Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | takafumi obara | -- | 2537 | 2024-01-11 07:24:25 | | | |
| 2 | Catherine Yang | Meta information modification | 2537 | 2024-01-11 07:50:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Obara, T.; Naito, H.; Nojima, T.; Hirayama, T.; Hongo, T.; Ageta, K.; Aokage, T.; Hisamura, M.; Yumoto, T.; Nakao, A. Hydrogen in Transplantation. Encyclopedia. Available online: https://encyclopedia.pub/entry/53719 (accessed on 07 February 2026).
Obara T, Naito H, Nojima T, Hirayama T, Hongo T, Ageta K, et al. Hydrogen in Transplantation. Encyclopedia. Available at: https://encyclopedia.pub/entry/53719. Accessed February 07, 2026.
Obara, Takafumi, Hiromichi Naito, Tsuyoshi Nojima, Takahiro Hirayama, Takashi Hongo, Kohei Ageta, Toshiyuki Aokage, Masaki Hisamura, Tetsuya Yumoto, Atsunori Nakao. "Hydrogen in Transplantation" Encyclopedia, https://encyclopedia.pub/entry/53719 (accessed February 07, 2026).
Obara, T., Naito, H., Nojima, T., Hirayama, T., Hongo, T., Ageta, K., Aokage, T., Hisamura, M., Yumoto, T., & Nakao, A. (2024, January 11). Hydrogen in Transplantation. In Encyclopedia. https://encyclopedia.pub/entry/53719
Obara, Takafumi, et al. "Hydrogen in Transplantation." Encyclopedia. Web. 11 January, 2024.
Copy Citation
Hydrogen gas, renowned for its antioxidant properties, has emerged as a novel therapeutic agent with applications across various medical domains, positioning it as a potential adjunct therapy in transplantation.
hydrogen
organ transplantation
ischemia reperfusion
1. Introduction
The latest findings in medical research regarding hydrogen unequivocally highlight substantial prospects for harnessing hydrogen as a therapeutic intervention. Extensive observations in both clinical and experimental studies distinctly demonstrate that hydrogen holds significant promise as an innovative therapy to address unmet patient needs across various etiologies [1][2][3][4][5][6][7][8][9][10]. Renowned for its antioxidant properties, hydrogen gas has emerged as a novel therapeutic agent with potential applications in various medical domains, including transplantation. In addition to its antioxidative attributes, hydrogen also exerts anti-inflammatory effects by modulating pro-inflammatory cytokines and signaling pathways. Moreover, hydrogen’s capacity to activate cytoprotective pathways enhances cellular resilience to stress.
2. Protective Mechanism of Hydrogen
2.1. Scavenging Free Radicals
Although various mechanisms for the cellular and tissue protection provided by hydrogen exposure have been suggested, hydrogen’s role as a scavenger of reactive oxygen species has been advocated. Ohsawa et al. reported that, in vitro, hydrogen selectively reduces peroxynitrite and hydroxyl radicals, which are very strong oxidants which react indiscriminately with nucleic acids, proteins, and lipids, resulting in lipid peroxidation, DNA fragmentation, and protein inactivation. Biochemical experiments using electron resonance spectroscopy spin traps and fluorescent probes suggest that the effects of hydrogen against hydroxyl radicals are more potent than those against peroxynitrite [11][12][13].
2.2. Protection of Mitochondrial Function
Hydrogen easily permeates biological membranes and diffuses into the nucleus, mitochondria, and cytosol, reaching target tissues. Treatment with hydrogen-rich saline significantly reduced the loss of mitochondrial membrane potential and preserved mitochondrial cytochrome c content [14]. In an open-label trial, Ito et al. investigated the effects of drinking hydrogen-enriched water for 12 weeks in patients with mitochondrial metabolism diseases, including progressive muscular dystrophy and mitochondrial myopathies, and observed significant improvements in lactate-to-pyruvate ratios [15]. Zhang et al. reported that hydrogen improved mitochondrial quality by upregulating heme oxygenase-1 (HO-1) expression through the nuclear factor erythroid 2-related factor 2 (Nrf2)/YY1 complex in vitro [16].
2.3. Anti-Inflammation
Hydrogen can modulate the production of inflammatory cytokines like interleukin (IL)-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α). These cytokines are key players in the inflammatory response of the human body. Hydrogen attenuates this inflammatory response by inhibiting the nuclear factor-kappa B (NF-κB) and p38 MAPK pathways [17]. Hydrogen can promote very early M1-to-M2 polarization without disturbing the functions of the M1 phenotype, suggesting that hydrogen could reduce inflammation by shifting early macrophage polarization in clinical settings [18]. Hydrogen’s anti-inflammatory impact on the inflammatory response was demonstrated to occur through the phosphatidylinositol 3 kinase/protein kinase B signaling pathway [19].
