
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Angulo | -- | 6366 | 2024-01-12 16:05:20 | | | |
| 2 | Lindsay Dong | + 7 word(s) | 6373 | 2024-01-15 02:00:14 | | |
Video Upload Options
The Warburg effect describes a change in the glucose metabolism in cancer cells, consuming excess glucose and converting it into lactate independently of the presence of oxygen. During this process, a wide variety of enzymes can modify their expression and activity to contribute to the mechanism of deregulated cancer metabolism. Therefore, the modulation of enzymes regulating aerobic glycolysis is a strategy for cancer treatment. Although numerous enzymes play a role in regulating aerobic glycolysis, hexokinase 2 (HK2), pyruvate dehydrogenase kinase (PDK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) are worth mentioning.
1. Introduction
2. Target Enzymes and the Reported Inhibitors
2.1. HK2 Modulators
2.1.1. Small Molecules
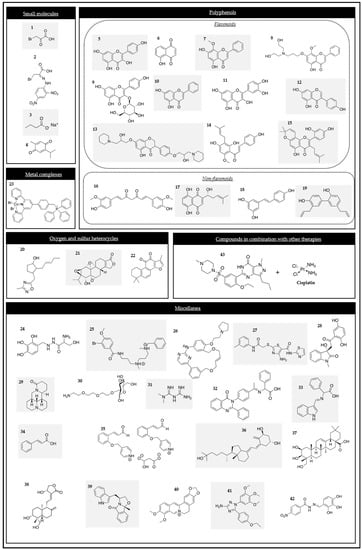
2.1.2. Polyphenols
-
Flavonoid phenolic compounds
-
Non-flavonoid phenolic compounds
2.1.3. Oxygen and Sulfur Heterocycles
2.1.4. Metal Complexes
2.1.5. Miscellanea
Benserazide (BEN) (compound 24 in Figure 1) was identified as a selective HK2 inhibitor after a structure-based virtual ligand screening of a library of 2924 US Food and Drug Administration (FDA)-approved drugs and a nutraceutical database. The molecular docking results showed that BEN adopted an extended conformation in which the pyrogallol group of the molecule occupied the binding site of the substrate glucose in HK2. BEN was specifically bound to HK2 as a competitive inhibitor.
High-throughput virtual screening is a widely used strategy for searching for new anti-cancer molecules. By using this method, several chemical scaffolds have been identified to be promising HK2 inhibitors. Among them, compound 25 (Figure 1) was highlighted due to its outstanding activity in tumor cell suppression and its capacity to be solubilized in aqueous environments.
2.1.6. Compounds in Combination with Other Therapies
2.2. PDK Modulators
2.2.1. Small Molecules
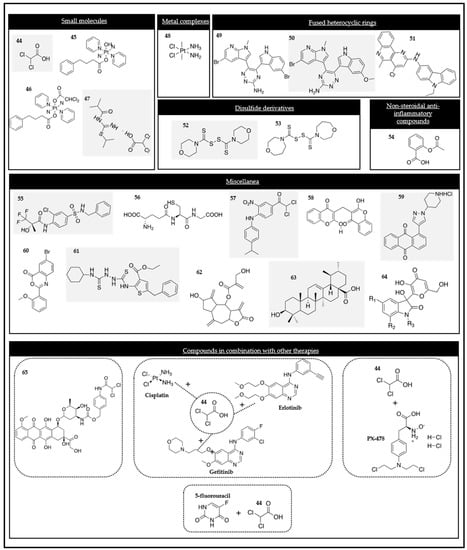
2.2.2. Metal Complexes
2.2.3. Fused Heterocyclic Rings
2.2.4. Disulfide Derivatives
2.2.5. Nonsteroidal Anti-Inflammatory Drugs
2.2.6. Miscellanea
2.2.7. Compounds in Combination with Other Therapies
Particularly, a binary prodrug named PDOX (compound 65 in Figure 2) was designed and investigated. It consisted of a combination of DCA (PDK inhibitor) and the anti-cancer drug doxorubicin (DOX). The prodrug was activated selectively by cancer-associated esterase to deliver DCA and DOX.
