
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maxime Taghavi | -- | 2394 | 2024-01-10 08:00:15 |
Video Upload Options
Antiphospholipid antibody (aPL)-persistent positivity is frequent in hemodialysis (HD) patients. Native arteriovenous fistula (AVF) complications such as stenosis and thrombosis are among the most important causes of morbidity and mortality in hemodialysis patients. The association between aPL positivity and AVF thrombosis seems to be well established.
1. Introduction
1.1. Hemodialysis and Vascular Access
1.2. Antiphospholipid Syndrome and Pathophysiology
1.3. Classification Criteria of Antiphospholipid Syndrome
|
2006 Revised Sapporo |
2023 ACR/EULAR |
|
|---|---|---|
|
Classification |
At least 1 clinical criterion AND 1 laboratory criterion |
3 points from clinical domains AND at least 3 points from laboratory domains |
|
Clinical criteria |
Entry criteria and scoring: count the highest weighted criterion towards the total score |
|
|
2 clinical criteria 1. Vascular thrombosis: One or more clinical episodes of arterial, venous, or small vessel thrombosis, in any tissue or organ 2. Pregnancy morbidity |
6 clinical domains 1. Macrovascular-Venous Thromboembolism 2. Macrovascular-Arterial Thrombosis 3. Microvascular 4. Obstetric 5. Cardiac Valve 6. Hematology |
|
|
Considered as non-criteria-manifestations: |
||
|
- Heart valve disease |
Yes |
No |
|
- Livedo racemosa |
Yes |
No |
|
- Thrombocytopenia |
Yes |
No |
|
- Nephropathy, |
Yes |
No |
|
- Neurological manifestations |
Yes |
Yes |
|
- Pulmonary/Adrenal hemorrhage |
Yes |
No |
|
Laboratory criteria |
||
|
Persistent positivity (at 12 weeks) |
Yes |
Yes |
|
Timeline of aPL positivity and clinical criteria |
Less than 5 years of clinical criteria |
Within 3 years of clinical criterion |
|
Thresholds of aCL and/or aβ2GPI |
aCL: >40 GPL or MPL, or >the 99th percentile aβ2GPI: >the 99th percentile |
aCL or aβ2GPI: Moderate 40–79 units High >80 units |
|
Antibodies for laboratory criteria: |
||
|
- Positive LA |
Yes |
Yes |
|
- IgG aCL or aβ2GPI |
Yes |
Yes |
|
- IgM aCL and/or aβ2GPI |
Yes |
Yes. If isolated: are not sufficient (weight only 1 point) |
