Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hugo Plácido da Silva | -- | 1403 | 2024-01-11 04:43:51 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, R.; Fred, A.; Plácido Da Silva, H. Approaches for Atrial Fibrillation Detection. Encyclopedia. Available online: https://encyclopedia.pub/entry/53714 (accessed on 28 February 2026).
Silva R, Fred A, Plácido Da Silva H. Approaches for Atrial Fibrillation Detection. Encyclopedia. Available at: https://encyclopedia.pub/entry/53714. Accessed February 28, 2026.
Silva, Rafael, Ana Fred, Hugo Plácido Da Silva. "Approaches for Atrial Fibrillation Detection" Encyclopedia, https://encyclopedia.pub/entry/53714 (accessed February 28, 2026).
Silva, R., Fred, A., & Plácido Da Silva, H. (2024, January 11). Approaches for Atrial Fibrillation Detection. In Encyclopedia. https://encyclopedia.pub/entry/53714
Silva, Rafael, et al. "Approaches for Atrial Fibrillation Detection." Encyclopedia. Web. 11 January, 2024.
Copy Citation
Atrial Fibrillation (AFib) is a Cardiovascular Disease (CVD) characterized by the uncoordinated activity of the heart chambers, with the atria exhibiting irregular and high-rate electrical activity. Autoencoders (AEs) can be used as an automatic feature extraction tool, tailoring the resulting features to a specific classification task. By coupling an encoder to a classifier, it is possible to reduce the dimension of the Electrocardiogram (ECG) heartbeat waveforms and classify them.
atrial fibrillation detection
ECG morphological features
supervised autoencoder
automatic feature extraction
1. Introduction
Atrial Fibrillation (AFib) is a Cardiovascular Disease (CVD) characterized by the uncoordinated activity of the heart chambers, with the atria exhibiting irregular and high-rate electrical activity. It is the most frequent type of cardiac arrhythmia in the Western World, with an estimated prevalence of 46.3 million individuals worldwide [1][2], and it is one of the leading causes of stroke, as it increases the risk of having one by four to five times [3]. In addition, recent reports suggest that both global incidence and prevalence are on the rise, putting a greater strain on healthcare systems [4][5].
AFib occurs because of a lack of synchrony in atrial contraction since disorganized electrical impulses propagate throughout both atria. This can be caused by the appearance of ectopic pacemaker foci, or by structural and biochemical changes that delay regular electrical pulses [6]. Over time, risk factors and lack of treatment can deteriorate the atria’s condition, and AFib can progress from a paroxysmal to a permanent state, in a process known as atrial remodeling [7][8]. Because persistent AFib is linked to a higher risk of a severe stroke [9], AFib early diagnosis and monitoring are crucial to prevent further complications.
Diagnosing AFib is commonly made using the Electrocardiogram (ECG) to monitor the heart’s electrical activity (Figure 1). Both its rhythm and waveform morphology are changed by the presence of an irregular heart rate, the replacement of P-waves by fibrillatory waves (F-waves), and the lack of an isoelectric baseline [10].