The PDK inhibitor DCA (compound 44) has also been explored in lung cancer in combination with commercial therapeutic drugs, particularly A549 and LNM35. Al-Azawi and co-workers demonstrated that DCA significantly enhanced the anti-cancer effect of cisplatin, gefitinib, and erlotinib. The additive effects on the inhibition of the mentioned cell lines due to the synergistic effect were shown.
Alongside the drug combination approach, new evidence for the synergism between the PDK inhibitor DCA and the HIF-1α inhibitor PX-478 was found. The group demonstrated that the combination of DCA and PX-478 produced synergistic effects in colorectal, lung, breast, cervical, liver, and brain cancer cell lines.
2.3. PK Modulators
2.3.1. Small Molecules
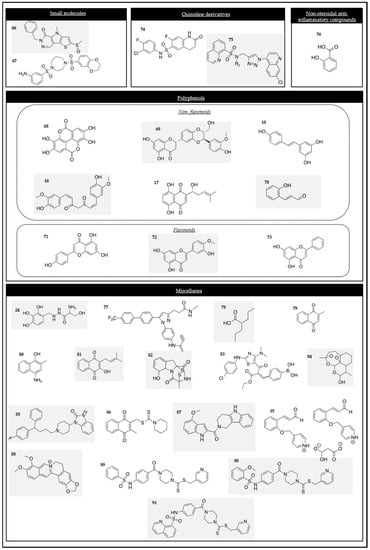
2.3.2. Polyphenolic Compounds
-
Non-flavonoids
-
Flavonoids
2.3.3. Quinoline Derivatives
2.3.4. Nonsteroidal Anti-Inflammatory Drugs
2.3.5. Miscellanea
2.4. LDH Enzyme Modulators
2.4.1. Small Molecules
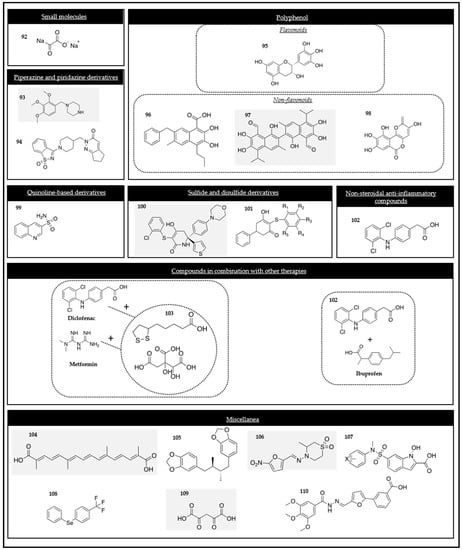
2.4.2. Pyridazine and Piperazine
2.4.3. Polyphenols
-
Flavonoids
-
Non-flavonoids
2.4.4. Quinoline-Based Derivatives
2.4.5. Sulfide and Disulfide Derivatives
2.4.6. Nonsteroidal Anti-Inflammatory Compounds
2.4.7. Compounds in Combination with Other Therapies
2.4.8. Miscellanea
References
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377.
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530.
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314.
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757.
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166.
- Benny, S.; Mishra, R.; Manojkumar, M.K.; Aneesh, T.P. From Warburg effect to Reverse Warburg effect; the new horizons of anti-cancer therapy. Med. Hypotheses 2020, 144, 110216.
- Zhong, J.; Lu, S.; Jia, X.; Li, Q.; Liu, L.; Xie, P.; Wang, G.; Lu, M.; Gao, W.; Zhao, T.; et al. Role of endoplasmic reticulum stress in apoptosis induced by HK2 inhibitor and its potential as a new drug combination strategy. Cell Stress Chaperones 2022, 27, 273–283.
- Zhao, B.; Aggarwal, A.; Marshall, J.A.; Barletta, J.A.; Kijewski, M.F.; Lorch, J.H.; Nehs, M.A. Glycolytic inhibition with 3-bromopyruvate suppresses tumor growth and improves survival in a murine model of anaplastic thyroid cancer. Surgery 2022, 171, 227–234.
