Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yiannis Deligiannakis | -- | 1386 | 2024-01-10 09:14:35 | | | |
| 2 | Constantinos Moularas | + 1 word(s) | 1387 | 2024-01-10 11:13:13 | | | | |
| 3 | Fanny Huang | Meta information modification | 1387 | 2024-01-11 08:01:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moularas, C.; Gemenetzi, A.; Deligiannakis, Y.; Louloudi, M. Plasmon-Assisted Molecular Catalysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/53656 (accessed on 07 February 2026).
Moularas C, Gemenetzi A, Deligiannakis Y, Louloudi M. Plasmon-Assisted Molecular Catalysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/53656. Accessed February 07, 2026.
Moularas, Constantinos, Aikaterini Gemenetzi, Yiannis Deligiannakis, Maria Louloudi. "Plasmon-Assisted Molecular Catalysis" Encyclopedia, https://encyclopedia.pub/entry/53656 (accessed February 07, 2026).
Moularas, C., Gemenetzi, A., Deligiannakis, Y., & Louloudi, M. (2024, January 10). Plasmon-Assisted Molecular Catalysis. In Encyclopedia. https://encyclopedia.pub/entry/53656
Moularas, Constantinos, et al. "Plasmon-Assisted Molecular Catalysis." Encyclopedia. Web. 10 January, 2024.
Copy Citation
The utilization of plasmonic nanomaterials in catalytic technologies is an emerging research field with foreseeable applications in energy-catalytic technologies. On this front, the coupling of plasmonic nanomaterials with molecular catalysts is a newly approached, thus far unexploited field.
nanoplasmonics
molecular catalysis
hot electrons
1. Introduction
The global energy crisis mandates the adoption of clean energy technologies, based on renewable sources such as sunlight. To this end, two key issues can be considered among forward-looking solutions: [i] one is exploitation of the full spectrum of solar photons, since the highest efficiency of sunlight energy conversion into chemical activity has so far almost exclusively been confined to the utilization of UV-absorbing TiO2-based nanomaterials [1]. In years, there mounting evidence has emerged that plasmonic nanomaterials can provide a decisive boost to photo-driven reactions [2]. In brief, the underlying physics of plasmonic nanostructures and their action as “antennae” can be outlined as follows: the incoming light can be concentrated in nanoscale volumes, thus giving rise to collective oscillations of electrons, a phenomenon known as localized surface plasmon resonance (LSPR) [3]. Consequently, locally intense electric fields (hot spots) at the particle–particle interface, form an ideal active site for chemical processes to occur [4][5]. The plasmonic effect also generates hot carriers [6], namely highly energetic photoinduced e−—h+ pairs. Moreover, the stored quanta of energy in the oscillating charge density during LSPR can be dissipated via several mechanisms and utilized in different ways [7], such as the photothermal effect, i.e., heat generation from the photo-excited plasmonic nanoparticles (PNPs) [8] (Figure 1). Thus, the plasmonic phenomenon manifests in different ways, and each of them can be utilized in different types of catalytic processes [9]. These properties of plasmonic nanoparticles render them highly attractive for various applications, including sensing, imaging, and enhancing light–matter interactions.