aCL: anticardiolipin antibody, aβ2GPI: anti-β2 glycoprotein I antibody, LA: lupus anticoagulant.
1.4. Antiphospholipid Antibodies in Hemodialysis Patients
2. AVF Maturation
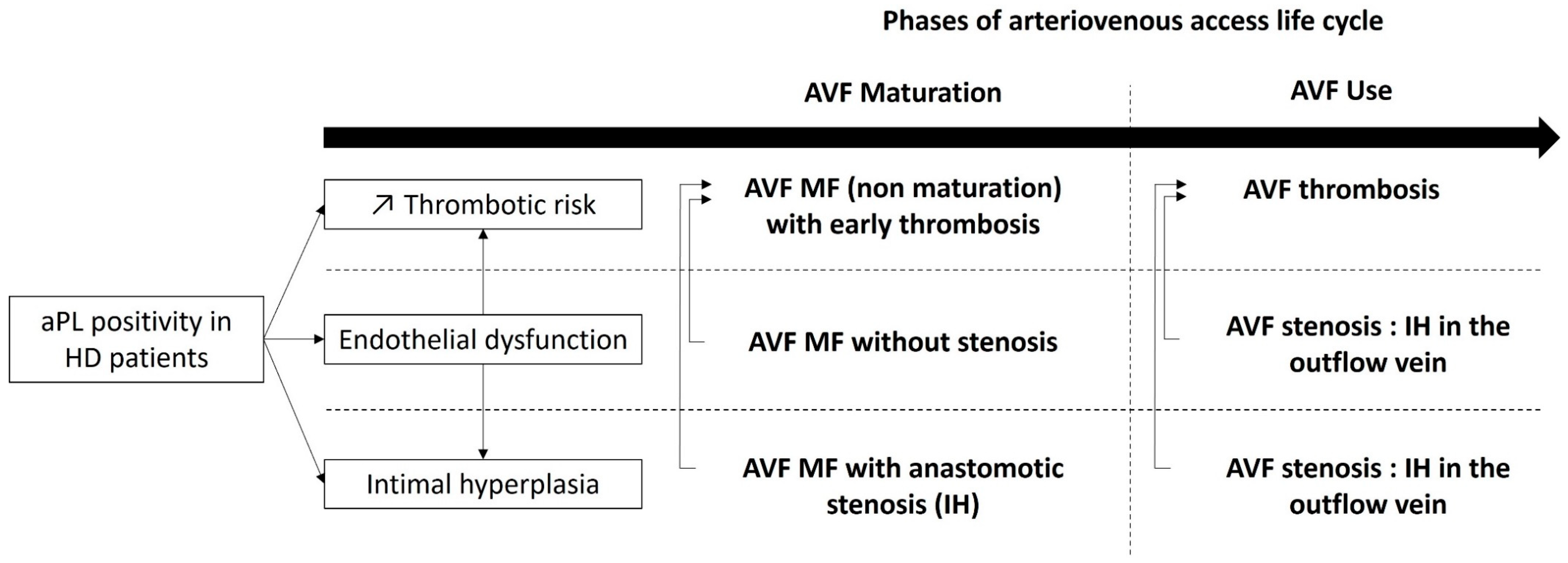
3. AVF Thrombosis
4. Stenosis and Intimal Hyperplasia
5. Mortality
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75 (Suppl. S1), A6–A7.
- Casserly, L.F.; Dember, L.M. Thrombosis in end-stage renal disease. Semin. Dial. 2003, 16, 245–256.
- Roy-Chaudhury, P.; Kelly, B.S.; Melhem, M.; Zhang, J.; Li, J.; Desai, P.; Munda, R.; Heffelfinger, S.C. Vascular access in hemodialysis: Issues, management, and emerging concepts. Cardiol. Clin. 2005, 23, 249–273.
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and novel therapies for vascular access in haemodialysis. Nat. Rev. Nephrol. 2020, 16, 586–602.
- Knight, J.S.; Branch, D.W.; Ortel, T.L. Antiphospholipid syndrome: Advances in diagnosis, pathogenesis, and management. BMJ 2023, 380, e069717.
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 2022, 44, 347–362.
- Velásquez, M.; Rojas, M.; Abrahams, V.M.; Escudero, C.; Cadavid, Á.P. Mechanisms of Endothelial Dysfunction in Antiphospholipid Syndrome: Association with Clinical Manifestations. Front. Physiol. 2018, 9, 1840.
- Cugno, M.; Borghi, M.O.; Lonati, L.M.; Ghiadoni, L.; Gerosa, M.; Grossi, C.; De Angelis, V.; Magnaghi, G.; Tincani, A.; Mari, D.; et al. Patients with antiphospholipid syndrome display endothelial perturbation. J. Autoimmun. 2010, 34, 105–110.
- Nochy, D.; Daugas, E.; Droz, D.; Beaufils, H.; Grünfeld, J.P.; Piette, J.C.; Bariety, J.; Hill, G. The intrarenal vascular lesions associated with primary antiphospholipid syndrome. J. Am. Soc. Nephrol. 1999, 10, 507–518.
- Canaud, G.; Bienaimé, F.; Tabarin, F.; Bataillon, G.; Seilhean, D.; Noël, L.-H.; Dragon-Durey, M.-A.; Snanoudj, R.; Friedlander, G.; Halbwachs-Mecarelli, L.; et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N. Engl. J. Med. 2014, 371, 303–312.
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306.
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.-C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann. Rheum. Dis. 2023, 82, 1258–1270.
