1. Skin Wound Healing
Skin wounds are physical injuries of the skin tissue, which lead it to break and open. Wound healing is a complex process, which requires a coordinated series of cellular and molecular events, which aim to restore the integrity and functionality of damaged tissues
[1][2][3]. It includes inflammation, cell migration, tissue formation, and remodeling, and due to lack of effectiveness or slow therapeutic action, new strategies are needed for efficient and fast wound healing
[4][5][6]. In this context, Morsy et al.
[7] developed a novel nanoemulgel formulation containing atorvastatin for wound healing application. Despite the fact that atorvastatin (ATR) is primarily prescribed as a lipid-lowering medication, to manage cholesterol levels, high cholesterol levels have been associated with impaired wound healing, which means that atorvastatin may indirectly contribute to improved wound healing outcomes
[8][9][10]. Furthermore, this drug also shows anti-inflammatory properties, and angiogenic effects, which promote the formation of new blood vessels, leading to an adequate blood supply that is crucial for tissue regeneration
[11][12][13]. Additionally, it has also been described as targeting and inhibiting the growth of microorganisms, including common wound pathogens, and has been linked to other pleiotropic effects, such as antioxidant properties, modulation of cellular signaling pathways, and promotion of cell proliferation and migration, which make this drug a quite relevant candidate due to multiple wound-healing-related beneficial effects
[11][14][15]. The incorporation of ATR into a nanoemulgel formulation intended to allow a controlled and sustained release at the wound site, maximizes its potential therapeutic effects. Hence, the developed nanoemulgel was prepared by using a combination of high-pressure homogenization and ultrasonication techniques. First, the gel was prepared by adding sodium carboxymethyl cellulose (CMC) to water and stirring continuously until gel formation. After that, a primary O/W emulsion was made, containing ATR solubilized in a mixture of liquid paraffin, Tween
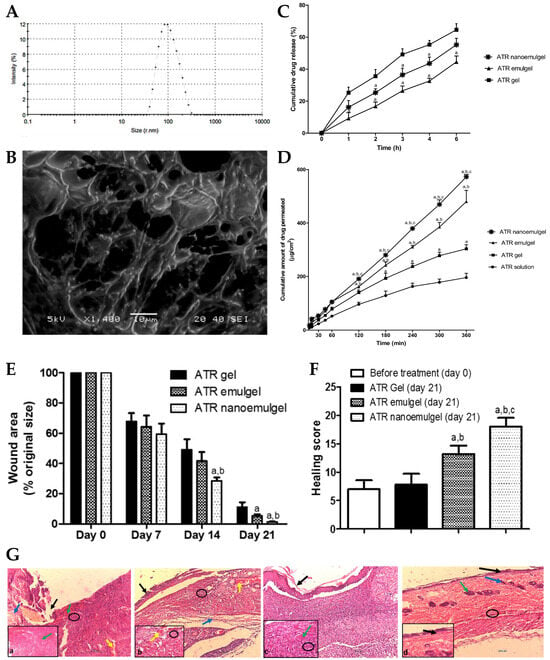
® 80, and propylene glycol, to which water was slowly added, and vortexed. Then, to this drug-loaded emulsion, the polymeric gel base was added and mixed for 5 min. Afterward, this primary emulgel was sonicated, for 10 min, in order to reduce its droplet size and finally obtain the required nanoemulgel. The developed formulations were characterized for particle size, PDI, zeta potential, viscosity, spreadability, in vitro drug release, stability, ex vivo permeation, and in vivo wound healing properties. The physicochemical characterization showed a small particle size of approximately 100 to 200 nm (
Figure 1A,B), a good homogeneity with a PDI value of less than 0.3, and the zeta potential was found to be within the range of −20 to −30 mV, indicating good stability and preventing particle aggregation. Additionally, the viscosity and spreadability of the nanoemulgel was determined to be in the range suitable for topical application, ensuring ease of spreading and adherence to the wound area. The in vitro drug release profile (
Figure 1C), determined across semipermeable cellulose membranes for 6 h, demonstrated a sustained release of the drug from the developed nanoemulgel over time, with a reduced initial burst release when compared to a CMC gel, and with a higher overall release when compared to an emulgel. Moreover, stability studies, which it underwent for a duration of 6 months, under storage conditions of 60% relative humidity and a temperature of 4 °C, indicated no noticeable alterations in several evaluated properties, such as color, appearance, spreading, or viscosity. In addition, an ex vivo permeation study (
Figure 1D), through excised rat skin, revealed that the ATR nanoemulgel had a higher permeation, both in what concerns cumulative amount and velocity, than the drug-loaded emulgel, gel, or solution, after 2 h, also exhibiting the shortest lag time. Furthermore, a histopathological analysis of rats’ skin, after nanoemulgel topical application in an in vivo study, supported its positive effect on wound healing, showing reduced inflammation and increased angiogenesis, with a marked improvement in the skin histological architecture, and considerable healing after 21 days of ATR nanoemulgel treatment (
Figure 1E–G). Additionally, although the developed gel-based formulations may encounter drawbacks such as limited residence time at the application site, the nanoemulgel formulation can address this concern by exhibiting enhanced retention in the affected area, and therefore prolonged retention, enabling a steady release of the drug, and facilitating extended contact with the skin surface.
Figure 1. (
A) Droplet size distribution of the developed ATR nanoemulgel; (
B) surface morphology of the developed ATR nanoemulgel; (
C) in vitro drug release profiles of the developed ATR nanoemulgel, compared to an emulgel and gel; (
D) ex vivo drug permeation profiles of the developed ATR nanoemulgel, compared to an emulgel, a gel, and a solution; (
E) wound area variation of rat skin after topical administration of the developed ATR formulations, after 0, 7, 14, and 21 treatment days; (
F,
G)—healing score (
F) and photomicrographs (
G) of rat skin before treatment (a), and after 21 days of topical administration of an ATR gel (b), an ATR emulgel (c), or an ATR nanoemulgel (d), where black arrows represent the absence of epidermal layer epithelization, blue arrows represent loss of collagen fibers normal arrangement in the dermal layer, green arrows represent severe congestion, yellow arrows represent hemorrhage, and black circles represent inflammatory cell infiltrations; ATR—atorvastatin; adapted from Morsy et al.