- Li, J.; Pan, J.; Liu, Y.; Luo, X.; Yang, C.; Xiao, W.; Li, Q.; Yang, L.; Zhang, X. 3-Bromopyruvic acid regulates glucose metabolism by targeting the c-Myc/TXNIP axis and induces mitochondria-mediated apoptosis in TNBC cells. Exp. Ther. Med. 2022, 24, 520.
- Roy, S.; Dukic, T.; Bhandary, B.; Tu, K.J.; Molitoris, J.; Ko, Y.H.; Shukla, H.D. 3-Bromopyruvate inhibits pancreatic tumor growth by stalling glycolysis, and dismantling mitochondria in a syngeneic mouse model. Am. J. Cancer Res. 2022, 12, 4977–4987.
- Beygi, F.; Mostoufi, A.; Mojaddami, A. Novel Hydrazone Derivatives of 3-Bromopyruvate: Synthesis, Evaluation of the Cytotoxic Effects, Molecular Docking and ADME Studies. Chem. Biodivers. 2022, 19, e202100754.
- Yu, Q.; Dai, W.; Ji, J.; Wu, L.; Feng, J.; Li, J.; Zheng, Y.; Li, Y.; Cheng, Z.; Zhang, J.; et al. Sodium butyrate inhibits aerobic glycolysis of hepatocellular carcinoma cells via the c-myc/hexokinase 2 pathway. J. Cell. Mol. Med. 2022, 26, 3031–3045.
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2305.
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515.
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834.
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015, 366, 141–149.
- Zheng, X.; Pan, Y.; Yang, G.; Liu, Y.; Zou, J.; Zhao, H.; Yin, G.; Wu, Y.; Li, X.; Wei, Z.; et al. Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur. J. Pharmacol. 2022, 931, 175226.
- Hu, C.; Xu, H.; Li, Z.; Liu, D.; Zhang, S.; Fang, F.; Wang, L. Juglone promotes antitumor activity against prostate cancer via suppressing glycolysis and oxidative phosphorylation. Phytother. Res. 2023, 37, 515–526.
- Li, L.; Ji, Y.; Zhang, L.; Cai, H.; Ji, Z.; Gu, L.; Yang, S. Wogonin inhibits the growth of HT144 melanoma via regulating hedgehog signaling-mediated inflammation and glycolysis. Int. Immunopharmacol. 2021, 101, 108222.
- Chen, X.; Wei, L.; Yang, L.; Guo, W.; Guo, Q.; Zhou, Y. Glycolysis inhibition and apoptosis induction in human prostate cancer cells by FV-429-mediated regulation of AR-AKT-HK2 signaling network. Food Chem. Toxicol. 2020, 143, 111517.
- Li, W.; Hao, J.; Zhang, L.; Cheng, Z.; Deng, X.; Shu, G. Astragalin Reduces Hexokinase 2 through Increasing miR-125b to Inhibit the Proliferation of Hepatocellular Carcinoma Cells in Vitro and in Vivo. J. Agric. Food Chem. 2017, 65, 5961–5972.
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44.
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-mTOR Pathway. Molecules 2019, 24, 1993.
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 2017, 117, 1518–1528.
- Tao, L.; Wei, L.; Liu, Y.; Ding, Y.; Liu, X.; Zhang, X.; Wang, X.; Yao, Y.; Lu, J.; Wang, Q.; et al. Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem. Pharmacol. 2017, 125, 12–25.
- Liu, W.; Li, W.; Liu, H.; Yu, X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508.
- Cho, A.R.; Park, W.Y.; Lee, H.J.; Sim, D.Y.; Im, E.; Park, J.E.; Ahn, C.H.; Shim, B.S.; Kim, S.H. Antitumor Effect of Morusin via G1 Arrest and Antiglycolysis by AMPK Activation in Hepatocellular Cancer. Int. J. Mol. Sci. 2021, 22, 10619.
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24.
- Zhang, Q.; Liu, Q.; Zheng, S.; Liu, T.; Yang, L.; Han, X.; Lu, X. Shikonin Inhibits Tumor Growth of ESCC by suppressing PKM2 mediated Aerobic Glycolysis and STAT3 Phosphorylation. J. Cancer 2021, 12, 4830–4840.
