
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nourhan Hassan | -- | 5246 | 2024-01-10 14:02:21 |
Video Upload Options
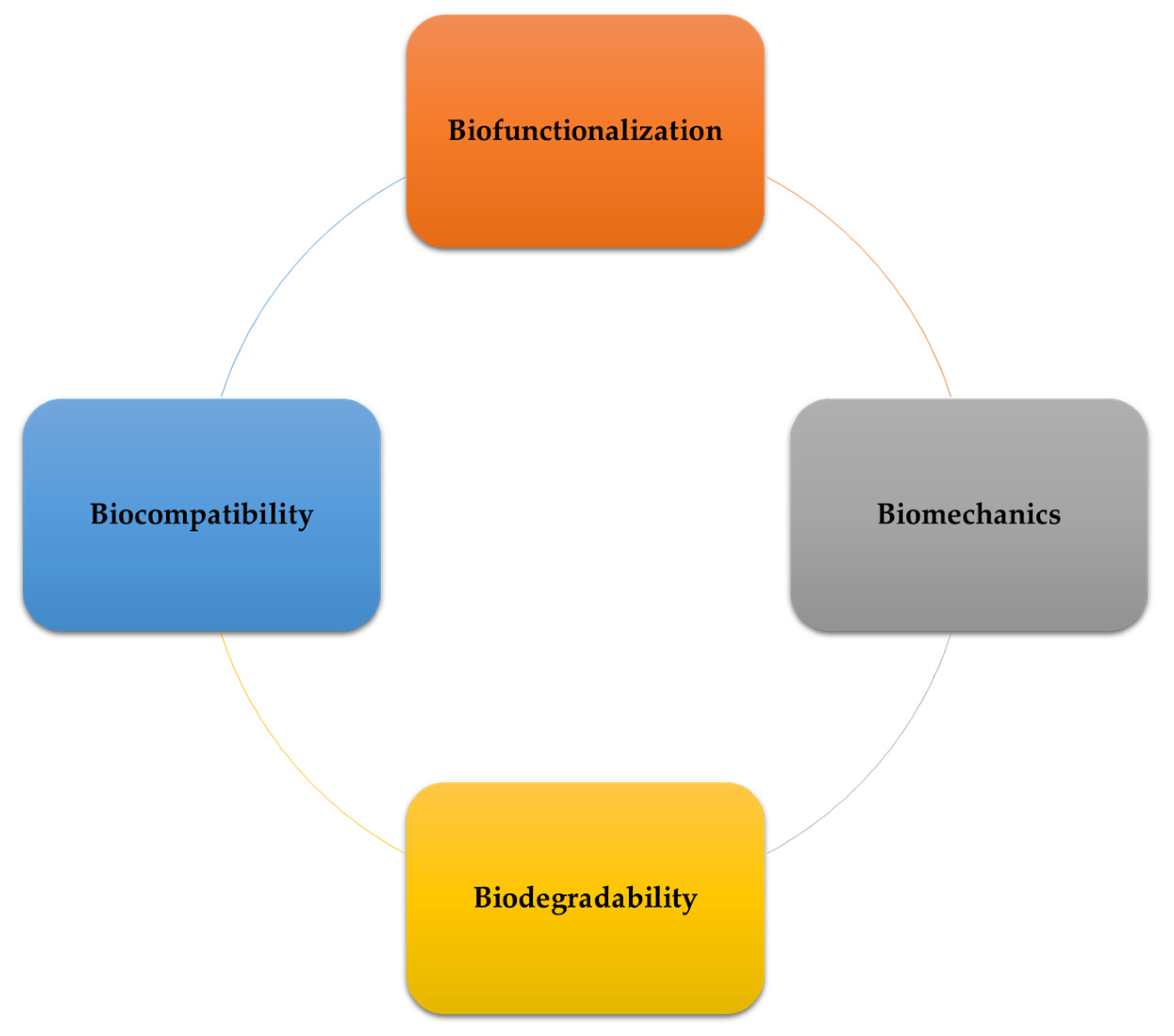
The utilization of materials in medical implants, serving as substitutes for non-functional biological structures, supporting damaged tissues, or reinforcing active organs, holds significant importance in modern healthcare, positively impacting the quality of life for millions of individuals worldwide. Biodegradable metals, including zinc (Zn), magnesium (Mg), iron, and others, present a new paradigm in the realm of implant materials. Research focuses on developing optimized materials that meet medical standards, encompassing controllable corrosion rates, sustained mechanical stability, and favorable biocompatibility. Achieving these objectives involves refining alloy compositions and tailoring processing techniques to carefully control microstructures and mechanical properties. Among the materials under investigation, Mg- and Zn-based biodegradable materials and their alloys demonstrate the ability to provide necessary support during tissue regeneration while gradually degrading over time. Furthermore, as essential elements in the human body, Mg and Zn offer additional benefits, including promoting wound healing, facilitating cell growth, and participating in gene generation while interacting with various vital biological functions.