[7], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
Wounds are strongly connected to health disorders such as immune system diseases, diabetes, chronic peripheral vascular disorders, and various infectious and inflammatory diseases. In this context, chronic wounds pose a significant healthcare challenge due to their slow healing and susceptibility to infections
[16][17]. Eucalyptol has been reported to function as a good penetrant in transdermal and topical drug delivery systems and is also claimed to possess antibacterial properties against human and food-borne pathogens
[18][19]. Hence, Rehman et al.
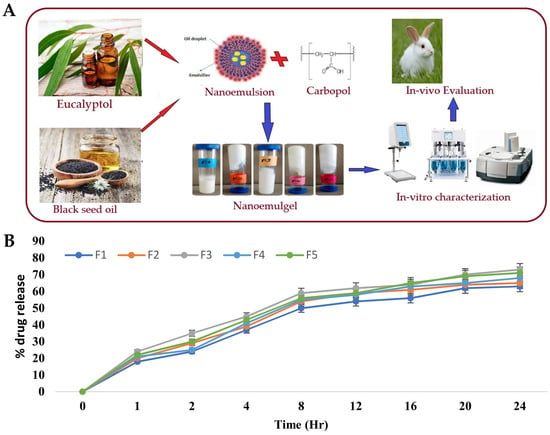
[20] developed a nanoemulgel for wound healing incorporating eucalyptus oil, obtained from
Eucalyptus globulus, into a nanoemulgel (
Figure 2A), developing an effective platform designed to enhance the stability, permeation, and controlled release of eucalyptol, one of the main constituents of eucalyptus oil, thereby promoting its therapeutic efficacy in wound healing. The preparation of the nanoemulgel was divided into two steps, with the first including the preparation of different primary O/W nanoemulsions by solvent emulsification diffusion method. The nanoemulsions were made of an aqueous phase containing the hydrophilic surfactant Tween
® 80 and distilled water, and an oil phase containing black seed oil, the hydrophobic surfactant Span
® 60, and the cosurfactant/cosolvent propylene glycol. These two phases were mixed together using a magnetic stirrer, and then the nanoemulsion was produced by droplet size reduction using a high-speed homogenizer. From the selected primary nanoemulsion, nanoemulgels were created, where Carbopol
® 940 was used as the gelling agent. A Carbopol gel was produced by adding it to distilled water and mixing until a clear solution was formed, and then the pre-prepared nanoemulsion was added to the gel, in ratio of 1:1, with the pH being adjusted to a value of 5–6 using triethanolamine. These nanoemulgels were subsequently subjected to characterization. For the stability studies, temperature tests and centrifugation were used, with all formulations being subjected to storage at different conditions, namely, 8 °C, 25 °C, 40 °C, and 40 °C, with 40% relative humidity, for 28 days. Results showed that all the formulations were stable under the studied conditions, with no phase separation being observed after subsequent centrifugation. A Fourier-transform infrared spectrophotometer analysis was also employed to investigate the chemical interactions and compatibility between the components. By analyzing the produced spectra, it was possible to identify specific functional groups and molecular vibrations, confirming the absence of any major chemical changes or incompatibilities that could potentially affect the stability or therapeutic properties of the formulations. It was also shown that the pH had a major effect on the stability of the systems, as triethanolamine, used to adjust the formulation’s pH to simulate the pH of the skin, affected the transparency and disrupted the internal structure of the formulations. Furthermore, organoleptic homogeneity tests were performed, where changes in color, phase separation, consistency, and liquefaction were observed. All the formulations were observed to be off-white in color, and smooth in terms of consistency, and showed also reasonable to good spreadability. Additionally, the drug content analysis showed that the drug was uniformly distributed throughout the nanoemulgels. In the study of in vitro drug release (
Figure 2B), the nanoemulgel which released the highest amount of drug was selected. The selected nanoemulgel’s particle size, PDI, and zeta potential were also determined. The droplet size was found to be around 139 ± 5.8 nm, the PDI was less than 0.45, and the zeta potential was measured to be −28.05 mV. After formulation physicochemical characterization, an in vivo study evaluated the wound-healing activity of the nanoemulgel in rabbits. The percentage of wound contraction was measured over a 15-day period, and they compared a negative control group, a nanoemulgel group, and a standard commercial product group. The results showed that the percentage of wound contraction for the nanoemulgel group on day 15 was 98.17%, indicating a significant improvement in wound healing compared to the negative control group (70.84%).
Figure 2. (
A) Schematic representation of the developed eucalyptus oil nanoemulgel, including partial composition and general indication of performed studies; (
B) in vitro eucalyptol release profiles of different nanoemulgels; adapted from Rehman et al.
[20], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
Algahtani et al.