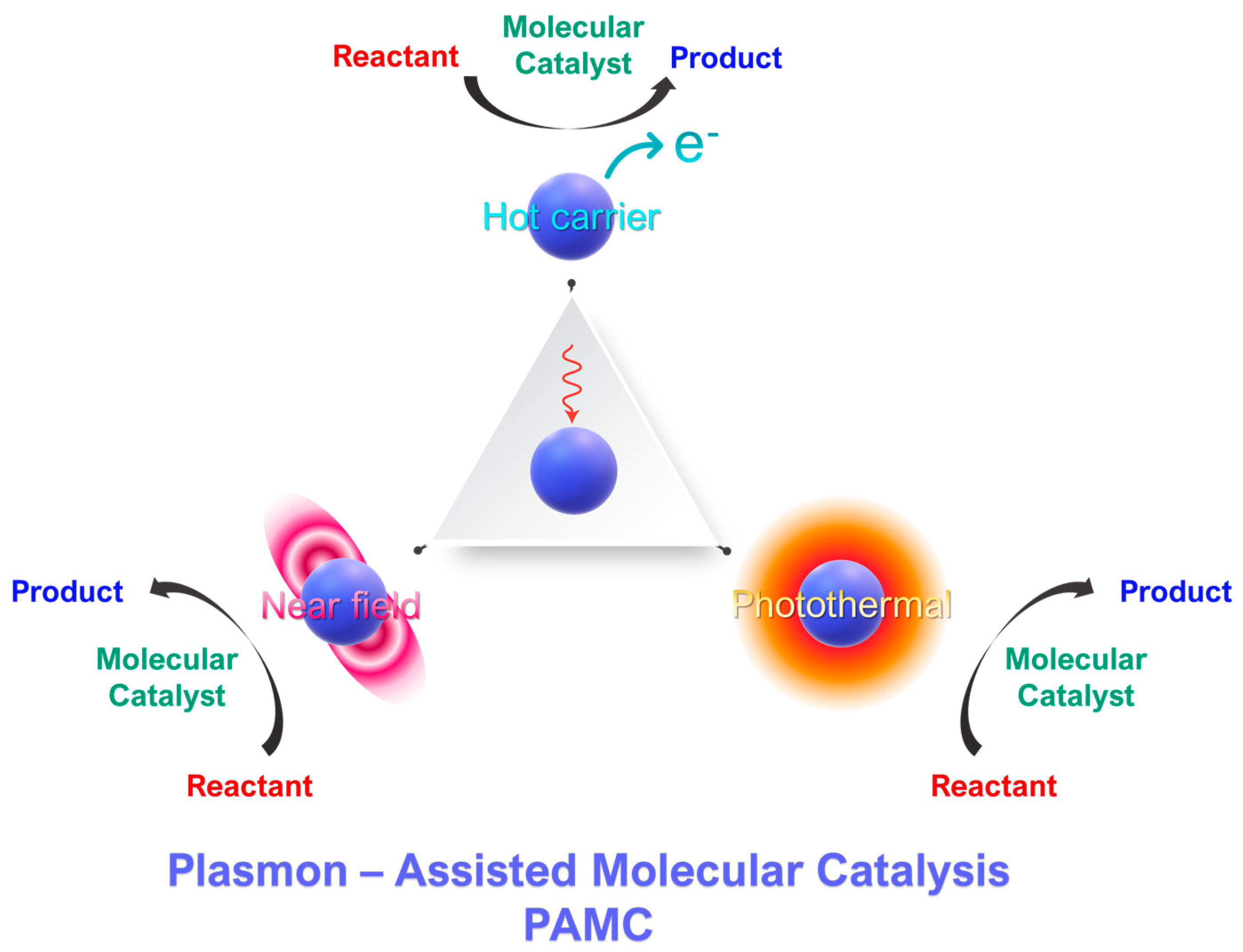
Figure 1. Concept of plasmon-assisted molecular catalysis (PAMC). Molecular catalysts perform a specific catalytic reaction. Photoexcited plasmonic nanoparticles can intervene in the catalytic process via three mechanisms: [i] generation of hot carriers and [ii] the photothermal effect of [iii] near-field effects.
In recent years, numerous review articles have been published focusing on catalytic applications where the plasmonic materials themselves act as catalysts for a wide range of catalytic reactions [10][11][12]. In particular, the local temperature increase around PNPs has led to, for example, applications in nanomedicine, for tumor targeting [13][14], or in catalysis for the thermal acceleration of reactions [15]. The LSPR-induced local electric field in close proximity to the PNPs is another intriguing property that has been utilized for surface-enhanced Raman scattering (SERS) spectroscopy [16], sensor-related applications [17][18], and catalysis [19][20]. Finally, in the context of catalysis, the generation of hot carriers by plasmonic nanostructures is the most widely exploited mechanism [21]. Gold (Au0), silver (Ag0), copper (Cu0), and aluminum (Al0) are the most common metals possessing measurable LSPR properties, giving birth to the field of plasmonic catalysis [22]. The coupling of plasmonic materials with catalytic metals (e.g., Pt, Pd, etc.) can promote the transfer of electrons from a PNP to the catalytic metal, for example, via LSPR-induced local electric fields in the PNP’s vicinity [23].