1. Introduction
2. Mechanotransduction in Skin
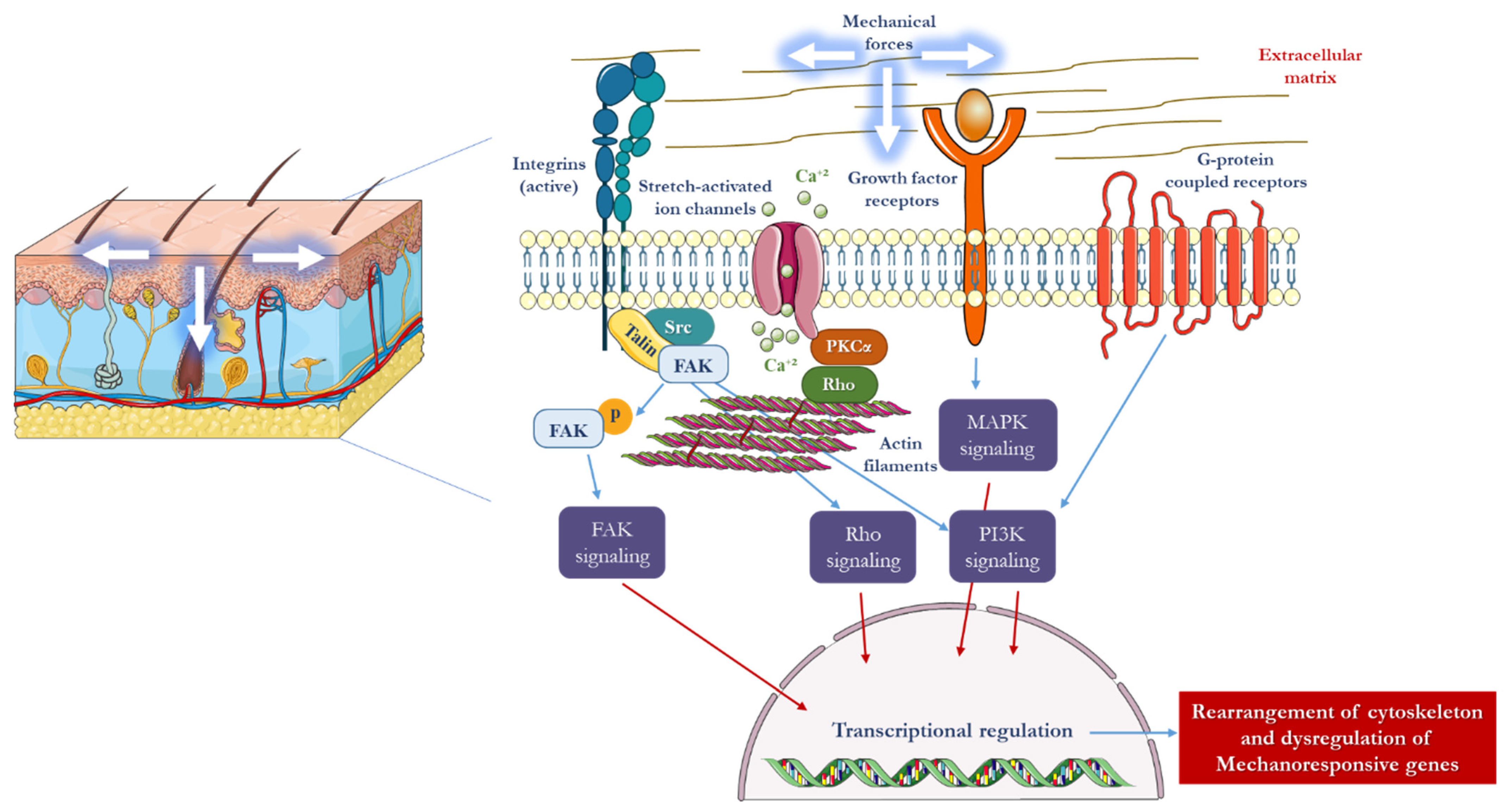
3. The Influence of Mechanical Forces on the Structure and Function of the Skin
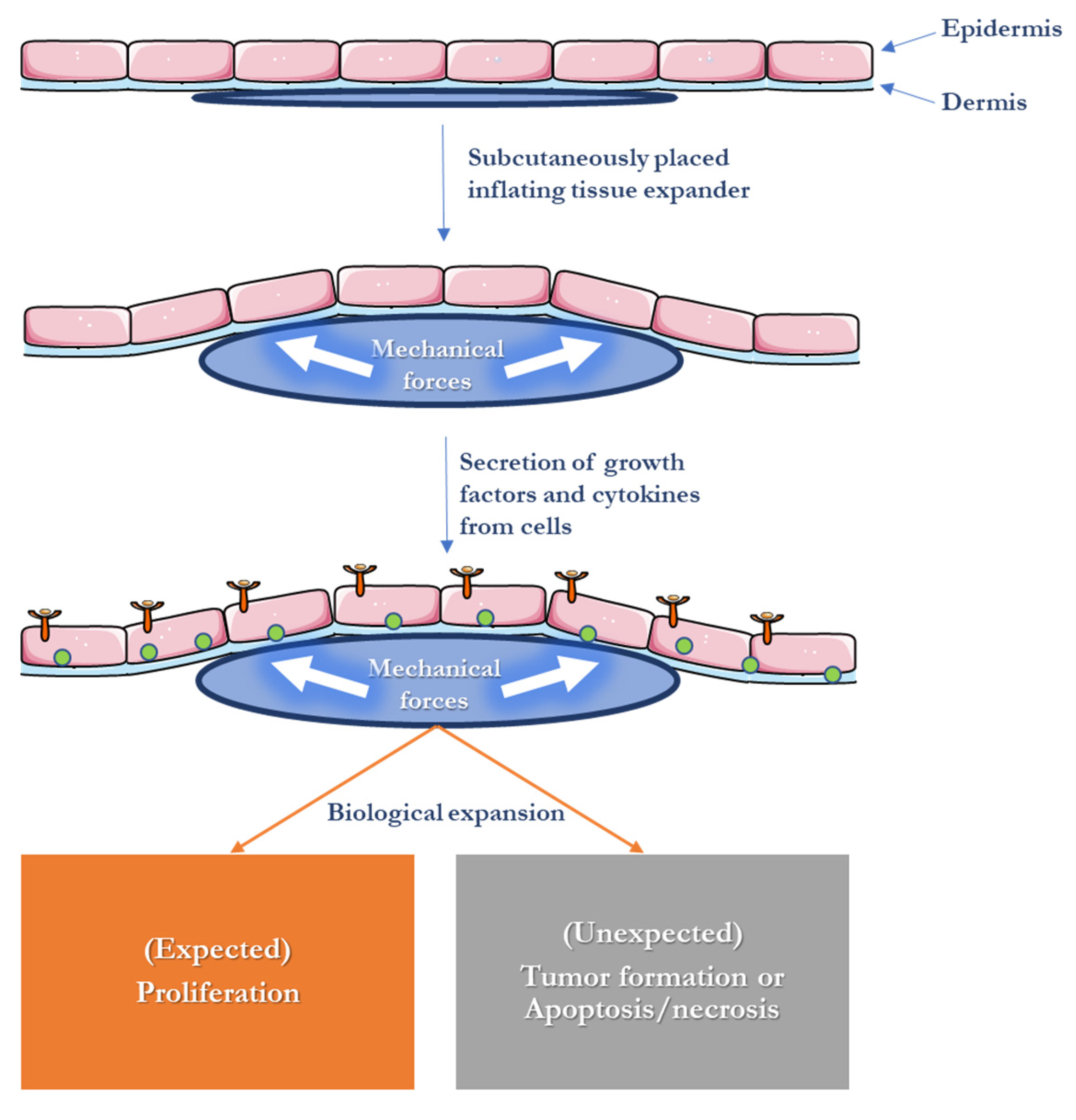
4. Skin Expansion in Reconstructive Surgery
5. Scaffold Fabrication Methods Used for Tissue Engineering
6. Materials Used for Skin Tissue Engineering
6.1. Natural Materials
6.2. Synthetic Bioresorbable Polymers
6.3. Absorbable Metallic Materials
7. Experimental Studies of Magnesium and Zinc in Soft Tissue Engineering
References
- Ingber, D. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991, 3, 841–848.
- Davidson, J.M.; Aquino, A.M.; Woodward, S.C.; Wilfinger, W.W. Sustained microgravity reduces intrinsic wound healing and growth factor responses in the rat. FASEB J. 1999, 13, 325–329.
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity Alters Cancer Growth and Progression. Curr. Cancer Drug Targets 2014, 14, 394–406.
- Farahani, R.M.; A DiPietro, L. Microgravity and the implications for wound healing. Int. Wound J. 2008, 5, 552–561.
- Evans, N.D.; Oreffo, R.; Healy, E.; Thurner, P.; Man, Y. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. 2013, 28, 397–409.
- Silver, F.H.; Siperko, L.M.; Seehra, G.P. Mechanobiology of force transduction in dermal tissue. Ski. Res. Technol. 2003, 9, 3–23.