- Liu, X.; Zhu, L.; Liu, H.; Cai, Q.; Yun, Z.; Sun, F.; Jia, Y.; Guo, J.; Li, C. Non-criteria antiphospholipid antibodies in antiphospholipid syndrome: Diagnostic value added. Front. Immunol. 2022, 13, 972012.
- Gracia-Tello, B.; Isenberg, D. Kidney disease in primary anti-phospholipid antibody syndrome. Rheumatology 2017, 56, 1069–1080.
- Ames, P.R.J.; Merashli, M.; Bucci, T.; Pastori, D.; Pignatelli, P.; Violi, F.; Bellizzi, V.; Arcaro, A.; Gentile, F. Antiphospholipid antibodies in end-stage renal disease: A systematic review and meta-analysis. Hemodial. Int. 2020, 24, 383–396.
- Chew, S.L.; Lins, R.L.; Daelemans, R.; Zachee, P.; De Clerck, L.S.; Vermylen, J. Are antiphospholipid antibodies clinically relevant in dialysis patients? Nephrol. Dial. Transplant. 1992, 7, 1194–1198.
- Roozbeh, J.; Serati, A.R.; Malekhoseini, S.A. Arteriovenous fistula thrombosis in patients on regular hemodialysis: A report of 171 patients. Arch. Iran. Med. 2006, 9, 26–32.
- Ozmen, S.; Danis, R.; Akin, D.; Batun, S. Anticardiolipin antibodies in hemodialysis patients with hepatitis C and their role in fistula failure. Clin. Nephrol. 2009, 72, 193–198.
- Brunet, P.; Aillaud, M.F.; San Marco, M.; Philip-Joet, C.; Dussol, B.; Bernard, D.; Juhan-Vague, I.; Berland, Y. Antiphospholipids in hemodialysis patients: Relationship between lupus anticoagulant and thrombosis. Kidney Int. 1995, 48, 794–800.
- Prakash, R.; Miller, C.C.; Suki, W.N. Anticardiolipin antibody in patients on maintenance hemodialysis and its association with recurrent arteriovenous graft thrombosis. Am. J. Kidney Dis. 1995, 26, 347–352.
- Serrano, A.; García, F.; Serrano, M.; Ramírez, E.; Alfaro, F.J.; Lora, D.; de la Cámara, A.G.; Paz-Artal, E.; Praga, M.; Morales, J.M. IgA antibodies against β2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012, 81, 1239–1244.
- Salmela, B.; Hartman, J.; Peltonen, S.; Albäck, A.; Lassila, R. Thrombophilia and arteriovenous fistula survival in ESRD. Clin. J. Am. Soc. Nephrol. 2013, 8, 962–968.
- Ghisdal, L.; Broeders, N.; Wissing, K.M.; Mena, J.M.; Lemy, A.; Wijns, W.; Pradier, O.; Donckier, V.; Racapé, J.; Vereerstraeten, P.; et al. Thrombophilic factors in Stage V chronic kidney disease patients are largely corrected by renal transplantation. Nephrol. Dial. Transplant. 2011, 26, 2700–2705.
- Dabit, J.Y.; Valenzuela-Almada, M.O.; Vallejo-Ramos, S.; Duarte-García, A. Epidemiology of Antiphospholipid Syndrome in the General Population. Curr. Rheumatol. Rep. 2022, 23, 85.
- Zonnebeld, N.; Huberts, W.; van Loon, M.M.; Delhaas, T.; Tordoir, J.H.M. Natural Vascular Remodelling After Arteriovenous Fistula Creation in Dialysis Patients With and Without Previous Ipsilateral Vascular Access. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 277–287.
- Gameiro, J.; Ibeas, J. Factors affecting arteriovenous fistula dysfunction: A narrative review. J. Vasc. Access. 2020, 21, 134–147.
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75 (Suppl. S2), S1–S164.