[21] also aimed to develop a novel topical nanoemulgel formulation, loaded with thymoquinone (TMQ), and evaluate its effectiveness in wound healing. TMQ is a bioactive compound derived from
Nigella sativa, and it has been widely studied for its potential therapeutic effects, including antioxidant, antimicrobial, anti-inflammatory, and wound healing properties. It has shown promise in promoting wound closure and tissue regeneration. However, its effectiveness in wound healing is limited by poor water solubility and low skin permeation
[22][23][24]. Hence, the study aimed to develop a nanosystem formulation, encapsulating TMQ within the internal phase’s oil droplets, using black seed oil as a natural carrier, and stabilized by a surfactant and cosurfactant mixture, with the addition of the aqueous phase leading to a primary O/W nanoemulsion. To prepare the TMQ-loaded nanoemulsion, a high-energy method was used, employing an ultrasonication technique. Initially, the researchers prepared a coarse emulsion by combining 5%
w/
w (50 mg/g) of TQM with the mixture of the oil phase and a surfactant/cosurfactant mixture (Kolliphor
® EL/Transcutol
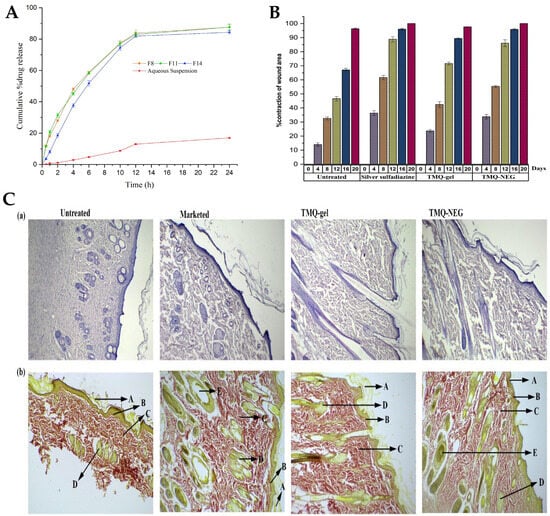
® HP). The components were mixed by using a vortex mixer, and then added to the aqueous phase, while continuously vortexing for 1 min. Then, in order to improve the emulsion’s properties, the coarse emulsion was subjected to ultrasonication, using an ultrasonic homogenizer in a water bath. This process helped to break down larger droplets into smaller ones, and enhance the stability of emulsion, transforming it into a nanoemulsion. The increase in surfactant mixture concentration decreased the mean droplet size, and the ultrasonication time also significantly influenced the mean droplet size and PDI of the nanoemulsions. When the ultrasonication time increased from 3 to 5 min, the mean droplet size decreased. Nevertheless, an ultrasonication time higher than that, leading to an excessive exposure to ultrasonication energy known as overprocessing, caused intense turbulence, leading to collisions between the nanoemulsion’s droplets and their subsequent coalescence, resulting in larger droplet sizes. The average globule sizes of selected nanoemulsions varied between 40.02 and 99.66 nm, and the PDI value between 0.052 and 0.542. Nanoemulsion viscosity was also measured, being between 71.04 mPas and 88.82 mPas, and drug content varied between 98.74% and 99.32%. The zeta potential of these primary nanoemulsions was measured to be in the range of −26.7 to −30.6 mV, which is expected to contribute to their stability, since high repulsive forces between the nanoemulsion droplets might help prevent their coalescence. Furthermore, the preliminary formulations’ stability was effectively assessed, and all were found to be stable when subjected to various tests, including heating–cooling cycles, centrifugation, and freeze–thaw cycles. The formulations that demonstrated thermodynamic stability were selected for the in vitro drug release studies (
Figure 3A) using the dialysis bag technique. The bags were filled with 1 mL of TMQ nanoemulsion formulation, and throughout the study, at specific time intervals, aliquots were withdrawn and replaced with PBS, up to 24 h. The aliquots were then analyzed by using UV-spectroscopy to quantify the amount of TMQ released from each formulation. After 12 h, more than 80% of the drug was released from all screened nanoemulsions, with the maximum cumulative drug release (at 24 h) varying between 84.3% and 87.1%. Additionally, comparing the TMQ nanoemulsions to a TMQ aqueous suspension, a significantly higher drug release was observed in the nanoemulsions. A selected drug-loaded nanoemulsion was then incorporated into a hydrogel system, creating the intended semisolid dosage form, a nanoemulgel. To form the nanoemulgel, the selected nanoemulsion was uniformly dispersed into a Carbopol
® 940 gel matrix. This step aimed to create a final concentration of 0.5% TQM in nanoemulgel form, with the desired consistency, making it more suitable for topical application and ensuring a patient-friendly experience. The pH of the developed nanoemulgel was found to be within the range of skin’s acid mantle (5.53), making it suitable for topical use, ensuring compatibility with the skin, and minimizing potential irritation. Moreover, the prepared nanoemulgel exhibited a similar rheological behavior to a placebo gel, demonstrating pseudoplastic behavior, with thixotropic properties, which is desirable for topical application. It also demonstrated excellent spreadability, making it suitable for topical application on wounded skin, and with the spreading area increasing proportionally with the applied force. Drug skin permeability and deposition investigation was conducted in an ex vivo study, using a Franz diffusion cell, on excised skin from Wistar rats, comparing the developed nanoemulgel to a conventional gel formulation. For that study, a shaved and excised dorsal skin sample from a Wistar rat was placed between the donor and receptor compartments of the Franz diffusion cell. Then, 500 mg of the test formulation was placed in the donor compartment, and the receptor compartment was filled with phosphate buffer. At various intervals, 1 mL aliquots were withdrawn and replaced with fresh media. Subsequently, these aliquots were diluted and quantified using a UV-spectrophotometer to estimate drug permeation through time. Results confirmed the expected enhancement in drug permeation, with the TMQ nanoemulgel showing a significantly enhanced cumulative drug permeation (549.16 μg/cm
2) when compared to the conventional gel form (120.75 μg/cm
2). Also, the percutaneous drug flux of TMQ from the nanoemulgel was approximately five times higher (23.14 µg/cm
2·h) than from the conventional gel form (4.78 µg/cm
2·h), as was the permeability coefficient (9.26 K × 10
−3 vs. 1.91 K × 10
−3). This enhanced permeation may be attributed to the presence of surfactant/cosurfactant mixture in the developed formulation, and to the small droplet size characteristic of the developed nanosystem. In addition, in order to estimate drug deposition in rat skin, the tape stripping technique was employed. After the 12 h ex vivo skin permeability study, the skin sample was removed from the assembly and washed with buffer. The first strips were discarded, and the subsequent 15 strips were used to remove the subcutaneous layer. The treated skin sample and the stripped tape were then chopped and incubated in ethanol to fully extract the drug. The incubated sample was sonicated and centrifugated before analyzing the extracted drug by using a UV-spectrophotometer, to determine the amount of drug deposited in the skin. The skin deposition of TMQ from the nanoemulgel form was significantly higher, measured to be 965.65 μg/cm
2, compared to 150.93 μg/cm
2 from the gel formulation. Moreover, the local accumulation efficiency of the nanoemulgel was higher by a factor of 1.4 when compared to the conventional gel, indicating a greater drug accumulation in the skin for localized and prolonged therapeutic action. Moreover, according to the in vivo studies performed in a Wistar rat wound model, the application of the nanoemulgel on the wounds resulted in accelerated wound closure, evidenced by reduced wound size, enhanced re-epithelization, and increased collagen deposition, with higher efficacy than control groups (no treatment, standard 1%
w/
w silver sulfadiazine cream, and TMQ conventional gel) (
Figure 3B). On the fourth day post-wounding, untreated rats displayed a hard thrombus swelling and exudates at the wound area. In contrast, animals from other groups exhibited a comparatively softer thrombus with reduced inflammation and no discharge. By the eighth day, reddish connective tissue, or granulation tissue, started forming in all groups. The complete epithelization time for the untreated group was 16.6 days. The groups with 1% silver sulfadiazine cream, TMQ conventional gel, and TMQ nanoemulgel had significantly shorter complete epithelization periods of 11.6, 14.33, and 10.33 days. A histopathological analysis (
Figure 3C), on day 20 after treatment, revealed that the TMQ-nanoemulgel-treated group displayed larger amounts of granulation tissue and fewer mononuclear inflammatory cells compared to animals treated with 1% silver sulfadiazine and TMQ conventional gel. These findings suggest that the developed TMQ nanoemulgel has a significant wound healing potential, comparable to the 1% silver sulfadiazine cream, making it a promising formulation for topical wound healing applications. Thus, a topical TMQ nanoemulgel formulation was developed, by combining biocompatible polymers, oils, surfactants, and cosurfactants, resulting in a stable nanoemulgel with enhanced drug delivery potential and, hence, improved therapeutic efficacy, making it a promising formulation for potential use in dermatological applications, and specifically for improved skin wound healing.