- Tranquillo, R.T.; Durrani, M.A.; Moon, A.G. Tissue engineering science: Consequences of cell traction force. Cytotechnology 1992, 10, 225–250.
- Takei, T.; Mills, I.; Arai, K.; Sumpio, B.E. Molecular Basis for Tissue Expansion: Clinical Implications for the Surgeon. Plast. Reconstr. Surg. 1998, 102, 247–258.
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nature 1999, 1, E131–E138.
- Wang, J.H.-C.; Thampatty, B.P. An Introductory Review of Cell Mechanobiology. Biomech. Model. Mechanobiol. 2006, 5, 1–16.
- Huang, S.; Ingber, D.E. Shape-Dependent Control of Cell Growth, Differentiation, and Apoptosis: Switching between Attractors in Cell Regulatory Networks. Exp. Cell Res. 2000, 261, 91–103.
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A Comparison of Conservative Versus Early Excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547–553; discussion 552–553.
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926.
- Karp, J.M.; Langer, R. Development and therapeutic applications of advanced biomaterials. Curr. Opin. Biotechnol. 2007, 18, 454–459.
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601.
- Tepole, A.B.; Ploch, C.J.; Wong, J.; Gosain, A.K.; Kuhl, E. Growing skin: A computational model for skin expansion in reconstructive surgery. J. Mech. Phys. Solids 2011, 59, 2177–2190.
- Neumann, C.G. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast. Reconstr. Surg. 1957, 19, 124–130.