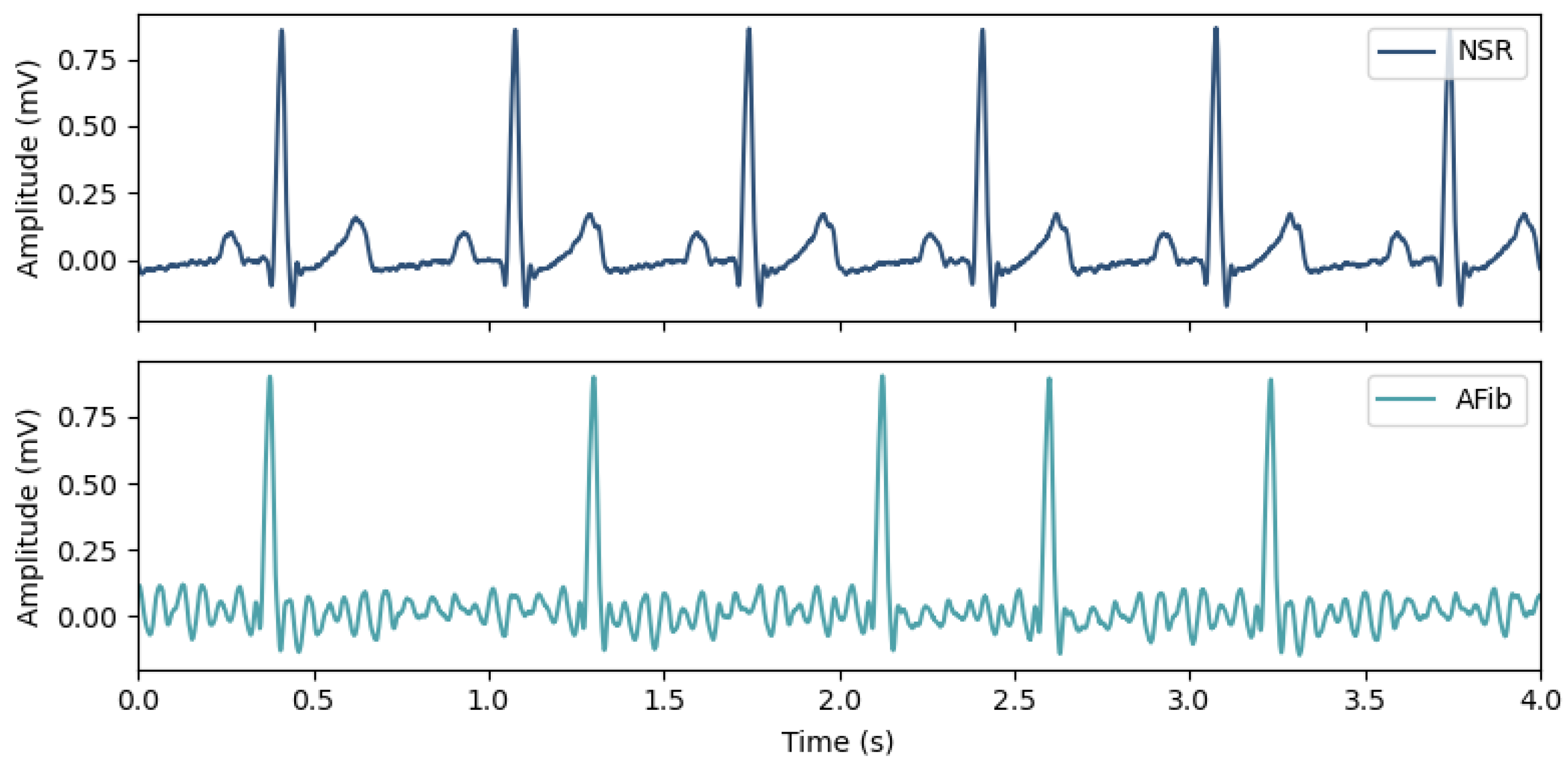
Figure 1. Morphological and rhythm changes between ECG recordings in Normal Sinus Rhythm (top) and in Atrial Fibrillation (bottom), where the absence of P-waves and the irregular heart rate are noticeable.
Since one cannot predict rare AFib events to occur while at the hospital, continuous monitoring of patients at risk can be achieved in other environments (e.g., home and/or ambulatory) by using implantable cardiac monitors, wearable patch monitors, and Holter monitoring systems [11][12][13]. Cardiovascular monitoring has also been extended to the general population using wrist-worn wearable devices, which can benefit from automatic detection of heart conditions [14]. More recently, off-the-person devices, increasingly dubbed as “invisibles”, are integrated with everyday use objects and do not require a conscious effort to perform data acquisition, providing a more pervasive way to detect CVDs [15][16][17][18].