From a forward-looking perspective, the combination of plasmonic nanoparticles with molecular catalysts is an emerging research field with significant potential for various catalytic applications.
Although the literature concerning this subject has, thus far, remained highly limited, it is possible to realize the potential of PAMC systems by studying the interaction between plasmonic nanoparticles and simpler moieties. For instance, there is extensive research on the interaction and catalytic behavior of plasmonic nanostructures with small molecules [24]. Considering all of the above, it is evident that, depending on the molecular moiety and its catalytic function, a different feature of plasmonic structures can be employed, from simply acting as a thermal source to inducing redox phenomena in more complex systems. For completeness, researchers can cite numerous interdisciplinary reviews focusing on the implementation of plasmonic materials as catalysts themselves in energy-related catalysis such as solar cells [25], CO2 reduction [26], environmental remediation [27], H2O splitting [28], photocatalysis [22][29][30], as well as plasmonic catalysts [31].
2. Plasmon-Assisted Molecular Catalysis (PAMC)
It is well established that compared to other catalytic systems, molecular catalysts display unique reaction mechanisms providing high selectivity and activity in challenging catalytic processes [32][33], and their usage has been extensively reviewed in numerous works [34][35]. On this front, the fundamentals of plasmon-derived mechanisms analyzed herein (hot carriers, hotspots, or thermal effects), as depicted in Figure 2, are able to enhance the activity of a molecular catalyst located in the proximity where these phenomena occur. In particular, researchers' group has presented some key studies [36][37] which show that the plasmonic hot electrons are the primary mechanism of the observed PAMC phenomena. In [36], researchers have shown that plasmon-generated hot electrons could reversibly stop/start the oxidative advancement of the Mn catalytic center via “pausing on demand” the oxidation catalytic process under light excitation [36]. The molecular catalysis studied was alkene epoxidation in the presence of a biomimetic Mn catalyst utilizing H2O2. This is based on the catalytic activation of H2O2 by the Mn catalyst forming a LMnIV = O transient intermediate. It was found that under the photoinduced action of plasmonic Ag0@SiO2 nanoparticles, the oxidation catalytic process can be reversibly switched off. When photoexcitation of the PNPs stops, the catalytic process recommences. Utilizing three types of plasmonic core–shell Ag0@SiO2 nanoparticles with a varying thickness (0.1–5 nm) SiO2 shell, it was shown that the intensity of the observed phenomenon changed. Using EPR spectroscopy, it was demonstrated that the key step related to the photoinduced pause of the catalytic process by the Ag0@SiO2 PNPs is the reversible inhibition of transient LMnIV = O intermediate formation [36]. Moreover, the SERS and redox potential data indicated that the Ag0@SiO2 PNPs present a moderate SERS effect on the LMnII catalyst, while the solution redox potential Eh decreases considerably [36]. Researchers' data showed that the plasmonic heating was insignificant [36]. Therefore, the reversible switch off of the catalytic process is a result of =hot electron generation by the Ag0@SiO2 PNPs, along with near-field generation. Overall, this work revealed a novel phenomenon, where plasmonics can act as a reversible switch for a molecular catalytic process.