- Wagh, M.; Dixit, V. Tissue expansion: Concepts, techniques and unfavourable results. Indian J. Plast. Surg. 2013, 46, 333–348.
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492.
- Wong, V.W.; Longaker, M.T.; Gurtner, G.C. Soft tissue mechanotransduction in wound healing and fibrosis. Semin. Cell Dev. Biol. 2012, 23, 981–986.
- Zöllner, A.M.; Tepole, A.B.; Kuhl, E. On the biomechanics and mechanobiology of growing skin. J. Theor. Biol. 2012, 297, 166–175.
- Wang, J.H.-C.; Thampatty, B.P.; Lin, J.-S.; Im, H.-J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391, 1–15.
- Derderian, C.A.; Bastidas, N.; Lerman, O.Z.; Bhatt, K.A.; Lin, S.-E.; Voss, J.; Holmes, J.W.; Levine, J.P.; Gurtner, G.C. Mechanical Strain Alters Gene Expression in an in Vitro Model of Hypertrophic Scarring. Ann. Plast. Surg. 2005, 55, 69–75; discussion 75.
- Silver, F.H.; Siperko, L.M. Mechanosensing and Mechanochemical Transduction: How Is Mechanical Energy Sensed and Converted Into Chemical Energy in an Extracellular Matrix? Crit. Rev. Biomed. Eng. 2003, 31, 78.
- Wong, V.; Levi, K.; Akaishi, S.; Schultz, G.; Dauskardt, R. Scar zones: Region-specific differences in skin tension may determine incisional scar formation. Plast. Reconstr. Surg. 2012, 129, 1272–1276.
- Montesano, R.; Orci, L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: Implications for wound healing. Proc. Natl. Acad. Sci. USA 1988, 85, 4894–4897.
- Halfter, W.; Liverani, D.; Vigny, M.; Monard, D. Deposition of extracellular matrix along the pathways of migrating fibroblasts. Cell Tissue Res. 1990, 262, 467–481.
- Sander, E.A.; Barocas, V.H.; Tranquillo, R.T. Initial Fiber Alignment Pattern Alters Extracellular Matrix Synthesis in Fibroblast-Populated Fibrin Gel Cruciforms and Correlates with Predicted Tension. Ann. Biomed. Eng. 2010, 39, 714–729.
- Wong, V.W.; Akaishi, S.; Longaker, M.T.; Gurtner, G.C. Pushing Back: Wound Mechanotransduction in Repair and Regeneration. J. Investig. Dermatol. 2011, 131, 2186–2196.
- Ko, K.S.; McCulloch, C.A. Intercellular Mechanotransduction: Cellular Circuits That Coordinate Tissue Responses to Mechanical Loading. Biochem. Biophys. Res. Commun. 2001, 285, 1077–1083.
- Geffeney, S.L.; Goodman, M.B. How We Feel: Ion Channel Partnerships that Detect Mechanical Inputs and Give Rise to Touch and Pain Perception. Neuron 2012, 74, 609–619.
- Powell, H.M.; McFarland, K.L.; Butler, D.L.; Supp, D.M.; Boyce, S.T. Uniaxial Strain Regulates Morphogenesis, Gene Expression, and Tissue Strength in Engineered Skin. Tissue Eng. Part A 2010, 16, 1083–1092.
- De Filippo, R.E.; Atala, A. Stretch and Growth: The Molecular and Physiologic Influences of Tissue Expansion. Plast. Reconstr. Surg. 2002, 109, 2450–2462.
- Tepole, A.B.; Gart, M.; Gosain, A.K.; Kuhl, E. Characterization of living skin using multi-view stereo and isogeometric analysis. Acta Biomater. 2014, 10, 4822–4831.
- Wilhelmi, B.J.; Blackwell, S.J.; Mancoll, J.S.; Phillips, L.G. Creep vs. Stretch: A Review of the Viscoelastic Properties of Skin. Ann. Plast. Surg. 1998, 41, 215–219.
- LoGiudice, J.; Gosain, A.K. Pediatric Tissue Expansion: Indications and Complications. J. Craniofacial Surg. 2003, 14, 866–872.
- Filho, P.T.B.; Neves, R.I.; Gemperli, R.; Kaweski, S.; Kahler, S.H.; Banducci, D.R.; Manders, E.K. Soft-Tissue Expansion in Lower Extremity Reconstruction. Clin. Plast. Surg. 1991, 18, 593–599.
- Beauchene, J.; Chambers, M.; Peterson, A.; Scott, P. Biochemical, biomechanical, and physical changes in the skin in an experimental animal model of therapeutic tissue expansion. J. Surg. Res. 1989, 47, 507–514.
- Pietramaggiori, G.; Liu, P.; Scherer, S.S.; Kaipainen, A.; Prsa, M.J.; Mayer, H.; Newalder, J.; Alperovich, M.; Mentzer, S.J.; Konerding, M.A.; et al. Tensile Forces Stimulate Vascular Remodeling and Epidermal Cell Proliferation in Living Skin. Ann. Surg. 2007, 246, 896–902.