- Beathard, G.A.; Lok, C.E.; Glickman, M.H.; Al-Jaishi, A.A.; Bednarski, D.; Cull, D.L.; Lawson, J.H.; Lee, T.C.; Niyyar, V.D.; Syracuse, D.; et al. Definitions and End Points for Interventional Studies for Arteriovenous Dialysis Access. Clin. J. Am. Soc. Nephrol. 2018, 13, 501–512.
- Huber, T.S.; Berceli, S.A.; Scali, S.T.; Neal, D.; Anderson, E.M.; Allon, M.; Cheung, A.K.; Dember, L.M.; Himmelfarb, J.; Roy-Chaudhury, P.; et al. Arteriovenous Fistula Maturation, Functional Patency, and Intervention Rates. JAMA Surg. 2021, 156, 1111–1118.
- Ma, S.; Duan, S.; Liu, Y.; Wang, H. Intimal Hyperplasia of Arteriovenous Fistula. Ann. Vasc. Surg. 2022, 85, 444–453.
- Siddiqui, M.A.; Ashraff, S.; Carline, T. Maturation of arteriovenous fistula: Analysis of key factors. Kidney Res. Clin. Pract. 2017, 36, 318–328.
- Somarathna, M.; Hwang, P.T.; Millican, R.C.; Alexander, G.C.; Isayeva-Waldrop, T.; Sherwood, J.A.; Brott, B.C.; Falzon, I.; Northrup, H.; Shiu, Y.-T.; et al. Nitric oxide releasing nanomatrix gel treatment inhibits venous intimal hyperplasia and improves vascular remodeling in a rodent arteriovenous fistula. Biomaterials 2022, 280, 121254.
- Singh, M.; Mahapatra, H.S.; Pursnani, L.; Muthukumar, B.; Neeraj Anant, I.; Kumar, A.; Kaur, N.; Singh, A.; Krishnan, C. on prediction of arterio-venous fistula maturation by flow mediated dilatation and AVF blood flow. J. Vasc. Access. 2023, 24, 443–451.
- Anapalli, S.R.; N, H.D.; Sarma, P.; Srikanth, L.; V, S.K. Thrombophilic risk factors and ABO blood group profile for arteriovenous access failure in end stage kidney disease patients: A single-center experience. Ren. Fail. 2022, 44, 34–42.
- Bolleke, E.; Seferi, S.; Rroji, M.; Idrizi, A.; Barbullushi, M.; Thereska, N. Exhausting multiple hemodialysis access failures. Med. Arch. 2014, 68, 361–363.
- Taghavi, M.; Demulder, A.; Mesquita, M.D.C.F.; Dernier, Y.; Nortier, J.; Collart, F.; Pozdzik, A. Underestimated Effect of Antiphospholipid Antibodies on Arteriovenous Fistula Maturation in Hemodialysis Patients, 09 May 2022, Preprint (Version 1). Research Square. Available online: https://www.researchsquare.com/article/rs-1606215/v1 (accessed on 9 May 2022).
- Evangelatos, G.; Kravvariti, E.; Konstantonis, G.; Tentolouris, N.; Sfikakis, P.P.; Tektonidou, M.G. Atherosclerosis progression in antiphospholipid syndrome is comparable to diabetes mellitus: A 3 year prospective study. Rheumatology 2022, 61, 3408–3413.
- Sorice, M.; Profumo, E.; Capozzi, A.; Recalchi, S.; Riitano, G.; Di Veroli, B.; Saso, L.; Buttari, B. Oxidative Stress as a Regulatory Checkpoint in the Production of Antiphospholipid Autoantibodies: The Protective Role of NRF2 Pathway. Biomolecules 2023, 13, 1221.
- Barbhaiya, M.; Taghavi, M.; Zuily, S.; Domingues, V.; Chock, E.Y.; Tektonidou, M.G.; Erkan, D.; Seshan, S.V.; New APS Classification Criteria Steering Committee and APS ACTION Collaborators. Efforts to Better Characterize “Antiphospholipid Antibody Nephropathy” for the 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria: Renal Pathology Subcommittee Report. J. Rheumatol. 2023, 50, jrheum.2022-1200.