2.4. Induction of Antioxidant Enzyme
Another potential mechanism underlying hydrogen’s cellular protective function may be an increase in antioxidant enzymes such as superoxide dismutase, catalase, or HO-1 [20]. Hydrogen-rich saline treatment considerably increased the antioxidant enzyme levels of serum superoxide dismutase and reduced glutathione [21].
2.5. Induction of Surfactant-Related Genes
Tanaka et al. investigated the changes in genes after hydrogen inhalation using a gene array analysis and showed that clara cell protein 16 (CC16) was the most upregulated gene in response to preloading hydrogen in lung grafts. CC16 is one of the major proteins secreted by the respiratory epithelium and has antioxidant and anti-inflammatory properties. Preloading hydrogen via mechanical ventilation also significantly increased other surfactant-related mRNAs, including HSD11b1, SCGB3A2, and SP-A [22].
2.6. Protection of Vascular Endothelial Cells
Nitric oxide (NO) plays a crucial role in maintaining the delicate equilibrium of factors that regulate vascular tone, blood flow, and coagulation, all of which are vital for the health of endothelial cells [23]. Hydrogen can enhance endothelial NO synthase activity, leading to increased circulating NO levels, thus providing protection to vascular endothelial cells. The incubation in a hydrogen-rich medium significantly improves cell viability and shields human umbilical vein endothelial cells from cellular damage induced by hydrogen peroxide [24]. Moreover, hydrogen effectively suppresses the release of cell adhesion molecules like intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1, as well as proinflammatory mediators, including high-mobility group box 1 protein, IL-1β, and TNF-α. Furthermore, hydrogen demonstrates the ability to elevate levels of the anti-inflammatory cytokine IL-10 [25][26].
2.7. Prevention of Apoptosis
Hydrogen’s anti-apoptotic functions have been proposed to occur via the inhibition of caspase-3 activation and the induction of anti-apoptotic gene B-cell lymphoma-2 (Bcl-2) [27]. Terasaki et al. reported that the activation of the pro-apoptotic gene Bax was reduced via hydrogen treatment [28]. The anti-apoptotic effects of hydrogen gas inhalation were partially mediated by the early activation of NF-κB during hydrogen treatment and correlated with decreased levels of Bax and elevated levels of the anti-apoptotic protein Bcl-2 [29]. Zhang et al. demonstrated that the upregulation of Bcl-2, NF-κB, HO-1, and zinc finger protein A20 was seen in rats where only the donors received hydrogen [30]. Meanwhile, in another study, the intraperitoneal administration of hydrogen-rich saline to a transplant recipient immediately after reperfusion protected them against acute kidney injury after liver transplantation, partly by reducing apoptosis, which is potentially involved in the modulation of p53-mediated autophagy [31].
2.8. Inhibition of Infiltrating Cell Migration
Accelerated atherosclerosis caused by the immune response is a primary cause of graft loss after organ transplantation. Smooth muscle cell proliferation and migration play important roles in the progression of intimal hyperplasia. Sun et al. demonstrated that the incubation in a hydrogen-rich medium suppressed smooth muscle cell migration using an in vitro rat smooth muscle cell (A7r5) culture model [24]. Matrix metalloproteinases (MMPs) are important mediators of intimal hyperplasia, and MMP-2 and MMP-9 promote the formation of neointima. In the same study, the oral intake of hydrogen-rich water effectively inhibited MMP-2 and MMP-9 in rat vein grafts [24].
2.9. Inhibition of Fibrosis
Type III collagen plays a significant role in the interstitia of solid organs and in the formation of granulation tissue following ischemic tissue damage. Terasaki et al. conducted a study showing that both the inhalation of 3% hydrogen gas and the oral consumption of hydrogen-enriched water reduced oxidative stress and apoptosis, which are indicators of acute lung damage, in mice exposed to irradiation [28]. This led to a decrease in the deposition of type III collagen and the development of lung fibrosis, which is a manifestation of late-stage damage. Therefore, hydrogen’s potential to mitigate fibrosis may prove effective in addressing chronic allograft injury.
2.10. Immunomodulation
Hydrogen has been found to influence various aspects of the immune response. By modulating cytokine profiles and immune cell interactions, hydrogen may promote an environment conducive to immune tolerance and decrease the likelihood of graft rejection. Using a mouse model, Itoh et al. demonstrated that drinking hydrogen-rich water could attenuate an immediate allergic reaction by inhibiting the phosphorylation of FcεRI-associated Lyn and its downstream signaling molecules, which subsequently reduced the generation of hydrogen peroxide and suppressed NADPH oxidase activity [32]. Hydrogen’s immunomodulatory effects have been investigated in both adaptive and innate immune responses. Hydrogen regulates T cell differentiation, dendritic cell function, and cytokine profiles, potentially leading to immune tolerance and prolonged graft survival [33]. In another study, T cell proliferation was significantly suppressed in the in vitro presence of hydrogen and accompanied by the lowered production of interferon-ɣ and IL-2 [34].