- Purnell, C.A.; Gart, M.S.; Buganza-Tepole, A.; Tomaszewski, J.P.; Topczewska, J.M.; Kuhl, E.; Gosain, A.K. Determining the Differential Effects of Stretch and Growth in Tissue-Expanded Skin: Combining Isogeometric Analysis and Continuum Mechanics in a Porcine Model. Dermatol. Surg. 2018, 44, 48–52.
- Huang, C.; Akaishi, S.; Ogawa, R. Mechanosignaling pathways in cutaneous scarring. Arch. Dermatol. Res. 2012, 304, 589–597.
- Hinz, B.; Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003, 14, 538–546.
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261.
- Ismavel, R.; Samuel, S.; Boopalan, P.R.J.V.C.; Chittaranjan, S.B. A Simple Solution for Wound Coverage by Skin Stretching. J. Orthop. Trauma 2011, 25, 127–132.
- Lancerotto, L.; Chin, M.S.; Freniere, B.; Lujan-Hernandez, J.R.; Li, Q.; Vasquez, A.V.; Bassetto, F.; Del Vecchio, D.A.; Lalikos, J.F.; Orgill, D.P. Mechanisms of Action of External Volume Expansion Devices. Plast. Reconstr. Surg. 2013, 132, 569–578.
- Ingber, D.E. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599.
- Orgill, D.P.; Bayer, L. Negative pressure wound therapy: Past, present and future. Int. Wound J. 2013, 10 (Suppl. S1), 15–19.
- Lancerotto, L.; Bayer, L.R.; Orgill, D.P. Mechanisms of action of microdeformational wound therapy. Semin. Cell Dev. Biol. 2012, 23, 987–992.
- Omar, M.T.; Alghadir, A.; Al-Wahhabi, K.K.; Al-Askar, A.B. Efficacy of shock wave therapy on chronic diabetic foot ulcer: A single-blinded randomized controlled clinical trial. Diabetes Res. Clin. Pract. 2014, 106, 548–554.
- Ottomann, C.; Stojadinovic, A.; Lavin, P.T.; Gannon, F.H.; Heggeness, M.H.; Thiele, R.; Schaden, W.; Hartmann, B. Prospective Randomized Phase II Trial of Accelerated Reepithelialization of Superficial Second-Degree Burn Wounds Using Extracorporeal Shock Wave Therapy. Ann. Surg. 2012, 255, 23–29.
- Ennis, W.J.; Foremann, P.; Mozen, N.; Massey, J.; Conner-Kerr, T.; Meneses, P. Ultrasound therapy for recalcitrant diabetic foot ulcers: Results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manag. 2005, 51, 24–39.
- Kloth, L.C. Electrical Stimulation for Wound Healing: A Review of Evidence from In Vitro Studies, Animal Experiments, and Clinical Trials. Int. J. Low. Extrem. Wounds 2005, 4, 23–44.
- El-Sabbagh, A.H. Negative pressure wound therapy: An update. Chin. J. Traumatol. 2017, 20, 103–107.
- Wiegand, C.; White, R. Microdeformation in wound healing. Wound Repair Regen. 2013, 21, 793–799.
- Nuutila, K.; Siltanen, A.; Peura, M.; Harjula, A.; Nieminen, T.; Vuola, J.; Kankuri, E.; Aarnio, P. Gene expression profiling of negative-pressure-treated skin graft donor site wounds. Burns 2013, 39, 687–693.
- McNulty, A.K.; Schmidt, M.; Feeley, T.; Kieswetter, K. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen. 2007, 15, 838–846.
- Greene, A.K.; Puder, M.; Roy, R.; Arsenault, D.; Kwei, S.; Moses, M.; Orgill, D. Microdeformational wound therapy: Effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann. Plast. Surg. 2006, 56, 418–422.
- Lu, F.; Ogawa, R.; Nguyen, D.T.; Chen, B.; Guo, D.; Helm, D.L.; Zhan, Q.; Murphy, G.F.; Orgill, D.P. Microdeformation of Three-Dimensional Cultured Fibroblasts Induces Gene Expression and Morphological Changes. Ann. Plast. Surg. 2011, 66, 296–300.
- Junker, J.P.; Kamel, R.A.; Caterson, E.; Eriksson, E.; Nuutila, K.; Patil, P.S.; Fathollahipour, S.; Inmann, A.; Pant, A.; Amini, R.; et al. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356.
- Walmsley, G.G.; Maan, Z.N.; Wong, V.W.; Duscher, D.; Hu, M.S.; Zielins, E.R.; Wearda, T.; Muhonen, E.; McArdle, A.; Tevlin, R.; et al. Scarless wound healing: Chasing the holy grail. Plast. Reconstr. Surg. 2015, 135, 907–917.
- Ud-Din, S.; Volk, S.W.; Bayat, A. Regenerative healing, scar-free healing and scar formation across the species: Current concepts and future perspectives. Exp. Dermatol. 2014, 23, 615–619.
- Nauta, A.; Gurtner, G.; Longaker, M. Wound healing and regenerative strategies. Oral Dis. 2011, 17, 541–549.
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43.
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013, 19, 555–564.
- Akaishi, S.; Akimoto, M.; Hyakusoku, H.; Ogawa, R. 142B: The relationship between keloid growth pattern and stretching tension-visual analysis using the finite element method. Ann Plast. Surg. 2008, 60, 445–451.