- Li, W.; Ma, X.; Li, N.; Liu, H.; Dong, Q.; Zhang, J.; Yang, C.; Liu, Y.; Liang, Q.; Zhang, S.; et al. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp. Cell Res. 2016, 349, 320–327.
- Uludağ, D.; Bay, S.; Sucu, B.O.; Şavluğ İpek, Ö.; Mohr, T.; Güzel, M.; Karakaş, N. Potential of Novel Methyl Jasmonate Analogs as Anticancer Agents to Metabolically Target HK-2 Activity in Glioblastoma Cells. Front. Pharmacol. 2022, 13, 828400.
- Li, M.; Shao, J.; Guo, Z.; Jin, C.; Wang, L.; Wang, F.; Jia, Y.; Zhu, Z.; Zhang, Z.; Zhang, F.; et al. Novel mitochondrion-targeting copper(II) complex induces HK2 malfunction and inhibits glycolysis via Drp1-mediating mitophagy in HCC. J. Cell Mol. Med. 2020, 24, 3091–3107.
- Liu, R.; Liu, X. Virtual Screening and Biological Activity Evaluation of New Potent Inhibitors Targeting Hexokinase-II. Molecules 2022, 27, 7555.
- Glenister, A.; Simone, M.I.; Hambley, T.W. A Warburg effect targeting vector designed to increase the uptake of compounds by cancer cells demonstrates glucose and hypoxia dependent uptake. PLoS ONE 2019, 14, e0217712.
- Nakayama, J.; Konno, Y.; Maruyama, A.; Tomita, M.; Makinoshima, H. Cinnamon bark extract suppresses metastatic dissemination of cancer cells through inhibition of glycolytic metabolism. J. Nat. Med. 2022, 76, 686–692.
- Huang, C.Y.; Weng, Y.T.; Li, P.C.; Hsieh, N.T.; Li, C.I.; Liu, H.S.; Lee, M.F. Calcitriol Suppresses Warburg Effect and Cell Growth in Human Colorectal Cancer Cells. Life 2021, 11, 963.
- Li, J.; Zou, Y.; Pei, M.; Zhang, Y.; Jiang, Y. Berberine inhibits the Warburg effect through TET3/miR-145/HK2 pathways in ovarian cancer cells. J. Cancer 2021, 12, 207–216.
- Jin, S.; Guo, Y.; Song, D.; Zhu, Z.; Zhang, Z.; Sun, Y.; Yang, T.; Guo, Z.; Wang, X. Targeting Energy Metabolism by a Platinum(IV) Prodrug as an Alternative Pathway for Cancer Suppression. Inorg. Chem. 2019, 58, 6507–6516.
- Pecoraro, C.; De Franco, M.; Carbone, D.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Moro, S.; et al. 1,2,4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2023, 249, 115134.
- Sun, W.; Xie, Z.; Liu, Y.; Zhao, D.; Wu, Z.; Zhang, D.; Lv, H.; Tang, S.; Jin, N.; Jiang, H.; et al. JX06 Selectively Inhibits Pyruvate Dehydrogenase Kinase PDK1 by a Covalent Cysteine Modification. Cancer Res. 2015, 75, 4923–4936.
- Peng, F.; Wang, J.H.; Fan, W.J.; Meng, Y.T.; Li, M.M.; Li, T.T.; Cui, B.; Wang, H.F.; Zhao, Y.; An, F.; et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 2018, 37, 1062–1074.
- Shen, M.; Wang, D.; Sennari, Y.; Zeng, Z.; Baba, R.; Morimoto, H.; Kitamura, N.; Nakanishi, T.; Tsukada, J.; Ueno, M.; et al. Pentacyclic triterpenoid ursolic acid induces apoptosis with mitochondrial dysfunction in adult T-cell leukemia MT-4 cells to promote surrounding cell growth. Med. Oncol. 2022, 39, 118.
- Almouhanna, F.; Blagojevic, B.; Can, S.; Ghanem, A.; Wölfl, S. Pharmacological activation of pyruvate kinase M2 reprograms glycolysis leading to TXNIP depletion and AMPK activation in breast cancer cells. Cancer Metab. 2021, 9, 5.