Figure 3. (
A) In vitro drug release profiles of the TMQ-loaded nanoemulsions, compared to a drug aqueous suspension; (
B) percentage of contraction of wound area variation, in the in vivo study, assessed for 20 days, with topical application of the developed TMQ nanoemulgel (TMQ-NEG), a conventional TMQ gel (TMQ-gel), a silver sulfadiazine formulation (Silver sulfadiazine), or no treatment (Untreated); (
C) histopathology analysis of the rat’s skin at day 20, newly healed, after topical application of the developed TMQ nanoemulgel (TMQ-NEG), a conventional TMQ gel (TMQ-gel), a silver sulfadiazine formulation (Marketed), or no treatment (Untreated), stained with hematoxylin-eosin (a) or Van Gieson (b), with arrows indicating the stratum corneum (A), the papillary dermis (B), collagen fibers (C), sebaceous glands (D), or hair follicles (E); adapted from Algahtani et al.
[21], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
Recently, natural compounds like curcumin have gained attention for their potential therapeutic effects on wound healing due to their anti-inflammatory, antioxidant, and antimicrobial properties. However, the clinical application of curcumin has been limited by its low solubility in aqueous media and poor skin permeability
[25][26][27]. To tackle these issues, Algahtani et al.
[28] developed a novel approach to enhance the wound healing potential of curcumin, through its formulation into a nanoemulgel, using the high-energy emulsification method of ultrasonication, known for its efficiency in producing nanosized droplets, in order to ensure uniform dispersion and stability of the nanoformulation. The first step involved the preparation of a preliminary O/W nanoemulsion, where curcumin was encapsulated within the oil droplets using Labrafac™ PG as the selected oil, and using a surfactant–cosurfactant system (Tween
® 80 and PEG 400). The droplet size of the curcumin-loaded nanoemulsion system was significantly influenced by the ratio of the Smix phase in the formulation components. Specifically, when the nanoemulsion system had a Smix ratio of 2:1, the droplet size was notably reduced, compared to the nanoemulsion system with a Smix ratio of 1:1. Additionally, the droplet size was found to be significantly influenced by the ultrasonication time. Specifically, 5 min of ultrasonication resulted in a notable reduction in droplet size, compared to only 3 min of ultrasonication, at constant Smix concentration and ratio. Additionally, the effect was more pronounced at a lower Smix concentration, rather than at higher ones. Nevertheless, the Smix ratio and ultrasonication time did not have a remarkable effect on the PDI. Selected preliminary nanoemulsions achieved a droplet size of less than 100 nm, with mean droplet sizes in a range from 49.61 to 84.23 nm, and PDI values from 0.10 to 0.23. Thermodynamic stress testing was also conducted on curcumin-loaded nanoemulsions, to assess their stability. The formulations exhibited high stability under heat–cooling cycles, freeze–thaw cycles, and centrifugation. This stability was attributed to the reasonably high zeta potential values, ranging from −15.96 ± 0.55 mV to −20.26 ± 0.65 mV, which minimized droplet coalescence and physical instability. The viscosity of the selected preliminary nanoemulsion systems was also evaluated, at room temperature, by rotational viscosimeter, with the results ranging from 83.74 mPas to 89.82 mPas. The difference in viscosity values was attributed to the increased concentration of surfactant between formulations (a reasonably viscous component). A UV-visible spectrophotometric analysis confirmed that the selected nanoemulsion formulations had high curcumin content, ranging from 98.86% to 99.23%. Thus, these formulations showed high drug encapsulation. With droplet sizes around 50 nm and desirable surface charges, the selected nanoemulsions were chosen for further investigation, as they were proven ideal for topical application, enabling improved skin permeability and deeper penetration. Hence, in vitro drug release was assessed, with all nanoemulsions being able to release up to 85% of curcumin within the first 12 h, while the release from aqueous suspension (control) was only around 10% at the same time. The small droplet size of the nanoemulsions could have been a critical factor in positively influencing the in vitro drug release, since it produces a large surface area for drug diffusion and, potentially, absorption to occur. The next step involved incorporating the curcumin nanoemulsion into a Carbopol