- Akaishi, S.; Ogawa, R.; Hyakusoku, H. Keloid and hypertrophic scar: Neurogenic inflammation hypotheses. Med. Hypotheses 2008, 71, 32–38.
- Ogawa, R.; Okai, K.; Tokumura, F.; Mori, K.; Ohmori, Y.; Huang, C.; Hyakusoku, H.; Akaishi, S. The relationship between skin stretching/contraction and pathologic scarring: The important role of mechanical forces in keloid generation. Wound Repair Regen. 2012, 20, 149–157.
- Ogawa, R. Mechanobiology of scarring. Wound Repair Regen. 2011, 19 (Suppl. S1), s2–s9.
- Ogawa, R.; Akaishi, S.; Kuribayashi, S.; Miyashita, T. Keloids and Hypertrophic Scars Can Now Be Cured Completely: Recent Progress in Our Understanding of the Pathogenesis of Keloids and Hypertrophic Scars and the Most Promising Current Therapeutic Strategy. J. Nippon. Med. Sch. 2016, 83, 46–53.
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2006, 4, 413–437.
- Rajendran, A.K.; Sankar, D.; Amirthalingam, S.; Kim, H.D.; Rangasamy, J.; Hwang, N.S. Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: A brief review. Biomater. Res. 2023, 27, 1–24.
- Butler, D.L.; Goldstein, S.A.; Guldberg, R.E.; Guo, X.E.; Kamm, R.; Laurencin, C.T.; McIntire, L.V.; Mow, V.C.; Nerem, R.M.; Sah, R.L.; et al. The Impact of Biomechanics in Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2009, 15, 477–484.
- Huang, C.-Y.; Mow, V.C.; Ateshian, G.A. The Role of Flow-Independent Viscoelasticity in the Biphasic Tensile and Compressive Responses of Articular Cartilage. J. Biomech. Eng. 2001, 123, 410–417.
- Walker, M.; Godin, M.; Harden, J.L.; Pelling, A.E. Time dependent stress relaxation and recovery in mechanically strained 3D microtissues. APL Bioeng. 2020, 4, 036107.
- Onal, S.; Alkaisi, M.M.; Nock, V. Microdevice-based mechanical compression on living cells. iScience 2022, 25, 105518.
- Ning, L.; Gil, C.J.; Hwang, B.; Theus, A.S.; Perez, L.; Tomov, M.L.; Bauser-Heaton, H.; Serpooshan, V. Biomechanical factors in three-dimensional tissue bioprinting. Appl. Phys. Rev. 2020, 7, 041319.
- Kaner, D.; Friedmann, A. Soft tissue expansion with self-filling osmotic tissue expanders before vertical ridge augmentation: A proof of principle study. J. Clin. Periodontol. 2010, 38, 95–101.
- Mertens, C.; Thiele, O.; Engel, M.; Seeberger, R.; Hoffmann, J.; Freier, K. The Use of Self-Inflating Soft Tissue Expanders Prior to Bone Augmentation of Atrophied Alveolar Ridges. Clin. Implant. Dent. Relat. Res. 2013, 17, 44–51.
- Johnson, T.M.; Lowe, L.; Brown, M.D.; Sullivan, M.J.; Nelson, B.R. Histology and Physiology of Tissue Expansion. J. Dermatol. Surg. Oncol. 1993, 19, 1074–1078.
- Rivera, R.; LoGiudice, J.; Gosain, A.K. Tissue expansion in pediatric patients. Clin. Plast. Surg. 2005, 32, 35–44.
- Uijlenbroek, H.J.J.; Liu, Y.; He, J.F.; Visscher, C.; van Waas, M.A.J.; Wismeyer, D. Expanding soft tissue with Osmed® tissue expanders in the goat maxilla. Clin. Oral Implant. Res. 2010, 22, 121–128.
- Berge, S.J.; Wiese, K.G.; von Lindern, J.J.; Niederhagen, B.; Appel, T.; Reich, R.H. Tissue expansion using osmotically active hydrogel systems for direct closure of the donor defect of the radial forearm flap. Plast. Reconstr. Surg. 2001, 108, 1–5, discussion 6–7.
- Ronert, M.A.M.; Hofheinz, H.M.; Manassa, E.M.; Asgarouladi, H.; Olbrisch, R.R.M. The Beginning of a New Era in Tissue Expansion: Self-Filling Osmotic Tissue Expander—Four-Year Clinical Experience. Plast. Reconstr. Surg. 2004, 114, 1025–1031.
- Obdeijn, M.C.; Nicolai, J.-P.A.; Werker, P.M. The osmotic tissue expander: A three-year clinical experience. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1219–1222.
- Chummun, S.; Addison, P.; Stewart, K.J. The osmotic tissue expander: A 5-year experience. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 2128–2132.
- Arneja, J.S.; Gosain, A.K. Giant congenital melanocytic nevi. Plast. Reconstr. Surg. 2009, 124 (Suppl. S1), 1e–13e.
- Moustafa, D.; Blundell, A.R.; Hawryluk, E.B. Congenital melanocytic nevi. Curr. Opin. Pediatr. 2020, 32, 491–497.