The hydrogen-related advantages described herein might hold significant implications for donors, grafts/organs, and recipients, manifesting anticipated organ-protective effects (Figure 1).
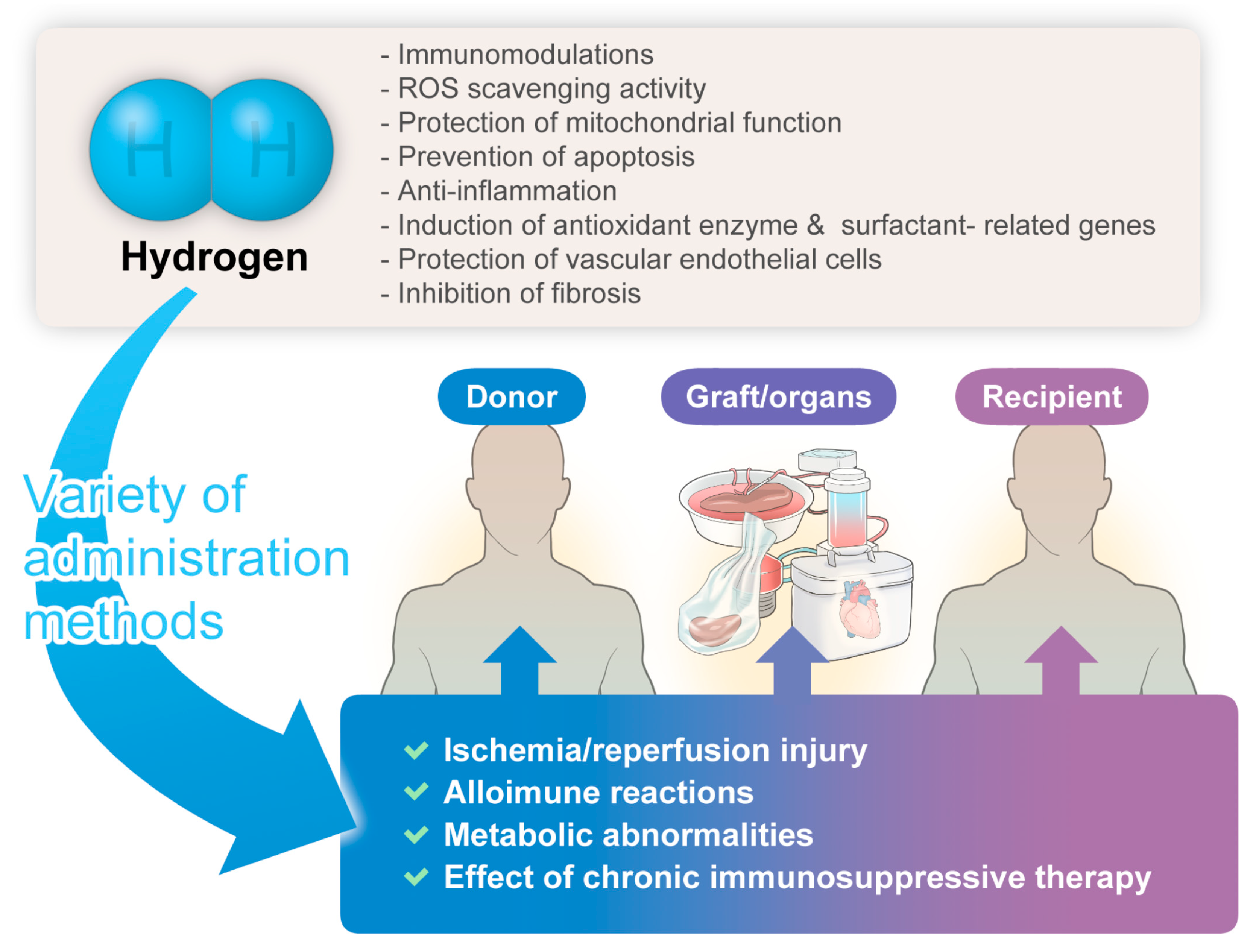
Figure 1. The significant benefits/potential of hydrogen gas therapy in transplantation medicine.