Because of the overwhelming amount of data generated by these long-term acquisitions, many Computer-Aided Diagnosis (CAD) tools have been developed over the last decades to assist in AFib detection. State-of-the-art CAD examples include algorithms based on Artificial Neural Networks (ANNs), Support Vector Machines (SVMs), and Decision-Trees (DTs) [19]. Most of these algorithms rely on ventricular and signal features extracted from ECG recordings, such as RR-Intervals (RRIs) and associated variability metrics, frequency content, among others. However, despite AFib being an atrial phenomenon, very few algorithms rely on atrial features (e.g., atrial wave morphology), mainly because of the relatively low amplitudes of the atrial activity and high sensitivity to noise [19].
Given the large number of features that can be extracted, feature selection can be computationally expensive (given that it is an NP-hard problem), and, depending on the number of chosen features, feature extraction can compromise the algorithms’ responsiveness. This way, algorithms that do not rely on feature engineering (e.g., only using morphology) or that use optimized features for a specific problem (e.g., using automatic feature extraction tools) can be advantageous for real-time (or near real-time) and resource-limited systems.
2. Without Feature Engineering
Feature extraction and feature selection are two of the main ML tasks since their purpose is to provide algorithms with meaningful and compact data. In AFib detection, Heart-Rate Variability (HRV) metrics are often used to measure the irregularity of RR-Intervals [19][20]. Examples of such ventricular features include: Root Mean Square of Successive Differences (RMSSD), Poincaré plots, Sample Entropy (SampEn), Turning Point Ratio (TPR), and Lyapunov exponents. In addition, signal features can include signal power, frequency content, statistical measures, Wavelet Transform, phase-space analysis, among others.
Using a Deep Learning (DL) approach, Baalman et al. [21] developed a feedforward neural network focused on classifying ECG beats (i.e., single cycle) as NSR or AFib. This approach demonstrated the capacity of DL models to automatically extract adapted features to a specific classification task. In addition, the authors used an attention mechanism to highlight the model’s hidden features, thus providing explainable results (in contrast with the typical black-box paradigm). Using their own ECG data recorded at 500 Hz, the authors achieved an accuracy of 96% and an F1-score of 94% using Lead II ECG recordings and, using Lead I recordings, the model achieved an accuracy of 93% and an F1-score of 90%.
Based on computer vision strategies, Fan et al. [22] proposed a fusion of two deep convolutional neural networks (DCNNs) with different filter sizes. Using ECG signals in one dimension, the output of both DCNNs is concatenated and fed into a set of 3 fully connected layers that produce an output for the AFib/NSR classification with a Softmax activation function. This model was tested in single-lead short ECG recordings from the CinC2017 dataset, and it achieved a 96.99% accuracy using a 5 s time window, and a peak accuracy of 98.13% using a 20 s input sequence.
To tackle the limitation of CNN-based models to analyze variable-duration ECG recordings, Zhang et. al [23] proposed a time-adaptive structure that consists of multiple densely connected CNNs (DenseNets) and a Bidirectional Long Short-Term Memory (Bi-LSTM) cell. Using 10 s cropped single-lead ECG fragments, the binary classification performance was 99% and 87% for the CinC2017 and AFDB datasets, respectively.
3. Autoencoder-Based
AEs are well-known for their ability to perform dimensionality reduction and have been used in numerous applications. However, their usage for AFib detection has been very scarce.
Yuan et al. [24] developed an approach for AFib detection from ECG records using a stacked Sparse Autoencoder (SAE) based on 84 selected features extracted from the RR-Intervals and P-wave measurements within a 10 s ECG window. The AE used to achieve data compression had 84 input nodes and 2 hidden layers with 300 nodes each. AFib detection was made by stacking a Softmax activation function to the extracted features of the AE. Using ECG recordings from the MIT-BIH databases, the model first achieved a detection accuracy of 75.6%, and, after fine-tuning the model, a 98.3% accuracy was reported.