- Formby, P.; Flint, J.; Gordon, W.T.; Fleming, M.; Andersen, R.C. Use of a Continuous External Tissue Expander in the Conversion of a Type IIIB Fracture to a Type IIIA Fracture. Orthopedics 2013, 36, e249–e251.
- Radovan, C. Breast Reconstruction after Mastectomy Using the Temporary Expander. Plast. Reconstr. Surg. 1982, 69, 207–208.
- Argenta, L.C.M.; Marks, M.W.M.; Grabb, W.C.M. Selective Use of Serial Expansion in Breast Reconstruction. Ann. Plast. Surg. 1983, 11, 188–195.
- Brobmann, G.F.; Huber, J. Effects of Different-Shaped Tissue Expanders on Transluminal Pressure, Oxygen Tension, Histopathologic Changes, and Skin Expansion in Pigs. Plast. Reconstr. Surg. 1985, 76, 731–745.
- Van Rappard, J.H.; Molenaar, J.; Van Doorn, K.; Sonneveld, G.J.; Borghouts, J.M. Surface-area increase in tissue expansion. Plast. Reconstr. Surg. 1988, 82, 833–839.
- Pietila, J.P. Tissue expansion and skin circulation. Simultaneous monitoring by laser Doppler flowmetry and transcutaneous oximetry. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 135–140.
- Van Damme, P.A.; Heidbüchel, K.L.; Kuijpers-Jagtman, A.-M.; Maltha, J.C.; Freihofer, H.P.M. Cranio-maxillo-facial tissue expansion, experimentally based or clinically empiric? A review of the literature. J. Cranio Maxillofac. Surg. 1992, 20, 61–69.
- Austad, E.D.; Rose, G.L. A self-inflating tissue expander. Plast. Reconstr. Surg. 1982, 70, 588–594.
- Wiese, K. Osmotically induced tissue expansion with hydrogels: A new dimension in tissue expansion? A preliminary report. J. Cranio-Maxillofac. Surg. 1993, 21, 309–313.
- Wiese, K.G.; Vogel, M.; Guthoff, R.; Gundlach, K.K. Treatment of congenital anophthalmos with self-inflating polymer expanders: A new method. J. Cranio-Maxillofac. Surg. 1999, 27, 72–76.
- Wiese, K.G.; Heinemann, D.E.H.; Ostermeier, D.; Peters, J.H. Biomaterial properties and biocompatibility in cell culture of a novel self-inflating hydrogel tissue expander. J. Biomed. Mater. Res. 2000, 54, 179–188.
- Hoffmann, J.F. Tissue expansion in the head and neck. Facial Plast. Surg. Clin. N. Am. 2005, 13, 315–324, vii.
- Downes, R.; Lavin, M.; Collin, R. Hydrophilic expanders for the congenital anophthalmic socket. Adv. Ophthalmic Plast. Reconstr. Surg. 1992, 9, 57–61.
- Bell, C.L.; Peppas, N.A. Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-g-ethylene glycol). Biomaterials 1996, 17, 1203–1218.
- Varga, J.; Janovak, L.; Varga, E.; Eros, G.; Dekany, I.; Kemeny, L. Acrylamide, Acrylic Acid and N-Isopropylacrylamide Hydrogels as Osmotic Tissue Expanders. Ski. Pharmacol. Physiol. 2009, 22, 305–312.
- Freeman, F.; Browe, D.; Nulty, J.; Von Euw, S.; Grayson, W.; Kelly, D. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cells Mater. 2019, 38, 168–187.
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymers 1994, 35, 1068–1077.
- Haugen, H.; Ried, V.; Brunner, M.; Will, J.; Wintermantel, E. Water as foaming agent for open cell polyurethane structures. J. Mater. Sci. Mater. Med. 2004, 15, 343–346.
- Parks, K.L.; Beckman, E.J. Generation of microcellular polyurethane foams via polymerization in carbon dioxide. II: Foam formation and characterization. Polym. Eng. Sci. 1996, 36, 2417–2431.
- Lee, K.-W.D.; Chan, P.K.; Feng, X. Morphology development and characterization of the phase-separated structure resulting from the thermal-induced phase separation phenomenon in polymer solutions under a temperature gradient. Chem. Eng. Sci. 2004, 59, 1491–1504.
- Thomson, R.C.; Wake, M.C.; Yaszemski, M.J.; Mikos, A.G. Biodegradable polymer scaffolds to regenerate organs. Adv. Polym. Sci. 1995, 122, 245–274.
- Liapis, A.; Bruttini, R. A theory for the primary and secondary drying stages of the freeze-drying of pharmaceutical crystalline and amorphous solutes: Comparison between experimental data and theory. Sep. Technol. 1994, 4, 144–155.
- Pikal, M.; Shah, S.; Roy, M.; Putman, R. The secondary drying stage of freeze drying: Drying kinetics as a function of temperature and chamber pressure. Int. J. Pharm. 1990, 60, 203–207.
- Vergnol, G.; Ginsac, N.; Rivory, P.; Meille, S.; Chenal, J.M.; Balvay, S.; Chevalier, J.; Hartmann, D.J. In vitro and in vivo evaluation of a polylactic acid-bioactive glass composite for bone fixation devices. J. Biomed. Mater. Res. B 2016, 104, 180–191.