- Wattanakit, K.; Cushman, M.; Stehman-Breen, C.; Heckbert, S.R.; Folsom, A.R. Chronic kidney disease increases risk for venous thromboembolism. J. Am. Soc. Nephrol. 2008, 19, 135–140.
- Go, A.S.; Bansal, N.; Chandra, M.; Lathon, P.V.; Fortmann, S.P.; Iribarren, C.; Hsu, C.-Y.; Hlatky, M.A.; ADVANCE Study Investigators. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J. Am. Coll. Cardiol. 2011, 58, 1600–1607.
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744.
- Sosińska-Zawierucha, P.; Bręborowicz, A. Uremic serum induces prothrombotic changes in venous endothelial cells and inflammatory changes in aortic endothelial cells. Ren. Fail. 2021, 43, 401–405.
- Montagnana, M.; Meschi, T.; Borghi, L.; Lippi, G. Thrombosis and occlusion of vascular access in hemodialyzed patients. Semin. Thromb. Hemost. 2011, 37, 946–954.
- Knoll, G.A.; Wells, P.S.; Young, D.; Perkins, S.L.; Pilkey, R.M.; Clinch, J.J.; Rodger, M.A. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J. Am. Soc. Nephrol. 2005, 16, 1108–1114.
- Grupp, C.; Troche-Polzien, I.; Stock, J.; Bramlage, C.; Müller, G.A.; Koziolek, M. Thrombophilic risk factors in hemodialysis: Association with early vascular access occlusion and patient survival in long-term follow-up. PLoS ONE 2019, 14, e0222102.
- Hadhri, S.; Rejeb, M.B.; Belarbia, A.; Achour, A.; Skouri, H. Hemodialysis duration, human platelet antigen HPA-3 and IgA isotype of anti-β2glycoprotein I antibodies are associated with native arteriovenous fistula failure in Tunisian hemodialysis patients. Thromb. Res. 2013, 131, e202–e209.
- Vazquez-Padron, R.I.; Duque, J.C.; Tabbara, M.; Salman, L.H.; Martinez, L. Intimal Hyperplasia and Arteriovenous Fistula Failure: Looking Beyond Size Differences. Kidney360 2021, 2, 1360–1372.
- Hu, H.; Patel, S.; Hanisch, J.J.; Santana, J.M.; Hashimoto, T.; Bai, H.; Kudze, T.; Foster, T.R.; Guo, J.; Yatsula, B.; et al. Future research directions to improve fistula maturation and reduce access failure. Semin. Vasc. Surg. 2016, 29, 153–171.
- Martínez-Flores, J.A.; Serrano, M.; Morales, J.M.; Serrano, A. Antiphospholipid Syndrome and Kidney Involvement: New Insights. Antibodies 2016, 5, 17.
- Nampoory, M.R.N.; Das, K.C.; Johny, K.V.; Al-Hilali, N.; Abraham, M.; Easow, S.; Saed, T.; Al-Muzeirei, I.A.; Sugathan, T.N.; Al Mousawi, M. Hypercoagulability, a serious problem in patients with ESRD on maintenance hemodialysis, and its correction after kidney transplantation. Am. J. Kidney Dis. 2003, 42, 797–805.
- Turmel-Rodrigues, L.; Mouton, A.; Birmelé, B.; Billaux, L.; Ammar, N.; Grézard, O.; Hauss, S.; Pengloan, J. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol. Dial. Transplant. 2001, 16, 2365–2371.
- Adler, S.; Szczech, L.; Qureshi, A.; Bollu, R.; Thomas-John, R. IgM anticardiolipin antibodies are associated with stenosis of vascular access in hemodialysis patients but do not predict thrombosis. Clin. Nephrol. 2001, 56, 428–434.
- Perl, L.; Netzer, A.; Rechavia, E.; Bental, T.; Assali, A.; Codner, P.; Mager, A.; Battler, A.; Kornowski, R.; Lev, E.I. Long-term outcome of patients with antiphospholipid syndrome who undergo percutaneous coronary intervention. Cardiology 2012, 122, 76–82.




