Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Perez-Araluce | -- | 1560 | 2024-01-09 15:47:38 | | | |
| 2 | Wendy Huang | Meta information modification | 1560 | 2024-01-10 07:33:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Detrimental Effects of Reactive Oxidative Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/53615 (accessed on 07 February 2026).
Perez-Araluce M, Jüngst T, Sanmartin C, Prosper F, Plano D, Mazo MM. Detrimental Effects of Reactive Oxidative Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/53615. Accessed February 07, 2026.
Perez-Araluce, Maria, Tomasz Jüngst, Carmen Sanmartin, Felipe Prosper, Daniel Plano, Manuel M. Mazo. "Detrimental Effects of Reactive Oxidative Stress" Encyclopedia, https://encyclopedia.pub/entry/53615 (accessed February 07, 2026).
Perez-Araluce, M., Jüngst, T., Sanmartin, C., Prosper, F., Plano, D., & Mazo, M.M. (2024, January 09). Detrimental Effects of Reactive Oxidative Stress. In Encyclopedia. https://encyclopedia.pub/entry/53615
Perez-Araluce, Maria, et al. "Detrimental Effects of Reactive Oxidative Stress." Encyclopedia. Web. 09 January, 2024.
Copy Citation
Oxidative stress is defined as a disturbance in the balance between oxidant production and antioxidant activity. Oxidative stress is characterized by an increase in reactive oxygen species or a decrease in antioxidants in the body. This imbalance leads to detrimental effects, including inflammation and multiple chronic diseases, ranging from impaired wound healing to highly impacting pathologies in the neural and cardiovascular systems, or the bone, amongst others.
oxidative stress
reactive oxygen
antioxidants
detrimental effects
inflammation
proteins
1. Introduction
The importance of oxidative stress and its effect on human health is becoming increasingly evident. An in-depth study of how it is generated, its mechanisms and consequences can be very useful for the treatment of numerous diseases. Progress in this field has led to the development of numerous strategies to tackle it, including the development of antioxidant materials as well as the development of new strategies for the effective delivery of antioxidants. This work aims to comprehensively show these biomaterial-based antioxidant strategies focusing on the treatment of chronic wounds, neurodegenerative, cardiovascular and bone diseases.
In chemistry, a free radical is a chemical species with one or more unpaired electrons. It can react with other molecules by donating its unpaired electron to another molecule or by accepting an electron from another molecule to become more stable. This reaction results in the formation of a new free radical and, thus, in a chain reaction that propagates until quenching [1]. Free radicals are formed physiologically. In the mitochondria, an oxygen molecule can be reduced to water because of the production of adenosine triphosphate (ATP) during the respiration process. The intermediate steps of oxygen reduction involve the formation of various free radicals or reactive oxygen species (ROS): the superoxide anion radical (O2-), hydrogen peroxide (H2O2) and the hydroxyl radical (OH) [2]. The formation of the superoxide anion radical stems from the reduction of oxygen by one electron, hydrogen peroxide by two electrons and the hydroxyl radical by three electrons. Furthermore, molecular oxygen can undergo electronic excitation to form singlet molecular oxygen (1O2). Additionally, various cellular sources contribute to ROS production, arising from different enzymes such as nitric oxide synthase, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and xanthine oxidase in mitochondria, as well as from peroxisomal constituents [3]. During protein folding and disulphide bond formation within the endoplasmic reticulum, oxidants are released [1]. Oxygen radicals can occur as alkyl or peroxyl radicals, as they can react with susceptible compounds, including lipids, proteins and/or DNA. Physiologically, the rate of quenching of free radicals is greater than that of the reactions between free radicals and other cellular components, so the basal concentration of free radicals is kept low [4].
ROS have emerged as versatile signalling molecules that orchestrate a wide spectrum of physiological processes. For instance, they play a crucial role in activating hypoxia-inducible factors (HIFs) [5], which, in turn, induce the expression of erythropoietin (EPO) to boost red blood cell production, vascular endothelial growth factor (VEGF) to stimulate angiogenesis and glycolytic enzymes to maintain ATP levels under hypoxic conditions [6]. On the other side, increased ROS in the body can induce autophagy through a variety of signalling pathways [7][8]. At low levels, increased ROS also activates the immune response [9][10]. The importance of ROS in the immune response is demonstrated by the fact that individuals with an inherited deficiency in some of the enzymes that produce physiological ROS develop chronic granulomatous disease (CGD) and are unable to defend themselves against common infections [11].