- Del Bakhshayesh, A.R.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705.
- Bracaglia, L.G.; Smith, B.T.; Watson, E.; Arumugasaamy, N.; Mikos, A.G.; Fisher, J.P. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017, 56, 3–13.
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2016, 45, 45–57.
- Lee, M.J.; Kim, S.E.; Park, J.; Ahn, G.Y.; Yun, T.H.; Choi, I.; Kim, H.; Choi, S. Curcumin-loaded biodegradable polyurethane scaffolds modified with gelatin using 3D printing technology for cartilage tissue engineering. Polym. Adv. Technol. 2019, 30, 3083–3090.
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145.
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848.
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83.
- Witkowska, D.; Rowińska-Żyrek, M. Biophysical approaches for the study of metal-protein interactions. J. Inorg. Biochem. 2019, 199, 110783.
- Churchfield, L.A.; Tezcan, F.A. Design and Construction of Functional Supramolecular Metalloprotein Assemblies. Acc. Chem. Res. 2019, 52, 345–355.
- Nastri, F.; D’alonzo, D.; Leone, L.; Zambrano, G.; Pavone, V.; Lombardi, A. Engineering Metalloprotein Functions in Designed and Native Scaffolds. Trends Biochem. Sci. 2019, 44, 1022–1040.
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16.
- Tenaud, I.; Sainte-Marie, I.; Jumbou, O.; Litoux, P.; Dreno, B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Brit. J. Dermatol. 1999, 140, 26–34.
- Huang, J.; Chen, L.; Yuan, Q.; Gu, Z.; Wu, J. Tofu-Based Hybrid Hydrogels with Antioxidant and Low Immunogenicity Activity for Enhanced Wound Healing. J. Biomed. Nanotechnol. 2019, 15, 1371–1383.
- Coelho, T.S.; Halicki, P.C.B.; Silva, L.; Vicenti, J.R.d.M.; Gonçalves, B.L.; da Silva, P.E.A.; Ramos, D.F. Metal-based antimicrobial strategies against intramacrophage Mycobacterium tuberculosis. Lett. Appl. Microbiol. 2020, 71, 146–153.
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6.
- Ashtiani, R.E.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid. Based Complement. Altern. Med. 2021, 2021, 3349433.
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639.
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393.
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141.
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799.
- Bulina, N.V.; Vinokurova, O.B.; Prosanov, I.Y.; Vorobyev, A.M.; Gerasimov, K.B.; Borodulina, I.A.; Pryadko, A.; Botvin, V.V.; Surmeneva, M.A.; Surmenev, R.A. Mechanochemical synthesis of strontium- and magnesium-substituted and cosubstituted hydroxyapatite powders for a variety of biomedical applications. Ceram. Int. 2022, 48, 35217–35226.
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193.
- Yang, L.; Arora, K.; Beard, W.A.; Wilson, S.H.; Schlick, T. Critical role of magnesium ions in DNA polymerase beta’s closing and active site assembly. J. Am. Chem. Soc. 2004, 126, 8441–8453.
- Panaghie, C.; Zegan, G.; Sodor, A.; Cimpoeșu, N.; Lohan, N.-M.; Istrate, B.; Roman, A.-M.; Ioanid, N. Analysis of Degradation Products of Biodegradable ZnMgY Alloy. Materials 2023, 16, 3092.
- Sazegar, G.; Reza, A.H.S.; Behravan, E. The effects of supplemental zinc and honey on wound healing in rats. Iran. J. Basic Med. Sci 2011, 14, 391–398.
- Lansdown, A. Influence of zinc oxide in the closure of open skin wounds. Int. J. Cosmet. Sci. 1993, 15, 83–85.
- Apelqvist, J.; Larsson, J.; Lstenstrom, A. Topical treatment of necrotic foot ulcers in diabetic patients: A comparative trial of DuoDerm and MeZinc. Br. J. Dermatol. 1990, 123, 787–792.
- Agren, M. Zinc oxide increases degradation of collagen in necrotic wound tissue. Br. J. Dermatol. 1993, 129, 221–222.
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Moreno-Eutimio, M.A.; Espinosa-Monroy, L.; Orozco-Amaro, T.; Torres-Ramos, Y.; Montoya-Estrada, A.; Hicks, J.J.; Rodríguez-Ayala, E.; Del Moral, P.; Moreno, J.; Cueto-García, J. Enhanced healing and anti-inflammatory effects of a carbohydrate polymer with zinc oxide in patients with chronic venous leg ulcers: Preliminary results. Arch. Med. Sci. 2016, 14, 336–344.
- Mirastschijski, U.; Haaksma, C.J.; Tomasek, J.J.; Ågren, M.S. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp. Cell Res. 2004, 299, 465–475.
- A Cabral-Pacheco, G.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; A Perez-Romero, B.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739.
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regener. 2007, 15, 2–16.
- Ågren, M.S.; Andersen, L.; Heegaard, A.M.; Jorgensen, L.N. Effect of parenteral zinc sulfate on colon anastomosis repair in the rat. Int. J. Color. Dis. 2008, 23, 857–861.