® 940 gel matrix, in order to form the curcumin nanoemulgel. The purpose was that this combination could take advantage of the benefits of both nanoemulsion and gel systems, both the high drug-loading capacity and improved skin permeation of nanoemulsions, and the enhanced stability and ease of application of gels. The drug concentration achieved in the final nanoemulgel preparation was 0.5%
w/
w of curcumin, and the formulation exhibited a favorable physicochemical profile, with a measured gel strength of 46.33 s, while the placebo gel system had a strength of 44.66 s. Additionally, the drug content uniformity of the curcumin nanoemulgel was calculated, showing a uniform dispersion of curcumin within the hydrogel, with a uniformity of 98.93%. Furthermore, the curcumin nanoemulgel exhibited a similar rheological profile to the placebo gel, with the incorporation of curcumin not affecting its rheological behavior, and exhibiting a thixotropic behavior, which is desirable for topical pharmaceutical dosage forms. Hence, the developed nanoemulgel proved to have the desired consistency for patient-friendly topical use, which aligned with small droplet size, appropriate zeta potential, a pH within the acceptable range for skin application, and optimal drug release properties, suggested its safe and effective application for wound healing. This adequacy for skin application was confirmed by the ex vivo skin permeation study results, conducted on excised rat skin, on Franz diffusion cells, to assess the ability of the curcumin nanoemulgel to penetrate the skin barrier. The results showed improved skin permeation of curcumin from the nanoemulgel formulation, further confirming its potential as an effective topical delivery system for wound healing applications. The cumulative amount of curcumin which permeated through the skin from the nanoemulgel was 773.82 μg/cm
2, versus only 156.90 μg/cm
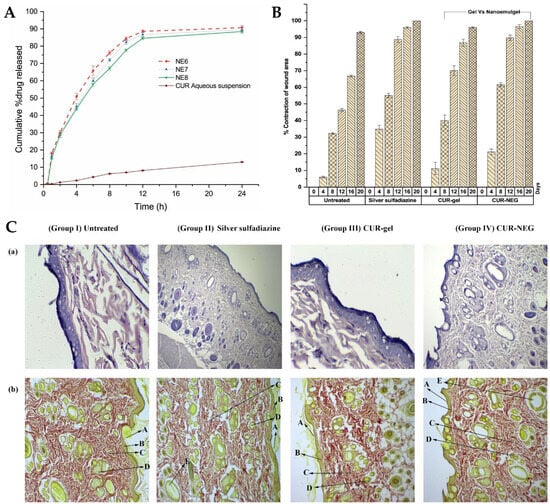
2 from the conventional gel formulation. In addition, the curcumin nanoemulgel showed a six-fold increase in percutaneous drug flux compared to the conventional curcumin gel. Similarly, the permeability coefficient from the curcumin nanoemulgel also increased approximately six-fold when compared to the conventional gel. Furthermore, the permeation enhancement ratio and local accumulation efficiency of curcumin from the nanoemulgel were significantly higher than from the gel, with a shorter lag time (0.75 h versus 2.37 h). Moreover, the in vivo wound-healing activity (
Figure 4A,B) from the nanoemulgel and gel formulations were evaluated and compared to a commonly used silver sulfadiazine formulation. The topical application of these formulations was performed and monitored, in Wistar rats, for 20 days. The results showed that all treated groups exhibited significant wound-healing activity compared to the untreated group. The curcumin nanoemulgel demonstrated almost equivalent wound-healing activity to the silver sulfadiazine gel, with both formulations leading to almost complete wound healing at day 20. Moreover, the histopathological evaluation (
Figure 4C) confirmed the enhanced wound-healing activity of curcumin from the nanoemulgel formulation, showing reduced inflammatory cells and extensive collagen fiber production.
Figure 4. (
A) In vitro cumulative drug release from the developed preliminary curcumin nanoemulsions, compared to the control (drug suspension); (
B) in vivo wound-healing activity, in a rat model, of the developed curcumin nanoemulgel (CUR-NEG), compared to a curcumin conventional gel (CUR-gel), a marketed control formulation (Silver sulfadiazine), or no treatment (Untreated), including contraction of wound area percentage; (
C) histopathology analysis of the rat’s skin tissue at day 20 after treatment, including indications for the
stratum corneum (A), the papillary dermis (B), collagen fibers (C), sebaceous glands (D), and hair follicles (E), (a) Stained with hematoxylin-eosin; (b) stained with vangeison to observe collagen formation (at 10× magnification); adapted from Algahtani et al.
[28], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
2. Skin and Skin Appendage Infections
The escalating global health threat posed by drug-resistant microbial and fungal infections necessitates the development of innovative and effective therapeutic strategies
[29][30]. In this context, miconazole nitrate is a broad-spectrum antifungal medication commonly used to treat various fungal infections, especially on the skin. However, its therapeutic efficacy can be limited by factors such as poor drug permeation and low bioavailability
[31][32]. Hence, a study by Tayah et al.
