl. Acad. Sci. USA 2019, 116, 18738–18744.
- Frascoli, M.; Coniglio, L.; Witt, R.; Jeanty, C.; Fleck-Derderian, S.; Myers, D.E.; Lee, T.H.; Keating, S.; Busch, M.P.; Norris, P.J.; et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci. Transl. Med. 2018, 10, eaan2263.
- Denney, J.M.; Nelson, E.; Wadhwa, P.; Waters, T.; Mathew, L.; Goldenberg, R.L.; Culhane, J.F. Cytokine profiling: Variation in immune modulation with preterm birth vs. uncomplicated term birth identifies pivotal signals in pathogenesis of preterm birth. J. Perinat. Med. 2021, 49, 299–309.
- Deng, W.; Yuan, J.; Cha, J.; Sun, X.; Bartos, A.; Yagita, H.; Hirota, Y.; Dey, S.K. Endothelial Cells in the Decidual Bed Are Potential Therapeutic Targets for Preterm Birth Prevention. Cell Rep. 2019, 27, 1755–1768.e1754.
- Xiao, H.; Siddiqui, J.; Remick, D.G. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006, 74, 5227–5235.
- Kacerovsky, M.; Musilova, I.; Hornychova, H.; Kutova, R.; Pliskova, L.; Kostal, M.; Jacobsson, B. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am. J. Obs. Gynecol. 2014, 211, 385.e1–385.e9.
- Park, H.; Park, K.H.; Kim, Y.M.; Kook, S.Y.; Jeon, S.J.; Yoo, H.-N. Plasma inflammatory and immune proteins as predictors of intra-amniotic infection and spontaneous preterm delivery in women with preterm labor: A retrospective study. BMC Pregnancy Childbirth 2018, 18, 146.
- Spinelli, M.; Boucard, C.; Di Nicuolo, F.; Haesler, V.; Castellani, R.; Pontecorvi, A.; Scambia, G.; Granieri, C.; Barnea, E.R.; Surbek, D.; et al. Synthetic PreImplantation Factor (sPIF) reduces inflammation and prevents preterm birth. PLoS ONE 2020, 15, e0232493.
- Chaemsaithong, P.; Romero, R.; Korzeniewski, S.J.; Martinez-Varea, A.; Dong, Z.; Yoon, B.H.; Hassan, S.S.; Chaiworapongsa, T.; Yeo, L. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: A step toward the early treatment of acute intra-amniotic inflammation/infection. J. Matern. Fetal Neonatal Med. 2016, 29, 360–367.
- Abuelghar, W.M.; Ellaithy, M.I.; Swidan, K.H.; Allam, I.S.; Haggag, H.M. Prediction of spontaneous preterm birth: Salivary progesterone assay and transvaginal cervical length assessment after 24 weeks of gestation, another critical window of opportunity. J. Matern. Fetal Neonatal Med. 2019, 32, 3847–3858.
- Bullerwell, C.E.; Robichaud, P.P.; Deprez, P.M.L.; Joy, A.P.; Wajnberg, G.; D’Souza, D.; Chacko, S.; Fournier, S.; Crapoulet, N.; Barnett, D.A.; et al. EBF1 drives hallmark B cell gene expression by enabling the interaction of PAX5 with the MLL H3K4 methyltransferase complex. Sci. Rep. 2021, 11, 1537.
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J.; Vazquez, A. EBF1 Gene mRNA Levels in Maternal Blood and Spontaneous Preterm Birth. Reprod. Sci. 2020, 27, 316–324.
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. J. Matern. Fetal Neonatal Med. 2022, 35, 1239–1247.
- Gupta, J.K.; Care, A.; Goodfellow, L.; Alfirevic, Z.; Muller-Myhsok, B.; Alfirevic, A. Genome and transcriptome profiling of spontaneous preterm birth phenotypes. Sci. Rep. 2022, 12, 1003.
- Bhattacharjee, E.; Thiruvengadam, R.; Ayushi; Das, C.; Bal, V.; Bhatnagar, S.; Das, B.; Desiraju, B.K.; Kshetrapal, P.; Misra, S.; et al. Genetic variants associated with spontaneous preterm birth in women from India: A prospective cohort study. Lancet Reg. Health Southeast Asia 2023, 14, 100190.
- Li, J.; Oehlert, J.; Snyder, M.; Stevenson, D.K.; Shaw, G.M. Fetal de novo mutations and preterm birth. PLoS Genet. 2017, 13, e1006689.
- Chien, C.W.; Lo, Y.S.; Wu, H.Y.; Hsuan, Y.; Lin, C.K.; Chen, Y.J.; Lin, W.; Han, C.L. Transcriptomic and Proteomic Profiling of Human Mesenchymal Stem Cell Derived from Umbilical Cord in the Study of Preterm Birth. Proteom. Clin. Appl. 2020, 14, e1900024.
- Bhavnani, S.K.; Dang, B.; Kilaru, V.; Caro, M.; Visweswaran, S.; Saade, G.; Smith, A.K.; Menon, R. Methylation differences reveal heterogeneity in preterm pathophysiology: Results from bipartite network analyses. J. Perinat. Med. 2018, 46, 509–521.
- Aslanidi, O.; Atia, J.; Benson, A.P.; van den Berg, H.A.; Blanks, A.M.; Choi, C.; Gilbert, S.H.; Goryanin, I.; Hayes-Gill, B.R.; Holden, A.V.; et al. Towards a computational reconstruction of the electrodynamics of premature and full term human labour. Prog. Biophys. Mol. Biol. 2011, 107, 183–192.