- Battisti, U.M.; Gao, C.; Akladios, F.; Kim, W.; Yang, H.; Bayram, C.; Bolat, I.; Kiliclioglu, M.; Yuksel, N.; Tozlu, O.O.; et al. Ellagic Acid and Its Metabolites as Potent and Selective Allosteric Inhibitors of Liver Pyruvate Kinase. Nutrients 2023, 15, 577.
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Sultana, T.; Adem, Ş. Identification of Natural Compounds as Inhibitors of Pyruvate Kinase M2 for Cancer Treatment. Molecules 2022, 27, 7113.
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323.
- Yoon, Y.J.; Kim, Y.H.; Jin, Y.; Chi, S.W.; Moon, J.H.; Han, D.C.; Kwon, B.M. 2′-hydroxycinnamaldehyde inhibits cancer cell proliferation and tumor growth by targeting the pyruvate kinase M2. Cancer Lett. 2018, 434, 42–55.
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50.
- Shan, S.; Shi, J.; Yang, P.; Jia, B.; Wu, H.; Zhang, X.; Li, Z. Apigenin Restrains Colon Cancer Cell Proliferation via Targeted Blocking of Pyruvate Kinase M2-Dependent Glycolysis. J. Agric. Food Chem. 2017, 65, 8136–8144.
- Aslan, E.; Adem, S. In vitro effects of some flavones on human pyruvate kinase isoenzyme M2. J. Biochem. Mol. Toxicol. 2015, 29, 109–113.
- Walsh, M.J.; Brimacombe, K.R.; Veith, H.; Bougie, J.M.; Daniel, T.; Leister, W.; Cantley, L.C.; Israelsen, W.J.; Vander Heiden, M.G.; Shen, M.; et al. 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg. Med. Chem. Lett. 2011, 21, 6322–6327.
- Marciniec, K.; Rzepka, Z.; Chrobak, E.; Boryczka, S.; Latocha, M.; Wrześniok, D.; Beberok, A. Design, Synthesis and Biological Evaluation of Quinoline-8-Sulfonamides as Inhibitors of the Tumor Cell-Specific M2 Isoform of Pyruvate Kinase: Preliminary Study. Molecules 2023, 28, 2509.
- Li, Z.; Yang, L.; Zhang, S.; Song, J.; Sun, H.; Shan, C.; Wang, D.; Liu, S. Valproic acid Suppresses Breast Cancer Cell Growth Through Triggering Pyruvate Kinase M2 Isoform Mediated Warburg Effect. Cell Transpl. 2021, 30, 9636897211027524.
- Sun, X.; Peng, Y.; Zhao, J.; Xie, Z.; Lei, X.; Tang, G. Discovery and development of tumor glycolysis rate-limiting enzyme inhibitors. Bioorg. Chem. 2021, 112, 104891.
- Chen, J.; Jiang, Z.; Wang, B.; Wang, Y.; Hu, X. Vitamin K(3) and K(5) are inhibitors of tumor pyruvate kinase M2. Cancer Lett. 2012, 316, 204–210.
- Tang, W.; Liu, Z.L.; Mai, X.Y.; Qi, X.; Li, D.H.; Gu, Q.Q.; Li, J. Identification of Gliotoxin isolated from marine fungus as a new pyruvate kinase M2 inhibitor. Biochem. Biophys. Res. Commun. 2020, 528, 594–600.
- Rihan, M.; Vineela Nalla, L.; Dharavath, A.; Patel, S.; Shard, A.; Khairnar, A. Boronic acid derivative activates pyruvate kinase M2 indispensable for redox metabolism in oral cancer cells. Bioorg. Med. Chem. Lett. 2022, 59, 128539.
- Jiang, M.; Wu, Y.; Qi, L.; Li, L.; Song, D.; Gan, J.; Li, Y.; Ling, X.; Song, C. Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma. Chem. Biol. Interact. 2021, 350, 109704.