[33] aimed to address these limitations by formulating miconazole into a nanoemulgel for topical application. Formulation composition was determined by selecting the excipients in which miconazole was most soluble, by dissolving it in various oils and surfactants. It was observed that olive oil and almond oil had the highest solubility, and among the surfactants, Tween
® 80 and Span
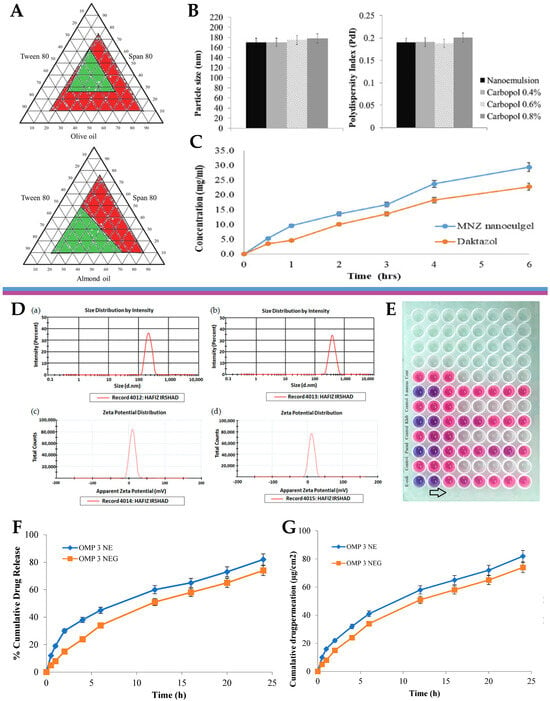
® 80 demonstrated the greatest ability to solubilize the drug. These oils and surfactants were selected as the drug vehicle, and hence preliminary O/W nanoemulsions were produced, using the self-emulsification technique. Optimized nanoemulsion formulations were chosen based on a ternary phase diagram (
Figure 5A), according to which it was evident that the almond-oil-based formulations displayed the smallest particle size (170 nm) and PDI values (0.193). Therefore, the formulation containing almond oil was selected for subsequent experiments. Miconazole nanoemulgel formulations were then prepared by incorporating the preliminary miconazole nanoemulsions into a Carbopol
® 940 hydrogel, with Carbopol at different concentrations. The particle size and PDI remained consistent when the transformation of the nanoemulsion into nanoemulgels occurred (
Figure 5B), with the three Carbopol concentrations (0.4%, 0.6%, and 0.8%) showing similar behavior, with particle sizes ranging from 170 to 180 nm. The zeta potential results confirmed the potential stability of the nanoemulgel formulations, with values just below −30 mV. Regarding the rheological properties of the nanoemulgel, its viscosity decreased with an increase in the shear rate, which indicated pseudoplastic behavior. The release of the drug was evaluated and compared to a market product, using the dialysis method. The results showed an inverse relationship between Carbopol concentration and release profile, which is in accordance with an increased viscosity. Hence, the formulation with the lowest Carbopol concentration (0.4%) exhibited the highest cumulative drug release. Furthermore, another in vitro drug release assay was also performed, this time using a Franz cell diffusion test (
Figure 5C), to measure the cumulative drug release from the selected miconazole nanoemulgel (0.4% Carbopol) and compare it to a conventional marketed Daktazol
® cream (same drug molecule). After 6 h, the developed miconazole nanoemulgel exhibited a cumulative drug release of 29.67%, while the conventional Daktazol
® cream achieved a release of 23.79%. Hence, the developed nanoemulgel achieved a higher drug release within the studied timeframe, while still retaining a controlled release profile. Then, the antifungal activity of the developed miconazole nitrate-loaded nanoemulgel was evaluated against selected fungal strains, and its performance was also compared to that of the conventional gel formulation. The antifungal activity was assessed by conducting an agar-based test on
Candida albicans, and the size of the inhibition zone was measured as an indicator of effectiveness. The miconazole nanoemulgel demonstrated the highest activity, with an inhibition zone of 40.9 ± 2.3 mm, showing significant improvements in antifungal activity when compared to the marketed formulation, hence suggesting a promising approach to overcome the limitations of current conventional gel formulations.
Figure 5. (
A) Ternary phase diagrams of the preliminary nanoemulsions containing either olive oil, Tween
® 80, and Span
® 80, or almond oil, Tween
® 80, and Span
® 80; (
B) droplet size and polydispersity index of the developed miconazole nitrate preliminary nanoemulsion and nanoemulgel formulations; (
C) in vitro drug release profiles, in Franz diffusion cells, of the developed miconazole nitrate nanoemulgel, compared to the marketed product, Daktazol
® cream; adapted from Tayah et al.
[33], reproduced with permission from Elsevier (license number 5671991093263); (
D) droplet size (a) and zeta potential (c) of the developed omeprazole-loaded nanoemulsion, and droplet size (b) and zeta potential (d) of the developed omeprazole-loaded nanoemulgel; (
E) minimum inhibitory concentration determination assay of the developed omeprazole-loaded nanoemulgel, against selected bacterial strains, using a 96-well microplate (arrow shows decrescent antimicrobial activity); (
F) cumulative drug release percentage from the developed omeprazole-loaded nanoemulsion and nanoemulgel formulations; (
G) cumulative drug permeation from the developed omeprazole-loaded nanoemulsion and nanoemulgel formulations; adapted from Ullah et al.
[34], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
Fungal infections pose a substantial public health concern, affecting a significant number of individuals globally. Conventional antifungal therapies often encounter challenges such as drug resistance and limited efficacy, and hence the need for the exploration of alternative treatment strategies arises
[35][36][37]. In this context, another study, by Vartak et al.
[38], focused on the development and characterization of a novel Ebselen nanoemulgel, intended for the effective treatment of topical fungal infections. Ebselen (EB) is a well-established antioxidant and anti-inflammatory synthetic compound; nevertheless, it has limited solubility in commonly used solvents
[39][40][41]. But since it has been proven to have reasonable solubility in dimethylacetamide (DMA), this cosolvent was selected to be part of the formulation’s composition. The rest of the excipients were also selected on a highest drug solubility basis, with Kolliphor
® ELP being selected as surfactant, and medium chain triglycerides (Captex
® 300 EP/NF) as oil phase. Additionally, in order to prevent drug precipitation and enhance formulation stability, a gelling polymer mixture was added to the external phase, hence producing an O/W nanoemulgel. Different polymers and polymer combinations were evaluated. This was achieved by mixing the components in a 5:7 ratio of oil to surfactant, and then mixing the resulting nanoemulsion in a 1:1 ratio with the gel bases, to create the final nanoemulgels. Hydroxypropyl methylcellulose (HPMC) K4M and Aquaphor, both present at a concentration of 0.5%
w/
w, formed a clear system when combined with 1 mg of EB in DMA. However, an increase in EB loading to approximately 2 mg caused immediate precipitation of EB. Similarly, when EB was added to a Carbopol