The body has antioxidant mechanisms to quench excessive ROS production [12]. Antioxidants can be classified into endogenous and exogenous ones. Endogenous antioxidants are generated by the body itself. They can be enzymatic, such as superoxide dismutase (SOD), catalase, glutathione peroxidase and glutathione reductase, or non-enzymatic, such as some metabolites (including lipoid acid, glutathione, L-arginine, melatonin and bilirubin) [13]. Exogenous antioxidants are supplied externally through food and drink intake, as these nutrients cannot be produced by the body and must be incorporated through the diet. They include molecules such as vitamins E and C, carotenoids, trace metals (selenium, zinc and manganese), flavonoids, omega 3 and 6 fatty acids, etc. [14]. The sources and generation of ROS and antioxidants are summarized and illustrated in Figure 1.
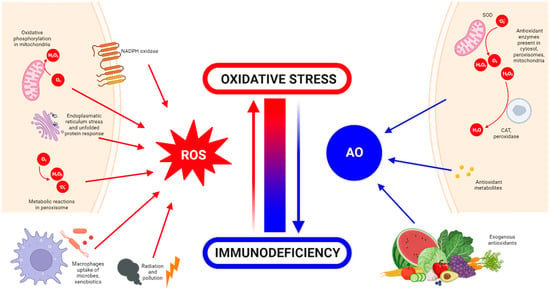
Figure 1. ROS and antioxidant (AO) generation and sources in the body. Made in ©BioRender-biorender.com.
Oxidative stress, first proposed in 1990 by Sohal and Allen [15], is defined as a disturbance in the balance between oxidant production and antioxidant activity [16]. This imbalance can arise either from excessive ROS generation or from a decline in the scavenging capacity or availability of antioxidants. The accumulation of ROS due to this imbalance leads to oxidative damage, affecting essential cellular macromolecules such as DNA, proteins and lipids. Over time, this damage can contribute to the development of chronic inflammation [17]. Inflammation has long been recognized as a primary driver of ROS overproduction, a physiological response triggered by detrimental stimuli and conditions such as infection or tissue injury. This response is aimed at neutralization of the damaging agent and homeostatic restoration of the tissues involved. Due to the complexity of the inflammatory response, it requires careful regulation to initiate, maintain, aggravate or modulate the inflammatory response. A restrained inflammatory response is generally considered beneficial but can be destructive when dysregulated and chronic. Chronic inflammation has been implicated in the pathogenesis of several diseases, including cancer, cardiovascular diseases and osteoarthritis, among others [18][19]. When the body is stimulated by exogenous and proinflammatory factors such as the exposure to excessive sunlight, pathogens or chemicals, ROS generated within the body can surpass the antioxidant defence capacity of cells, leading to a disruption in redox homeostasis [7][20].
2. Detrimental Effects of ROS in the Body
Oxidative stress has been demonstrated to play a key role in the pathobiology of multiple organs, including the lungs, brain, skin, joints, bones, kidneys, eyes, heart and blood vessels. Increased ROS is also related to multiorgan diseases such as cancer, aging, diabetes, inflammation and infections [18]. These diseases align with the consequences of elevated ROS levels within cells. As previously mentioned, ROS can inflict detrimental effects, including DNA or RNA damage [21], membrane lipid and phospholipid peroxidation, and protein oxidation [22]. This damage is often irreversible and prevents cells from performing their physiological functions.
Amongst nucleic acids, mitochondrial DNA is often more susceptible to oxidative damage caused by ROS generated by the respiratory chain, largely owing to its proximity [21]. Mitochondrial DNA mutations have been linked to various disorders, particularly neurodegenerative diseases [23]. Nuclear DNA and RNA can also be targeted by ROS. The primary mechanism of ROS-induced DNA damage involves the reaction between the hydroxyl radical and the double bonds of DNA’s nitrogenous bases, resulting in hydroxylated adduct formation. The most studied bioproduct of this reaction is 8-oxoguanine or 8-hydroxyguanine from guanine [24]. The hydroxyl adducts generated by hydroxyl radical attack can themselves react further with DNA, initiating a cascade of oxidative damage and leading to the formation of additional ROS. The hydroxyl radical, in addition to reacting with the nitrogenous bases, can also react with sugar by subtracting oxygen from the 2′-deoxyribose residues causing strand breaks (Figure 2a) [25]. These types of alterations have been linked to some diseases such as cancer [26]. Furthermore, with these types of modifications, ROS can also attack nucleosomes leading to DNA unpacking and fragmentation. These genetic and epigenetic alterations can result in the deregulation of oncogenes and tumour suppressor genes [27][28].
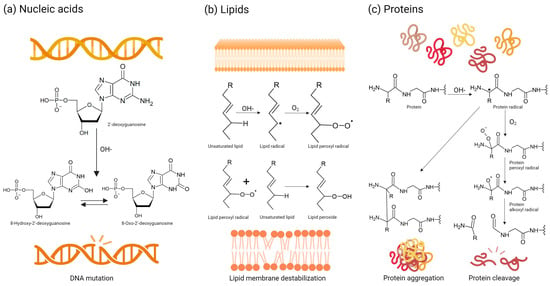
Figure 2. Main reactions between ROS and biomolecules, and detrimental effects of ROS in the body. (a) DNA alterations by ROS. (b) Lipid peroxidation by ROS. (c) Protein modifications by ROS. Made in ©BioRender-biorender.com.