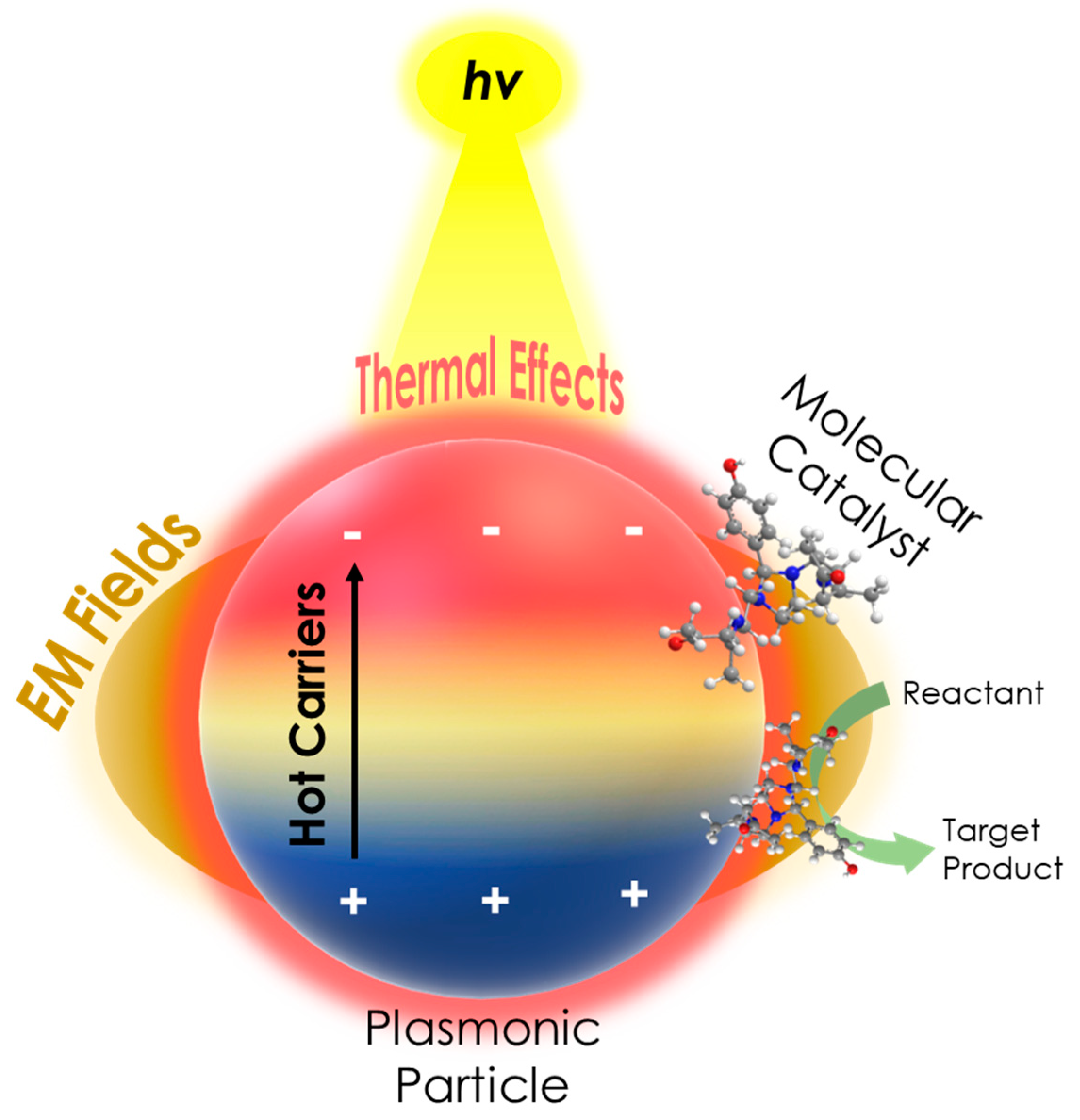
Figure 2. A schematic of the PAMC concept, illustrating the enhanced catalytic activity of a molecular catalyst in the presence of a plasmonic particle under irradiation, due to plasmonic mechanisms, i.e., hot carrier, EM fields (hotspots), and thermal effects.