- Tong, W.C.; Choi, C.Y.; Kharche, S.; Holden, A.V.; Zhang, H.; Taggart, M.J. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PLoS ONE 2011, 6, e18685.
- Bastos, L.F.; Lobo, M.F.; van Meurs, W.L.; Ayres-de-Campos, D. An intrauterine pressure generator for educational simulation of labour and delivery. Med. Eng. Phys. 2010, 32, 740–745.
- Abraham, A.; Le, B.; Kosti, I.; Straub, P.; Velez-Edwards, D.R.; Davis, L.K.; Newton, J.M.; Muglia, L.J.; Rokas, A.; Bejan, C.A.; et al. Dense phenotyping from electronic health records enables machine learning-based prediction of preterm birth. BMC Med. 2022, 20, 333.
- Maner, W.L.; Garfield, R.E. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann. Biomed. Eng. 2007, 35, 465–473.
- Most, O.; Langer, O.; Kerner, R.; David, G.B.; Calderon, I. Can myometrial electrical activity identify patients in preterm labor? Am. J. Obs. Gynecol. 2008, 199, 378.e1–378.e6.
- Chen, L.; Xu, H. Deep neural network for semi-automatic classification of term and preterm uterine recordings. Artif. Intell. Med. 2020, 105, 101861.
- Anumba, D.O.C.; Stern, V.; Healey, J.T.; Dixon, S.; Brown, B.H. Value of cervical electrical impedance spectroscopy to predict spontaneous preterm delivery in asymptomatic women: The ECCLIPPx prospective cohort study. Ultrasound Obs. Gynecol. 2021, 58, 293–302.
- Olbrich, P.; Pavón, A.; Rosso, M.L.; Molinos, A.; de Felipe, B.; Sanchez, B.; Praena-Fernández, J.M.; Jimenez, F.; Obando, I.; Neth, O. Association of human beta-defensin-2 serum levels and sepsis in preterm neonates. Pediatr. Crit. Care Med. 2013, 14, 796–800.
- Goldenberg, R.L.; Andrews, W.W.; Mercer, B.M.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.; Caritis, S.N.; Roberts, J.M.; Miodovnik, M.; et al. The preterm prediction study: Granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obs. Gynecol. 2000, 182, 625–630.
- Goldenberg, R.L.; Iams, J.D.; Mercer, B.M.; Meis, P.J.; Moawad, A.; Das, A.; Miodovnik, M.; Vandorsten, P.J.; Caritis, S.N.; Thurnau, G.; et al. The Preterm Prediction Study: Toward a multiple-marker test for spontaneous preterm birth. Am. J. Obs. Gynecol. 2001, 185, 643–651.
- Hu, S.; Zhong, H.; Huang, W.; Zhan, W.; Yang, X.; Tang, B.; Chen, K.; Wang, J.; Hu, T.; Zhang, C.; et al. Rapid and visual detection of Group B streptococcus using recombinase polymerase amplification combined with lateral flow strips. Diagn. Microbiol. Infect. Dis. 2019, 93, 9–13.
- Liang, Y.; Jin, X.; Yuan, F.; Li, Z.; Chen, S. Comparison of rRNA-based and DNA-based nucleic acid amplifications for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum in urogenital swabs. BMC Infect. Dis. 2018, 18, 651.
- Golparian, D.; Unemo, M. Antimicrobial resistance prediction in Neisseria gonorrhoeae: Current status and future prospects. Expert Rev. Mol. Diagn. 2022, 22, 29–48.
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020, 10, 308.
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935.
- Routine Testing for Group B Strep: The GBS3 Trial. Available online: https://www.gbs3trial.ac.uk/home.aspx (accessed on 20 November 2023).
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B streptococcus infection during pregnancy and infancy: Estimates of regional and global burden. Lancet Glob. Health 2022, 10, e807–e819.
- Cappelletti, M.; Presicce, P.; Feiyang, M.; Senthamaraikannan, P.; Miller, L.A.; Pellegrini, M.; Sim, M.S.; Jobe, A.H.; Divanovic, S.; Way, S.S.; et al. The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection. PLoS Biol. 2021, 19, e3001385.
- Presicce, P.; Park, C.W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018, 3, e98306.
- Fenlon, S.N.; Chee, Y.C.; Chee, J.L.Y.; Choy, Y.H.; Khng, A.J.; Liow, L.T.; Mehershahi, K.S.; Ruan, X.; Turner, S.W.; Yao, F.; et al. Sequencing of E. coli strain UTI89 on multiple sequencing platforms. BMC Res. Notes 2020, 13, 487.
- Zaga-Clavellina, V.; Garcia-Lopez, G.; Flores-Herrera, H.; Espejel-Nuñez, A.; Flores-Pliego, A.; Soriano-Becerril, D.; Maida-Claros, R.; Merchant-Larios, H.; Vadillo-Ortega, F. In vitro secretion profiles of interleukin (IL)-1beta, IL-6, IL-8, IL-10, and TNF alpha after selective infection with Escherichia coli in human fetal membranes. Reprod. Biol. Endocrinol. 2007, 5, 46.
- Watt, S.; Lanotte, P.; Mereghetti, L.; Moulin-Schouleur, M.; Picard, B.; Quentin, R. Escherichia coli strains from pregnant women and neonates: Intraspecies genetic distribution and prevalence of virulence factors. J. Clin. Microbiol. 2003, 41, 1929–1935.
- Lee, J.; Romero, R.; Kim, S.M.; Chaemsaithong, P.; Park, C.W.; Park, J.S.; Jun, J.K.; Yoon, B.H. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J. Matern. Fetal Neonatal Med. 2016, 29, 707–720.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
856
Revisions:
4 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