3. Hydrogen Applications in Transplantation
3.1. Hydrogen Gas Inhalation
Since hydrogen is a gaseous molecule, a safe concentration of hydrogen inhalation would be a straightforward delivery method for employing hydrogen as a therapeutic tool. Buchholz et al. demonstrated that hydrogen inhalation by recipients at a 2% concentration significantly dampened transplant-induced muscularis inflammation, mitigated bowel dysfunction, and prevented bowel dysmotility in rat intestinal transplant models [35]. In a lung transplantation brain death rat model, donor and recipient ventilation with 2% hydrogen hindered oxidative injuries by increasing the actions of superoxide dismutase and other antioxidants to protect lung function [36]. Kawamura et al. also indicated the efficacy of donor treatment with 2% hydrogen for three hours on lung allograft function in rats [37]. Zhang et al. demonstrated that one hour of donor treatment with 2% hydrogen inhalation significantly reduced liver injury after transplantation in a rat orthotopic liver transplant model [30].
3.2. Lung Inflation during Cold Preservation
Among transplantable organs, the lung is unique because it is an organ that contains air, allowing for the incorporation of hydrogen into the air within the alveoli. Lung inflation with 3% hydrogen gas during the cold ischemia phase alleviated lung graft injury, determined by inhibiting apoptosis and inflammatory responses, and improved graft function [38][39]. Similarly, Duan et al. investigated the efficacy of lung inflation during cold ischemia with 3% hydrogen + 40% oxygen + 57% nitrogen in a rat model and demonstrated that hydrogen exposure during cold ischemia improved donor lung quality by mitigating mitochondrial structural anomalies, enhancing mitochondrial function, and reducing apoptosis, inflammation, and oxidative stress, which may be achieved through activation of the Nrf2/HO-1 pathway [40].
3.3. Preservation Solution
The ongoing optimization of organ preservation solutions has always been an important component of donor protection for lung transplantation. Various ways of dissolving hydrogen gas in organ preservation solutions have been developed, including the use of electrolysis, a hydrogen gas cylinder, or a hydrogen-generating agent. The use of a hydrogen-rich preservation solution (more than 1.0 ppm) diminishes IRI in rat lungs during cold ischemia through anti-inflammatory and antioxidant effects [41]. Abe et al. demonstrated that 24 to 48 h of organ preservation in hydrogen-rich University of Wisconsin solution attenuated renal cold IRI in a syngeneic rat kidney transplantation model and was associated with less interstitial macrophage infiltration, tubular apoptosis, and oxidative stress in the kidney grafts, better renal graft function, and longer graft survival compared to simple cold storage [42].
Buchholz et al. indicated that luminal preservation, cold graft storage, and vascular flush in a hydrogen-bubbled preservation solution significantly preserved mucosal graft morphology and diminished graft malondialdehyde levels, showing significant reduction potential and weakened proinflammatory molecular responses within the re-perfused intestinal graft in rats [43].
Similarly, a hydrogen gas-containing organ preservation solution impeded the development of acute injuries in a donor’s kidney after cardiac death in a preclinical miniature pig model with an optimal immunosuppressive protocol based on the human clinical setting. A marginal kidney processed using a hydrogen gas-containing preservation solution was engrafted for longer than 100 days [44]. Kobayashi et al. studied a practical method of quickly dissolving hydrogen gas in organ preservation solutions using a canister containing a hydrogen-absorbing alloy [45]. After 30 min of warm ischemic injury induced by circulatory arrest, the donor kidneys were harvested and perfused for five minutes with a hydrogen-containing cold ET-Kyoto (ETK) solution in a miniature pig kidney transplantation model. Preservation in a hydrogen-containing solution for either one or four hours resulted in better renal function with more blood flow [45]. In another study, Kayawake et al. demonstrated that a hydrogen-rich preservation solution lessened IRI in a canine left lung transplantation model following 23 h of cold ischemia in an ETK solution. The graft function in this study, determined using blood gas analysis, significantly improved in the hydrogen-treated group, which was associated with lesser extents of lung edema and histopathological injury [46].
3.4. Hydrogen Flush after Cold Storage
Protective effects can be achieved by subjecting hydrogen to a single flush ex vivo, even without placing it in a storage solution. In a previous study, hydrogen flush after 24 h of cold ischemia significantly lowered transaminases, high-mobility group box protein 1 release, and portal venous pressure compared to vehicle-treated controls. The portal venous route maintained the sinusoidal endothelia, and the arterial route attenuated biliary damage [47].