In [37], researchers have shown that photoexcited core–shell Ag0@SiO2 PNPs can dramatically enhance production of H2 via a formic acid dehydrogenation (FADH) reaction, catalyzed by the molecular catalyst [Fe(BF4)2∙6H2O/P(CH2CH2PPh2)3, PP3]. This is based on the catalytic activation of HCOOH by the catalyst forming a Fe-hydride transient intermediate. An almost 10-fold increase in H2 gas production rate was achieved in the presence of photoexcited PNPs, while the TONs were boosted by ~400% and the TOFs by ~600% [37]. Through selective excitation at wavelengths (λex) ranging over the photo-response profile of the Ag0@SiO2 NPs, it was demonstrated that the enhancement on FADH is maximal at λex = 405 nm, namely the peak of the photo-plasmonic response of the Ag0@SiO2 NPs [37]. The study of the solution redox potential (Eh) under catalytic conditions showed that the excitation of the Ag0@SiO2 PNPs results in hot electron injection into the reaction solution. The hot electron injection rates and the ensuing FADH rates could be controlled by varying the SiO2-shell thickness of the Ag0@SiO2 PNPs in the range of 3 nm to 5 nm. Thermoplasmonic effects, albeit not macroscopically observed, seem to play a secondary role, if any [37]. This work demonstrated the possibility to approach industrial-scale H2 production rates via FADH, using low-cost Fe-based molecular catalysts and without any sacrificial cocatalysts.
Lu et al. have found a strong synergistic relation between gold nanoparticles and cobalt porphyrin which induces highly efficient photocatalytic hydrogen evolution [38]. This was another example where the catalytic activity of molecular catalysts near plasmonic nanostructures may be enhanced dramatically. The authors [38] developed a photocatalytic system for the hydrogen evolution reaction (HER) by combining a cobalt-porphyrin molecular catalyst together with plasmonic gold nanoparticles. After optimization, the HER rate and turnover frequency (TOF) reached 3.21 mol g−1 h−1 and 4650 h−1, respectively. It has been demonstrated that the lifetime of plasmon-generated hot carriers is prolonged at the AuNP-CoTPyP interface, and transferred to the LUMO of CoTPyP hot carriers favoring catalytic HER. Moreover, this catalytic system could remain stable after 45 h of catalytic cycles and being illuminated for two weeks.
References
- Banerjee, A. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures. Nanotechnol. Sci. Appl. 2011, 4, 35.
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple experimental procedures to distinguish photothermal from hot-carrier processes in plasmonics. Light. Sci. Appl. 2020, 9, 108.
- Araujo, T.P.; Quiroz, J.; Barbosa, E.C.M.; Camargo, P.H.C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110–122.
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980.
- Sheikholeslami, S.; Jun, Y.; Jain, P.K.; Alivisatos, A.P. Coupling of Optical Resonances in a Compositionally Asymmetric Plasmonic Nanoparticle Dimer. Nano Lett. 2010, 10, 2655–2660.
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34.
- Baffou, G.; Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907.
- Kim, M.; Lee, J.; Nam, J. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471.
- Gellé, A.; Moores, A. Plasmonic nanoparticles: Photocatalysts with a bright future. Curr. Opin. Green Sustain. Chem. 2019, 15, 60–66.
- Zhao, J.; Wang, J.; Brock, A.J.; Zhu, H. Plasmonic heterogeneous catalysis for organic transformations. J. Photochem. Photobiol. C Photochem. Rev. 2022, 52, 100539.
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230.
- Jain, V.; Kashyap, R.K.; Pillai, P.P. Plasmonic Photocatalysis: Activating Chemical Bonds through Light and Plasmon. Adv. Opt. Mater. 2022, 10, 2200463.
- Sotiriou, G.A. Biomedical applications of multifunctional plasmonic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 19–30.
- Brooks, J.L.; Warkentin, C.L.; Saha, D.; Keller, E.L.; Frontiera, R.R. Toward a mechanistic understanding of plasmon-mediated photocatalysis. Nanophotonics 2018, 7, 1697–1724.
- Baffou, G. Thermoplasmonics; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781108418324.
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117.
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521.
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic Hydrogen Sensing with Nanostructured Metal Hydrides. ACS Nano 2014, 8, 11925–11940.