® 974P and Poloxamer 407 gel, at different gelling concentrations and loading levels, precipitation of the drug occurred. In contrast, the nanoemulgel prepared using Soluplus
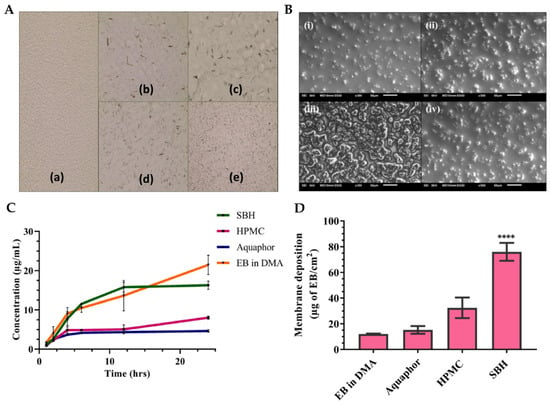
® showed a delayed precipitation effect. This polymer was also proven to be an effective solubilizer for EB, hence playing a crucial role as both a solubility and drug-loading enhancer, and a formulation viscosity enhancer and stabilizer. Optical microscopy (
Figure 6A) and scanning electron microscopy (SEM) (
Figure 6B) images of EB-loaded nanoemulgels supported the stability of the Soluplus gel, when compared to all other gel compositions, not showing any drug precipitation. In contrast, the HPMC K4M nanoemulgels displayed distinct precipitation of EB throughout the system, manifesting as large, irregularly shaped crystals with small oil globules. In the optimized Soluplus
® spontaneously formed nanoemulgel, EB was present at 1%
w/
w, and the nanosystem revealed a droplet size of 54.82 ± 1.26 nm and zeta potential of −1.69 mV. Furthermore, the findings of rheological studies showed that the optimized EB-loaded nanoemulgel displayed a non-Newtonian fluidic behavior. The observed decrease in viscosity with increasing rotational speed confirms the pseudoplastic behavior of the nanoemulgel, rendering it an ideal choice for topical application. In the in vitro drug release study (
Figure 6C), HPMC K4M and Aquaphor nanoemulgels initially showed similar drug release, but after 24 h, the HMPC K4M gel exhibited a three-times higher drug release. Interestingly, the release profile of EB in a DMA solution was similar to that of the Soluplus
® nanoemulgel, which demonstrated approximately two- and four-times higher release than the HPMC and Aquaphor formulations, respectively, after 24 h. Additionally, the optimized nanoemulgel exhibited a controlled release profile. Furthermore, the membrane deposition study (
Figure 6D), which quantified the drug entrapped within the used membrane, showed that the nanoemulgel prepared using Soluplus
® displayed a significantly higher drug deposition, compared to all other formulations, from 2.3- to 5-fold higher. Relating to the formulations’ antifungal activity, resazurin, a redox indicator, was employed to assess the viability of fungal organisms, more specifically, multi-drug-resistant
Candida albicans and
Candida tropicalis, in culture plates.
Figure 6. (
A) Optical microscopy images of the EB-loaded preliminary nanoemulgels, containing either Soluplus
® (a), HPMC (b), Poloxamer 407 (c), Carbopol
® 974P (d), or Aquaphor (e); (
B) scanning electron microscopy images of the optimized nanoemulgels, either drug-loaded Soluplus
® formulation (i), Soluplus
® vehicle (ii), drug-loaded HPMC formulation (iii), or HPMC vehicle (iv); (
C,
D) in vitro cumulative drug release (
C) and membrane drug deposition (
D) of the different EB-loaded nanoemulgels; ****
p < 0.0001; DMA—dimethylacetamide; EB—Ebselen; HPMC—hydroxypropyl methylcellulose; SBH—Soluplus
®; adapted from Vartak et al.
[38], reproduced with permission from Elsevier (license number 5672000024708).
Another study, by Almostafa et al.
[42], also focused on the development of a novel approach to combat skin bacterial infections, using a nanoemulgel formulation containing fusidic acid (FA) and myrrh oil. FA is a potent antibiotic, effective against a panoply of Gram-positive bacteria, but it has poor water solubility, which poses a significant challenge for its effective formulation
[43][44][45]. Myrrh oil, a traditional herbal extract, was used to modify and enhance the transdermal delivery of FA. In addition to these components, Tween
® 80 was used as a surfactant, Transcutol
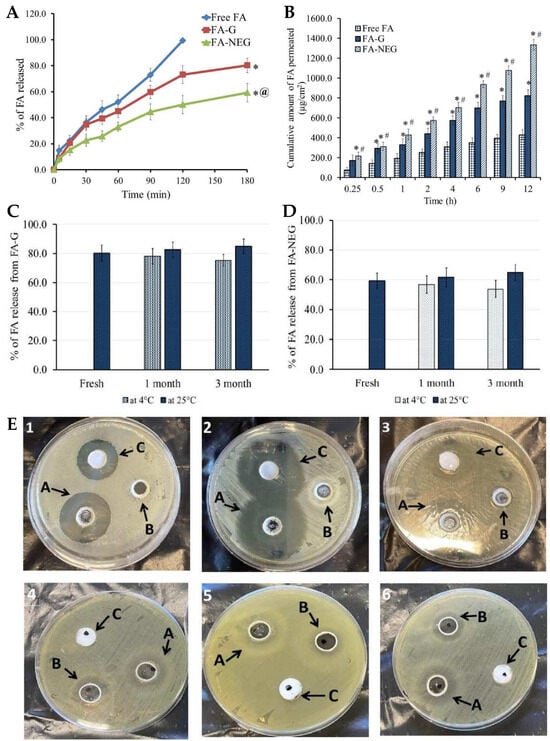
® P as a cosurfactant, and CMC as a viscosifying and gelling agent. In initial studies, it was observed that increasing oil concentrations led to a relative increase in particle size for all preliminary nanoemulsions. Contrarily, a decrease in nanoemulsion particle size was observed with an increase in surfactant concentration, while keeping the oil concentration constant, due to a reduction in surface tension. The particle size of all developed preliminary nanoemulsions ranged from 116 to 226 nm. Moreover, the in vitro release of FA from the fabricated nanoemulsion formulations was evaluated, and results showed that the percentage of FA released from all nanoemulsion formulations ranged from 40.1 to 75.6%, exhibiting a controlled release profile. Increasing oil concentration resulted in a decrease in the percentage of FA released, due to the resulting larger particle size, since it provides a smaller surface area. On the other hand, increasing Tween 80 and Transcutol P concentrations led to a substantial increase in the percentage of FA release, which can be attributed not only to these excipients’ strong solubilizing ability, but also to the resulting smaller particle size. Hence, overall, the particle size of the formulations was revealed to have a crucial role in the drug release process, as systems with smaller particle sizes achieved maximum drug release. The preliminary nanoemulsion formulation was hence transformed into a nanoemulgel by adding CMC to the external phase, and this formulation was also characterized for relevant parameters. The pH value for the FA nanoemulgel formulation was found to be within an acceptable range at 6.61, making it potentially safe for topical application and minimizing the risk of skin irritation. The viscosity was also measured to assess the formulations’ rheological behavior, as this influences drug diffusion and in vitro release. The viscosity of the developed nanoemulgel was found to be 25265.0 cP, higher than that of the corresponding CMC gel formulation, 15245.0 cP, which was also measured for comparison purposes. Hence, both fell within an appropriate range for topical application. The spreadability, which determines whether the uniform application of the formulation on the skin is possible or not, was also determined. Results showed that the FA gel exhibited a spreadability of 40.5 mm, while the nanoemulgel formulation had a spreadability of 33.6 mm, both indicating excellent spreadability despite the observed difference between the two formulations. The formulations were also studied for stability during storage at 4 °C and 25 °C, for 1 and 3 months, and both showed nonsignificant variation in physical properties under all studied conditions, compared to their freshly prepared counterparts. The in vitro drug release (