3.5. Ex Vivo Perfusion
Ex vivo lung perfusion allows for the evaluation and recovery of an ex vivo donor lung by perfusion with normothermic perfusate. Haam et al. reported that lung graft ventilation after cardiac death with 2% hydrogen gas for four hours during ex vivo perfusion significantly mitigated inflammation-related lung injury and improved lung function in a porcine model [48]. Noda et al. used a rat lung ex vivo perfusion model and ventilated the lungs with air supplemented with 2% hydrogen for four hours. Hydrogen administration decreased proinflammatory changes through the upregulation of HO-1, promoted mitochondrial biogenesis, and significantly reduced lactate production. In addition, the expression of hypoxia-inducible factor-1 in the hydrogen-treated lungs was significantly attenuated. Thus, the preconditioning of lung grafts with inhaled hydrogen diminished these proinflammatory changes, promoted mitochondrial biogenesis in the lungs throughout the procedure, and resulted in better post-transplant graft function [49][50]. Ishikawa et al. reported that 90 min of reperfusion with oxygenated buffer with hydrogen at 37° on an isolated perfused rat liver apparatus significantly reduced the apoptosis, energy depletion, liver enzyme leakage, redox status, impaired microcirculation, and necrosis associated with increased bile production. The phosphorylation of cytoplasmic MKK4 and JNK were suppressed by means of hydrogen treatment [51].
3.6. Hydrogen Exposure Using a Hydrogen Bath
Noda et al. invented a unique cold storage device with a hydrogen-rich water bath, which enabled water saturation with hydrogen and maintained saturated hydrogen levels and a consistent temperature throughout the procedure. The grafts stored with the hydrogen-rich water bath also had a higher adenosine triphosphate content and less mitochondrial damage, which were associated with efficiently ameliorated myocardial injury [24]. The use of a hydrogen-rich water bath in which hydrogen was dissolved into a solution for liver graft tissues enabled superior morphologic and functional protection against IRI in a rat liver transplant model [52].
3.7. Venous/Intraperitoneal Injection
Luo et al. demonstrated that the intravenous administration of hydrogen-saturated saline to the recipient enhances the migration and proliferation capacity of bone marrow mesenchymal stem cells (BMSCs) to repair spinal cord injury by reducing the inflammatory response and oxidative stress in the injured area, suggesting that hydrogen and BMSC co-delivery is an effective method of improving BMSC transplantation in the treatment of spinal cord injury [53]. Intravenous hydrogen-rich saline administered via the tail vein at the beginning of reperfusion significantly diminished the severity of pancreatic IRI in rats, possibly by decreasing inflammation and oxidative stress [54].
3.8. Intraluminal Administration
The small intestine is a unique organ because it has both luminal and vascular routes through which preservation solutions can be administered. In addition to the vessel walls, the epithelial cell layers forming the mucosa and covering the inner part of the lumen are also highly susceptible to IRI and, thus, a potential therapeutic target [55]. Yamamoto et al. demonstrated that the intraluminal administration of hydrogen-rich saline regulated the loss of the transmembrane protein ZO-1 in the graft intestine and modulated IRI to the transplanted intestine in rats [56].
3.9. Oral Intake of a Hydrogen-Rich Solution
Oral consumption of hydrogen-rich solutions could be implemented easily into everyday clinical practice [57]. Solubilized hydrogen may be valuable since it is a safe, easily administered, and portable method of delivering hydrogen to the human body. Cardinal et al. showed that the oral intake of water containing dissolved hydrogen resulted in a sustained increase in the hydrogen levels in the serum and kidney, a better kidney allograft function over a 60-day follow-up period, and a reduction in the markers of inflammation and tissue oxidation. Noda et al. showed that drinking hydrogen-rich water prolongs the survival of cardiac allografts and decreases intimal hyperplasia in aortic allografts [48]. Furthermore, in another study, T cell proliferation was significantly restrained in the presence of hydrogen in vitro, accompanied by a lower production of interferon-ɣ and IL-2. In yet another study, hydrogen treatment was also associated with higher graft ATP levels and higher activity of the mitochondrial respiratory chain enzymes [58].
References
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109.
- Snijder, P.M.; van den Berg, E.; Whiteman, M.; Bakker, S.J.; Leuvenink, H.G.; van Goor, H. Emerging role of gasotransmitters in renal transplantation. Am. J. Transplant. 2013, 13, 3067–3075.
- Xie, F.; Song, Y.; Yi, Y.; Jiang, X.; Ma, S.; Ma, C.; Li, J.; Zhanghuang, Z.; Liu, M.; Zhao, P.; et al. Therapeutic Potential of Molecular Hydrogen in Metabolic Diseases from Bench to Bedside. Pharmaceuticals 2023, 16, 541.
- Zhu, B.; Cui, H.; Xu, W. Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis. Cancer Cell Int. 2021, 21, 70.
- Chen, J.B.; Kong, X.F.; Mu, F.; Lu, T.Y.; Lu, Y.Y.; Xu, K.C. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med. Gas Res. 2020, 10, 75–80.