- Jiang, P.; Dong, Y.; Yang, L.; Zhao, Y.; Xie, W. Hot Electron-Induced Carbon–Halogen Bond Cleavage Monitored by in Situ Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2019, 123, 16741–16746.
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002.
- Khurgin, J.B. Hot carriers generated by plasmons: Where are they generated and where do they go from there? Faraday Discuss. 2019, 214, 35–58.
- Camargo, P.H.C.; Cortés, E. Plasmonic Catalysis; Wiley: New York, NY, USA, 2021; ISBN 9783527347506.
- Swearer, D.F.; Zhao, H.; Zhou, L.; Zhang, C.; Robatjazi, H.; Martirez, J.M.P.; Krauter, C.M.; Yazdi, S.; McClain, M.J.; Ringe, E.; et al. Heterometallic antenna-reactor complexes for photocatalysis. Proc. Natl. Acad. Sci. USA 2016, 113, 8916–8920.
- Joshi, G.; Mir, A.Q.; Layek, A.; Ali, A.; Aziz, S.T.; Khatua, S.; Dutta, A. Plasmon-Based Small-Molecule Activation: A New Dawn in the Field of Solar-Driven Chemical Transformation. ACS Catal. 2022, 12, 1052–1067.
- Erwin, W.R.; Zarick, H.F.; Talbert, E.M.; Bardhan, R. Light trapping in mesoporous solar cells with plasmonic nanostructures. Energy Environ. Sci. 2016, 9, 1577–1601.
- Verma, R.; Belgamwar, R.; Polshettiwar, V. Plasmonic Photocatalysis for CO2 Conversion to Chemicals and Fuels. ACS Mater. Lett. 2021, 3, 574–598.
- Wang, D.; Pillai, S.C.; Ho, S.-H.; Zeng, J.; Li, Y.; Dionysiou, D.D. Plasmonic-based nanomaterials for environmental remediation. Appl. Catal. B Environ. 2018, 237, 721–741.
- Du, L.; Shi, G.; Zhao, Y.; Chen, X.; Sun, H.; Liu, F.; Cheng, F.; Xie, W. Plasmon-promoted electrocatalytic water splitting on metal–semiconductor nanocomposites: The interfacial charge transfer and the real catalytic sites. Chem. Sci. 2019, 10, 9605–9612.
- Smith, J.G.; Faucheaux, J.A.; Jain, P.K. Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today 2015, 10, 67–80.
- Kazuma, E.; Kim, Y. Mechanistic Studies of Plasmon Chemistry on Metal Catalysts. Angew. Chemie Int. Ed. 2019, 58, 4800–4808.
- da Silva, A.; Rodrigues, T.; Wang, J.; Camargo, P. Plasmonic catalysis with designer nanoparticles. Chem. Commun. 2022, 58, 2055–2074.
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77.
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369.
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433.
- Stathi, P.; Solakidou, M.; Louloudi, M.; Deligiannakis, Y. From Homogeneous to Heterogenized Molecular Catalysts for H2 Production by Formic Acid Dehydrogenation: Mechanistic Aspects, Role of Additives, and Co-Catalysts. Energies 2020, 13, 733.
- Gemenetzi, A.; Moularas, C.; Belles, L.; Deligiannakis, Y.; Louloudi, M. Reversible Plasmonic Switch in a Molecular Oxidation Catalysis Process. ACS Catal. 2022, 12, 9908–9921.
- Gemenetzi, A.; Deligiannakis, Y.; Louloudi, M. Controlled Photoplasmonic Enhancement of H2 Production via Formic Acid Dehydrogenation by a Molecular Fe Catalyst. ACS Catal. 2023, 13, 9905–9917.
- Sheng, H.; Wang, J.; Huang, J.; Li, Z.; Ren, G.; Zhang, L.; Yu, L.; Zhao, M.; Li, X.; Li, G.; et al. Strong synergy between gold nanoparticles and cobalt porphyrin induces highly efficient photocatalytic hydrogen evolution. Nat. Commun. 2023, 14, 1528.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
585
Revisions:
3 times
(View History)
Update Date:
11 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