A similar approach was followed by Chen and Ying [25], where a stacked SAE receives 19 features extracted from the ECG records, including statistical measures, parameters from the Hilbert–Huang transform, and Wavelet decomposition features. After training, the AE is then coupled to a Softmax activation function to detect AFib; a 96.0% accuracy was achieved.
4. For Real-Time Implementation
Detecting AFib in real-time is challenging, requiring algorithms that are highly sensitive and specific while also being robust to noise and artifacts, with low computational requirements. Additionally, designing embedded systems for AFib detection must take into account hardware constraints, such as memory allocation and processing power, as well as the efficiency and number of operations of the algorithm.
To address these challenges, Chen and colleagues [26] proposed an edge computing framework for AFib detection based on an embedded platform that mainly uses feature engineering to collect ECG signals in real-time. The model used for AFib detection is lightweight and achieved an F1-score of about 90% with only 18 multiplications and 18 additions. Directly analyzing data at the edge can reduce the response time, making the proposed solution feasible for analysis and training on the edge.
In addition, Andersen et al. [27] proposed a DL-based approach for real-time automatic detection of AFib in long-term ECG recordings. Their proposed model, a combination of CNNs and RNNs, achieved high sensitivity and specificity of 98.98% and 96.95%, respectively, after being trained and validated on three different databases with a total of 89 subjects. The model also demonstrated computational efficiency, analyzing 24 h of ECG recordings in less than one second. The proposed algorithm was tested on unseen datasets, resulting in 98.96% and 86.04% for specificity and sensitivity, respectively, and was found to outperform existing state-of-the-art models evaluated on standard benchmark ECG datasets. By learning data-driven features to distinguish AFib from the remaining rhythms in the signals, the proposed method eliminates the need for traditional feature engineering.
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528.
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20.
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical Atrial Fibrillation and the Risk of Stroke. N. Engl. J. Med. 2012, 366, 120–129.
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global Epidemiology of Atrial Fibrillation: An Increasing Epidemic and Public Health Challenge. Int. J. Stroke 2021, 16, 217–221.
- Zhang, J.; Johnsen, S.P.; Guo, Y.; Lip, G.Y.H. Epidemiology of Atrial Fibrillation: Geographic and Ecological Risk Factors, Age, Sex, Genetics. Card. Electrophysiol. Clin. 2021, 13, 1–23.
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial Remodeling and Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2008, 1, 62–73.
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345.
- Kato, T.; Yamashita, T.; Sagara, K.; Iinuma, H.; Fu, L.T. Progressive Nature of Paroxysmal Atrial Fibrillation. Circ. J. 2004, 68, 568–572.
- Hagii, J.; Metoki, N.; Saito, S.; Shiroto, H.; Sasaki, S.; Takahashi, K.; Hitomi, H.; Baba, Y.; Yamada, N.; Seino, S.; et al. Persistent or Permanent Atrial Fibrillation is Associated with Severe Cardioembolic Stroke in Patients with Non-valvular Atrial Fibrillation. Thromb. J. 2021, 19, 22.
- Podrid, P.; Malhotra, R.; Kakkar, R.; Noseworthy, P. Podrid’s Real-World ECGs: Volume 4, Arrhythmias: A Master’s Approach to the Art and Practice of Clinical ECG Interpretation; Cardiotext Publishing: Minneapolis, MN, USA, 2015.
- Hickey, K.T.; Riga, T.C.; Mitha, S.A.; Reading, M.J. Detection and Management of Atrial Fibrillation Using Remote Monitoring. Nurse Pract. 2018, 43, 24–30.
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917.
- Perino, A.C.; Gummidipundi, S.E.; Lee, J.; Hedlin, H.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Gardner, R.M.; Cheung, L.; Hung, G.; et al. Arrhythmias Other Than Atrial Fibrillation in Those With an Irregular Pulse Detected With a Smartwatch: Findings From the Apple Heart Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e010063.