- Niu, Y.; Nie, Q.; Dong, L.; Zhang, J.; Liu, S.F.; Song, W.; Wang, X.; Wu, G.; Song, D. Hydrogen Attenuates Allergic Inflammation by Reversing Energy Metabolic Pathway Switch. Sci. Rep. 2020, 10, 1962.
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982.
- Yang, F.; Lei, Y.; Liu, R.; Luo, X.; Li, J.; Zeng, F.; Lu, S.; Huang, X.; Lan, Y. Hydrogen: Potential Applications in Solid Organ Transplantation. Oxid. Med. Cell Longev. 2021, 2021, 6659310.
- Quan, L.; Zheng, B.; Zhou, H. Protective effects of molecular hydrogen on lung injury from lung transplantation. Exp. Biol. Med. 2021, 246, 1410–1418.
- Hasegawa, T.; Ito, M.; Hasegawa, S.; Teranishi, M.; Takeda, K.; Negishi, S.; Nishiwaki, H.; Takeda, J.I.; LeBaron, T.W.; Ohno, K. Molecular Hydrogen Enhances Proliferation of Cancer Cells That Exhibit Potent Mitochondrial Unfolded Protein Response. Int. J. Mol. Sci. 2022, 23, 2888.
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Huang, C.S.; Kawamura, T.; Lee, S.; Tochigi, N.; Shigemura, N.; Buchholz, B.M.; Kloke, J.D.; Billiar, T.R.; Toyoda, Y.; Nakao, A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit. Care. 2010, 14, R234.
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85.
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia--reperfusion injury by protecting mitochondrial function in rats. J. Surg. Res. 2014, 192, 564–572.
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med. Gas Res. 2011, 1, 24.
- Zhang, Y.; Chen, J.; Wu, H.; Li, L.; Yang, X.; Lai, K.; Bao, J.; Xie, K.; Yu, Y. Hydrogen regulates mitochondrial quality to protect glial cells and alleviates sepsis-associated encephalopathy by Nrf2/YY1 complex promoting HO-1 expression. Int. Immunopharmacol. 2023, 118, 110009.
- Zhang, G.; Li, Z.; Meng, C.; Kang, J.; Zhang, M.; Ma, L.; Zhou, H. The Anti-inflammatory Effect of Hydrogen on Lung Transplantation Model of Pulmonary Microvascular Endothelial Cells During Cold Storage Period. Transplantation 2018, 102, 1253–1261.
- Zhao, P.; Cai, Z.; Zhang, X.; Liu, M.; Xie, F.; Liu, Z.; Lu, S.; Ma, X. Hydrogen Attenuates Inflammation by Inducing Early M2 Macrophage Polarization in Skin Wound Healing. Pharmaceuticals 2023, 16, 885.
- Song, D.; Liu, X.; Diao, Y.; Sun, Y.; Gao, G.; Zhang, T.; Chen, K.; Pei, L. Hydrogen-rich solution against myocardial injury and aquaporin expression via the PI3K/Akt signaling pathway during cardiopulmonary bypass in rats. Mol. Med. Rep. 2018, 18, 1925–1938.
- Wang, X.; An, Z.; Liao, J.; Ran, N.; Zhu, Y.; Ren, S.; Meng, X.; Cui, N.; Yu, Y.; Fan, H. The Role and Mechanism of Hydrogen-Rich Water in the Cucumis sativus Response to Chilling Stress. Int. J. Mol. Sci. 2023, 24, 6702.
- Hu, Y.; Feng, X.; Chen, J.; Wu, Y.; Shen, L. Hydrogen-rich saline alleviates early brain injury through inhibition of necroptosis and neuroinflammation via the ROS/HO-1 signaling pathway after traumatic brain injury. Exp. Ther. Med. 2022, 23, 126.
- Tanaka, Y.; Shigemura, N.; Kawamura, T.; Noda, K.; Isse, K.; Stolz, D.B.; Billiar, T.R.; Toyoda, Y.; Bermudez, C.A.; Lyons-Weiler, J.; et al. Profiling molecular changes induced by hydrogen treatment of lung allografts prior to procurement. Biochem. Biophys. Res. Commun. 2012, 425, 873–879.
- Hu, Y.; Ma, Z.; Guo, Z.; Zhao, F.; Wang, Y.; Cai, L.; Yang, J. Type 1 Diabetes Mellitus is an Independent Risk Factor for Pulmonary Fibrosis. Cell Biochem. Biophys. 2014, 70, 1385–1391.
- Sun, Q.; Kawamura, T.; Masutani, K.; Peng, X.; Sun, Q.; Stolz, D.B.; Pribis, J.P.; Billiar, T.R.; Sun, X.; Bermudez, C.A.; et al. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc. Res. 2012, 94, 144–153.