The unsaturated fatty acids in cell membranes can be oxidized by ROS, resulting in the formation of lipid peroxides. Elevated levels of lipid peroxides are linked to atherosclerosis, heart failure, Alzheimer’s disease, rheumatoid arthritis, cancer and various immunological disorders [29]. Free radicals that come into contact with lipids initiate oxidation of the lipid chains, resulting in the formation of hydroperoxidized lipids and alkyl radicals. In the context of cell membranes, this effect is particularly significant due to the substantial presence of lipids. The alkyl radicals formed during the process are extremely reactive and can react with other polyunsaturated fatty acids, causing the chain reaction to continue until two free radicals react with each other. The structure and physical properties of the membrane are altered by these chemical changes, affecting its integrity, fluidity and, in turn, function (Figure 2b) [30].
Proteins are also affected by oxidative stress. Both non-enzymatic and enzymatic proteins are indispensable for the structural integrity and functional processes of the body. ROS can lead to dysregulation of enzyme catalytic activity and metabolic pathways. Proteins are made up of amino acids, which can be oxidized by ROS. The peptide bond that joins amino acids can be affected by ROS, resulting in its cleavage. Finally, oxidative stress can also cause protein aggregation (Figure 2c). Depending on the affected tissue, these effects have been linked to neurodegenerative diseases, rheumatoid arthritis and other diseases [31].
Dietary supplementation of antioxidants has been attempted to reduce the effects of inflammation and reduce oxidative stress, but their low bioavailability and stability are major drawbacks. Indeed, in the same way as ROS, antioxidants are highly reactive, making storage difficult due to their rapid oxidation. Similarly, their reactivity, even after in vivo administration, can lead to low bioavailability [32]. To improve the bioavailability of potential antioxidant compounds, materials and biomaterials have been devised, particularly in the domain of tissue engineering, to facilitate sustained and prolonged exposure of antioxidant compounds.
References
- Juan, C.; Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Bolisetty, S.; Jaimes, E.A. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344.
- Magnani, F.; Mattevi, A. Structure and Mechanisms of ROS Generation by NADPH Oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97.
- Lichtenberg, D.; Pinchuk, I. Oxidative Stress, the Term and the Concept. Biochem. Biophys. Res. Commun. 2015, 461, 441–444.
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A.; Chan, D.A.; Giaccia, A.J.; Richard, D.E. Hypoxia-Inducible Factor-1 Activation in Nonhypoxic Conditions: The Essential Role of Mitochondrial-Derived Reactive Oxygen Species. Mol. Biol. Cell 2010, 21, 3247–3257.
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-Dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J. Biol. Chem. 2007, 282, 2871–2879.
- Gao, Q. Oxidative Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 179–198.
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ 2015, 22, 377–388.
- Tal, M.C.; Sasai, M.; Lee, H.K.; Yordy, B.; Shadel, G.S.; Iwasaki, A. Absence of Autophagy Results in Reactive Oxygen Species-Dependent Amplification of RLR Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 2770–2775.
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR Signalling Augments Macrophage Bactericidal Activity through Mitochondrial ROS. Nature 2011, 472, 476–480.
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. NOX2-Dependent Regulation of Inflammation. Physiol. Behav. 2017, 176, 139–148.
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130.
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553.
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging In Vivo. Free Radic. Biol. Med. 2014, 66, 24–35.
- Sohal, R.S.; Allen, R.G. Oxidative Stress as a Causal Factor in Differentiation and Aging: A Unifying Hypothesis. Exp. Gerontol. 1990, 25, 499–522.
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295.
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167.
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435.
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659.
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19.
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742.
- Abuja, P.M.; Albertini, R. Methods for Monitoring Oxidative Stress, Lipid Peroxidation and Oxidation Resistance of Lipoproteins. Clin. Chim. Acta 2001, 306, 1–17.
- Picca, A.; Calvani, R.; Coelho-junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537.
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Env. Sci. Health C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139.
- Muftuoglu, M.; Mori, M.P.; Souza-Pinto, N.C. de Formation and Repair of Oxidative Damage in the Mitochondrial DNA. Mitochondrion 2014, 17, 164–181.
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219.
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28.
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative Stress, DNA Methylation and Carcinogenesis. Cancer Lett. 2008, 266, 6–11.
- Ramana, K.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease. Oxid. Med. Cell. Longev. 2013, 2013, 583438.
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular Dynamic Simulations of Oxidized Skin Lipid Bilayer and Permeability of Reactive Oxygen Species. Sci. Rep. 2019, 9, 4496.
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734.
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
921
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
10 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