- Serhani, M.A.; El Kassabi, H.; Ismail, H.; Nujum Navaz, A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796.
- da Silva, H.P. The Biosignal C.A.O.S.: Reflections on the Usability of Physiological Sensing for Human-Computer Interaction Practitioners and Researchers. In Proceedings of the Converging Clinical and Engineering Research on Neurorehabilitation II; Ibáñez, J., González-Vargas, J., Azorín, J.M., Akay, M., Pons, J.L., Eds.; Biosystems & Biorobotics; Springer: Cham, Switzerland, 2017; pp. 807–811.
- da Silva, H.P.; Carreiras, C.; Lourenço, A.; Fred, A.; das Neves, R.C.; Ferreira, R. Off-the-person Electrocardiography: Performance Assessment and Clinical Correlation. Health Technol. 2015, 4, 309–318.
- Silva, A.S.; Correia, M.V.; Silva, H.P. Invisible ECG for High Throughput Screening in eSports. Sensors 2021, 21, 7601.
- dos Santos Silva, A.; Almeida, H.; da Silva, H.P.; Oliveira, A. Design and Evaluation of a Novel Approach to Invisible Electrocardiography (ECG) in Sanitary Facilities Using Polymeric Electrodes. Sci. Rep. 2021, 11, 6222.
- Wesselius, F.J.; van Schie, M.S.; De Groot, N.M.S.; Hendriks, R.C. Digital Biomarkers and Algorithms for Detection of Atrial Fibrillation Using Surface Electrocardiograms: A Systematic Review. Comput. Biol. Med. 2021, 133, 104404.
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258.
- Baalman, S.W.E.; Schroevers, F.E.; Oakley, A.J.; Brouwer, T.F.; Stuijt, W.v.d.; Bleijendaal, H.; Ramos, L.A.; Lopes, R.R.; Marquering, H.A.; Knops, R.E.; et al. A Morphology Based Deep Learning Model for Atrial Fibrillation Detection Using Single Cycle Electrocardiographic Samples. Int. J. Cardiol. 2020, 316, 130–136.
- Fan, X.; Yao, Q.; Cai, Y.; Miao, F.; Sun, F.; Li, Y. Multiscaled Fusion of Deep Convolutional Neural Networks for Screening Atrial Fibrillation from Single Lead Short ECG Recordings. IEEE J. Biomed. Health Inform. 2018, 22, 1744–1753.
- Zhang, X.; Jiang, M.; Polat, K.; Alhudhaif, A.; Hemanth, J.; Wu, W. Detection of Atrial Fibrillation from Variable-Duration ECG Signal Based on Time-Adaptive Densely Network and Feature Enhancement Strategy. IEEE J. Biomed. Health Inform. 2023, 27, 944–955.
- Yuan, C.; Yan, Y.; Zhou, L.; Bai, J.; Wang, L. Automated Atrial Fibrillation Detection Based on Deep Learning Network. In Proceedings of the 2016 IEEE International Conference on Information and Automation (ICIA), Ningbo, China, 1–3 August 2016; pp. 1159–1164.
- Chen, L.; He, Y. Identification of Atrial Fibrillation from Electrocardiogram Signals Based on Deep Neural Network. J. Med Imaging Health Inform. 2019, 9, 838–846.
- Chen, J.; Zheng, Y.; Liang, Y.; Zhan, Z.; Jiang, M.; Zhang, X.; da Silva, D.S.; Wu, W.; Albuquerque, V.H.C.d. Edge2Analysis: A Novel AIoT Platform for Atrial Fibrillation Recognition and Detection. IEEE J. Biomed. Health Inform. 2022, 26, 5772–5782.
- Andersen, R.S.; Peimankar, A.; Puthusserypady, S. A Deep Learning Approach for Real-Time Detection of Atrial Fibrillation. Expert Syst. Appl. 2019, 115, 465–473.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
413
Revision:
1 time
(View History)
Update Date:
11 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