- Chen, H.; Xie, K.; Han, H.; Li, Y.; Liu, L.; Yang, T.; Yu, Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int. Immunopharmacol. 2015, 28, 643–654.
- Aoyama-Ishikawa, M.; Seishu, A.; Kawakami, S.; Maeshige, N.; Miyoshi, M.; Ueda, T.; Usami, M.; Nakao, A.; Kotani, J. Intravenous immunoglobulin-induced neutrophil apoptosis in the lung during murine endotoxemia. Surg. Infect. 2014, 15, 36–42.
- Kawamura, T.; Huang, C.S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation 2010, 90, 1344–1351.
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Urushiyama, H.; Amenomori, S.; Kaneko-Togashi, M.; Kuwahara, N.; et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L415–L426.
- Huang, C.S.; Kawamura, T.; Peng, X.; Tochigi, N.; Shigemura, N.; Billiar, T.R.; Nakao, A.; Toyoda, Y. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem. Biophys. Res. Commun. 2011, 408, 253–258.
- Zhang, C.B.; Tang, Y.C.; Xu, X.J.; Guo, S.X.; Wang, H.Z. Hydrogen gas inhalation protects against liver ischemia/reperfusion injury by activating the NF-kappaB signaling pathway. Exp. Ther. Med. 2015, 9, 2114–2120.
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-Rich Saline Attenuates Acute Kidney Injury After Liver Transplantation via Activating p53-Mediated Autophagy. Transplantation 2016, 100, 563–570.
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149.
- Yuan, L.; Chen, X.; Qian, L.; Shen, J.; Cai, J. Administration of hydrogen-rich saline in mice with allogeneic hematopoietic stem-cell transplantation. Med. Sci. Monit. 2015, 21, 749–754.
- Noda, K.; Shigemura, N.; Tanaka, Y.; Kawamura, T.; Hyun Lim, S.; Kokubo, K.; Billiar, T.R.; Bermudez, C.A.; Kobayashi, H.; Nakao, A. A novel method of preserving cardiac grafts using a hydrogen-rich water bath. J. Heart Lung Transplant. 2013, 32, 241–250.
- Buchholz, B.M.; Kaczorowski, D.J.; Sugimoto, R.; Yang, R.; Wang, Y.; Billiar, T.R.; McCurry, K.R.; Bauer, A.J.; Nakao, A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008, 8, 2015–2024.
- Zhou, H.; Fu, Z.; Wei, Y.; Liu, J.; Cui, X.; Yang, W.; Ding, W.; Pan, P.; Li, W. Hydrogen inhalation decreases lung graft injury in brain-dead donor rats. J. Heart Lung Transplant. 2013, 32, 251–258.
- Kawamura, T.; Huang, C.S.; Peng, X.; Masutani, K.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Toyoda, Y.; Nakao, A. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery 2011, 150, 240–249.
- Zheng, P.; Kang, J.; Xing, E.; Zheng, B.; Wang, X.; Zhou, H. Lung Inflation With Hydrogen During the Cold Ischemia Phase Alleviates Lung Ischemia-Reperfusion Injury by Inhibiting Pyroptosis in Rats. Front. Physiol. 2021, 12, 699344.
- Liu, R.; Fang, X.; Meng, C.; Xing, J.; Liu, J.; Yang, W.; Li, W.; Zhou, H. Lung inflation with hydrogen during the cold ischemia phase decreases lung graft injury in rats. Exp. Biol. Med. 2015, 240, 1214–1222.
- Duan, L.; Quan, L.; Zheng, B.; Li, Z.; Zhang, G.; Zhang, M.; Zhou, H. Inflation using hydrogen improves donor lung quality by regulating mitochondrial function during cold ischemia phase. BMC Pulm. Med. 2023, 23, 213.
- Saito, M.; Chen-Yoshikawa, T.F.; Takahashi, M.; Kayawake, H.; Yokoyama, Y.; Kurokawa, R.; Hirano, S.I.; Date, H. Protective effects of a hydrogen-rich solution during cold ischemia in rat lung transplantation. J. Thorac. Cardiovasc. Surg. 2020, 159, 2110–2118.
- Abe, T.; Li, X.K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H.; et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012, 94, 14–21.
- Buchholz, B.M.; Masutani, K.; Kawamura, T.; Peng, X.; Toyoda, Y.; Billiar, T.R.; Bauer, A.J.; Nakao, A. Hydrogen-enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation 2011, 92, 985–992.
- Nishi, K.; Iwai, S.; Tajima, K.; Okano, S.; Sano, M.; Kobayashi, E. Prevention of Chronic Rejection of Marginal Kidney Graft by Using a Hydrogen Gas-Containing Preservation Solution and Adequate Immunosuppression in a Miniature Pig Model. Front. Immunol. 2020, 11, 626295.