- Nagasawa, I.; Muroi, M.; Kawatani, M.; Ohishi, T.; Ohba, S.I.; Kawada, M.; Osada, H. Identification of a Small Compound Targeting PKM2-Regulated Signaling Using 2D Gel Electrophoresis-Based Proteome-wide CETSA. Cell Chem. Biol. 2020, 27, 186–196.e184.
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26.
- Zhai, X.; Yang, Y.; Wan, J.; Zhu, R.; Wu, Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol. Rep. 2013, 30, 2983–2991.
- Abdelrahman, R.S.; Shawky, N.M. Trimetazidine, a metabolic modulator, attenuates silica-induced pulmonary fibrosis and decreases lactate levels and LDH activity in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23071.
- Atlı Şekeroğlu, Z.; Şekeroğlu, V.; Işık, S.; Aydın, B. Trimetazidine alone or in combination with gemcitabine and/or abraxane decreased cell viability, migration and ATP levels and induced apoptosis of human pancreatic cells. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101632.
- Mishra, D.; Banerjee, D. Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvironment. Cancers 2019, 11, 750.
- He, Y.; Chen, X.; Yu, Y.; Li, J.; Hu, Q.; Xue, C.; Chen, J.; Shen, S.; Luo, Y.; Ren, F.; et al. LDHA is a direct target of miR-30d-5p and contributes to aggressive progression of gallbladder carcinoma. Mol. Carcinog. 2018, 57, 772–783.
- Xian, Z.Y.; Liu, J.M.; Chen, Q.K.; Chen, H.Z.; Ye, C.J.; Xue, J.; Yang, H.Q.; Li, J.L.; Liu, X.F.; Kuang, S.J. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015, 36, 8093–8100.
- Farabegoli, F.; Vettraino, M.; Manerba, M.; Fiume, L.; Roberti, M.; Di Stefano, G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur. J. Pharm. Sci. 2012, 47, 729–738.
- Billiard, J.; Dennison, J.B.; Briand, J.; Annan, R.S.; Chai, D.; Colón, M.; Dodson, C.S.; Gilbert, S.A.; Greshock, J.; Jing, J.; et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013, 1, 19.
- Boudreau, A.; Purkey, H.E.; Hitz, A.; Robarge, K.; Peterson, D.; Labadie, S.; Kwong, M.; Hong, R.; Gao, M.; Del Nagro, C.; et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 2016, 12, 779–786.
- Han, J.H.; Kim, M.; Kim, H.J.; Jang, S.B.; Bae, S.J.; Lee, I.K.; Ryu, D.; Ha, K.T. Targeting Lactate Dehydrogenase A with Catechin Resensitizes SNU620/5FU Gastric Cancer Cells to 5-Fluorouracil. Int. J. Mol. Sci. 2021, 22, 5406.
- Granchi, C.; Fortunato, S.; Meini, S.; Rizzolio, F.; Caligiuri, I.; Tuccinardi, T.; Lee, H.Y.; Hergenrother, P.J.; Minutolo, F. Characterization of the Saffron Derivative Crocetin as an Inhibitor of Human Lactate Dehydrogenase 5 in the Antiglycolytic Approach against Cancer. J. Agric. Food Chem. 2017, 65, 5639–5649.
- Chung, T.W.; Kim, E.Y.; Han, C.W.; Park, S.Y.; Jeong, M.S.; Yoon, D.; Choi, H.J.; Jin, L.; Park, M.J.; Kwon, Y.J.; et al. Machilin A Inhibits Tumor Growth and Macrophage M2 Polarization Through the Reduction of Lactic Acid. Cancers 2019, 11, 963.
- Cabanillas Stanchi, K.M.; Bruchelt, G.; Handgretinger, R.; Holzer, U. Nifurtimox reduces N-Myc expression and aerobic glycolysis in neuroblastoma. Cancer Biol. Ther. 2015, 16, 1353–1363.
- Granchi, C.; Roy, S.; Giacomelli, C.; Macchia, M.; Tuccinardi, T.; Martinelli, A.; Lanza, M.; Betti, L.; Giannaccini, G.; Lucacchini, A.; et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J. Med. Chem. 2011, 54, 1599–1612.