- Kobayashi, E.; Sano, M. Organ preservation solution containing dissolved hydrogen gas from a hydrogen-absorbing alloy canister improves function of transplanted ischemic kidneys in miniature pigs. PLoS ONE 2019, 14, e0222863.
- Kayawake, H.; Chen-Yoshikawa, T.F.; Saito, M.; Yamagishi, H.; Yoshizawa, A.; Hirano, S.I.; Kurokawa, R.; Date, H. Protective Effects of a Hydrogen-Rich Preservation Solution in a Canine Lung Transplantation Model. Ann. Thorac. Surg. 2021, 111, 246–252.
- Tamaki, I.; Hata, K.; Okamura, Y.; Nigmet, Y.; Hirao, H.; Kubota, T.; Inamoto, O.; Kusakabe, J.; Goto, T.; Tajima, T.; et al. Hydrogen Flush After Cold Storage as a New End-Ischemic Ex Vivo Treatment for Liver Grafts Against Ischemia/Reperfusion Injury. Liver Transpl. 2018, 24, 1589–1602.
- Haam, S.; Lee, S.; Paik, H.C.; Park, M.S.; Song, J.H.; Lim, B.J.; Nakao, A. The effects of hydrogen gas inhalation during ex vivo lung perfusion on donor lungs obtained after cardiac death. Eur. J. Cardiothorac. Surg. 2015, 48, 542–547.
- Noda, K.; Shigemura, N.; Tanaka, Y.; Bhama, J.; D’Cunha, J.; Kobayashi, H.; Luketich, J.D.; Bermudez, C.A. Hydrogen Preconditioning During Ex Vivo Lung Perfusion Improves the Quality of Lung Grafts in Rats. Transplantation 2014, 98, 499–506.
- Dark, J. Hydrogen in lung reconditioning—More than just inflation. Transplantation 2014, 98, 497–498.
- Ishikawa, T.; Shimada, S.; Fukai, M.; Kimura, T.; Umemoto, K.; Shibata, K.; Fujiyoshi, M.; Fujiyoshi, S.; Hayasaka, T.; Kawamura, N.; et al. Post-reperfusion hydrogen gas treatment ameliorates ischemia reperfusion injury in rat livers from donors after cardiac death: A preliminary study. Surg. Today. 2018, 48, 1081–1088.
- Uto, K.; Sakamoto, S.; Que, W.; Shimata, K.; Hashimoto, S.; Sakisaka, M.; Narita, Y.; Yoshii, D.; Zhong, L.; Komohara, Y.; et al. Hydrogen-rich solution attenuates cold ischemia-reperfusion injury in rat liver transplantation. BMC Gastroenterol. 2019, 19, 25.
- Luo, S.; Wu, J.; Qiu, Y.; Xiao, B.; Xi, Y.; Yang, C.; Narita, Y.; Yoshii, D.; Zhong, L.; Komohara, Y.; et al. Hydrogen Promotes the Effectiveness of Bone Mesenchymal Stem Cell Transplantation in Rats with Spinal Cord Injury. Stem Cells Int. 2023, 2023, 8227382.
- Luo, Z.L.; Cheng, L.; Ren, J.D.; Fang, C.; Xiang, K.; Xu, H.T.; Tang, L.J.; Wang, T.; Tian, F.Z. Hydrogen-rich saline protects against ischemia/reperfusion injury in grafts after pancreas transplantations by reducing oxidative stress in rats. Mediat. Inflamm. 2015, 2015, 281985.
- Obara, T.; Yamamoto, H.; Aokage, T.; Igawa, T.; Nojima, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nakao, A.; Motterlini, R.; et al. Luminal Administration of a Water-soluble Carbon Monoxide-releasing Molecule (CORM-3) Mitigates Ischemia/Reperfusion Injury in Rats Following Intestinal Transplantation. Transplantation 2021, 106, 1365–1375.
- Yamamoto, H.; Aokage, T.; Igawa, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nojima, T.; Nakao, A.; Naito, H. Luminal preloading with hydrogen-rich saline ameliorates ischemia-reperfusion injury following intestinal transplantation in rats. Pediatr. Transplant. 2020, 24, e13848.
- Simon, A.R. Hydrogen-supplemented drinking water, just soda or an elixir of life? Transpl. Int. 2012, 25, 1211–1212.
- Noda, K.; Tanaka, Y.; Shigemura, N.; Kawamura, T.; Wang, Y.; Masutani, K.; Sun, X.; Toyoda, Y.; Bermudez, C.A.; Nakao, A. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl. Int. 2012, 25, 1213–1222.
More
Information
Subjects:
Transplantation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
571
Revisions:
2 times
(View History)
Update Date:
11 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