Figure 7A) of FA from the developed gel and nanoemulgel formulations was also evaluated and compared to an FA suspension. The results revealed that the drug was completely released from the suspension after 120 min, reaching 99.5% of cumulative release. In contrast, the FA gel and nanoemulgel formulations released 80.3% and 59.3% of FA after 180 min, exhibiting a more controlled release profile, as is intended for topically applied formulations. These results can be explained by the substantially increased viscosity of the gel and, especially, the nanoemulgel formulations, which slowed down the diffusion of the drug from the formulations and into and through the membrane. Additionally, the nanoemulgel formulation could have acted as a drug reservoir, where the drug passed from the inner phase to the outer one, further slowing the release rate. Furthermore, FA release from both preparations remained consistent during storage (
Figure 7C,D), when compared to freshly prepared formulations. The results confirm the stability of the formulations and demonstrate the efficacy of nanoemulgels as nanocarriers. Skin permeability studies (
Figure 7B) were also conducted, with the permeability of FA when incorporated into different formulations being evaluated using excised animal skin. The results further supported the potential of the developed formulations, since they showed that the FA nanoemulgel exhibited the highest permeability, followed by the FA gel, and only then the FA suspension, which exhibited the lowest skin permeability. This proven superiority of the nanoemulgel and gel formulations when compared to the drug suspension could be due to the incorporation of Transcutol P, a known effective permeation enhancer, especially effective in increasing permeation through the skin (more specifically, the
stratum corneum), which certainly contributed to the significantly improved drug permeation profile through rat skin. The safety of the developed formulations was also tested, with the animals’ back skin being treated with the test formulations, and then undergoing a thorough examination to check for any sensitivity reactions. Fortunately, no signs of inflammation, irritation, erythema, or edema were observed on the inspected area during the entire 7-day study period. These results indicate that the formulations are potentially safe and well tolerated by the skin, without causing any adverse reactions or sensitivity issues. Moreover, the antibacterial activity of the developed FA nanoemulgel was evaluated (
Figure 7E) against various microorganisms and compared to a placebo nanoemulgel and a common marketed cream. The FA nanoemulgel showed significant antibacterial activity against
Staphylococcus Aureus,
Bacillus subtilis, and
Enterococcus faecalis, with a larger inhibition zone than the placebo or the marketed cream. Moreover, both the FA-loaded nanoemulgel and the placebo nanoemulgel exhibited significant inhibition zones against
Candida albicans,
Shigella, and
Escherichia coli, while the marketed cream showed a negative effect against these bacteria. The combination of FA and myrrh oil in the developed nanoemulgel likely contributed to this enhanced antibacterial activity. In conclusion, FA was successfully incorporated into a nanoemulgel prepared with myrrh essential oil, showing good physical properties for topical application, enhanced skin permeation, no skin irritation, and potent antibacterial and antifungal activity, against several different types of microorganisms, with the combination of FA and myrrh essential oil showing synergistic effects. Hence, the results demonstrated that the developed nanoemulgel showed promise as a potential topical or transdermal drug delivery system for the treatment of skin bacterial or fungal infections, offering a new platform for innovative and effective topical treatments, and providing a basis for future research and clinical applications in dermatology.
Figure 7. (
A) In vitro FA release profiles from the developed nanoemulgel (FA-NEG), compared to a conventional gel (FA-G) and a drug suspension (Free FA), where *
p < 0.05 compared to the drug suspension, and @
p < 0.05 compared to the conventional gel; (
B) ex vivo FA permeation profiles, across rat skin, from the developed nanoemulgel (FA-NEG), compared to a conventional gel (FA-G) and a drug suspension (Free FA), where *
p < 0.05 compared to the drug suspension, and #
p < 0.05 compared to the conventional gel; (
C,
D) variation of the in vitro drug release during stability studies, under storage at 4 °C and 25 °C for 1 and 3 months, for the developed nanoemulgel (C) and conventional gel (D) formulations; (
E) inhibition zone diameter photographs after treatment with the developed FA-loaded nanoemulgel (A), placebo nanoemulgel (B), or marketed FA formulation (C), on
Bacillus subtilis (1),
Staphylococcus aureus (2),
Enterococcus faecalis (3),
Candida albicans (4),
Shigella (5), and
Escherichia coli (6); FA—fusidic acid; adapted from Almostafa et al.
[42], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
3. Skin Cancer
Skin cancer is a prevalent form of cancer with increasing incidence rates worldwide. Conventional treatment options often come with limitations, such as systemic side effects and inadequate skin permeation
[46][47][48]. To fight these limitations, Nagaraja et al.
[49] also developed a novel topical nanoemulgel formulation, incorporating nanosized droplets encapsulating the drug, chrysin, a flavonoid with proven anticancer potential, into a gel matrix for improved drug delivery and extended release, aiming for it to be an effective skin cancer treatment
[50][51][52]. Stable lipid-based nanoemulsifying preconcentrates containing chrysin, made of Capryol
® 90 (oil and hydrophobic surfactant), Tween
® 80 (hydrophilic surfactant), and Transcutol
® HP (cosurfactant and cosolvent), were designed to be easily reconstituted in a gel base made of Pluronic
® F127 (gelling agent). The formulation components were selected in order to achieve the highest chrysin solubilization and, consequently, drug strength in the final formulation. Additionally, a surfactant mixture of 2:1 of Tween
® 80 to Transcutol
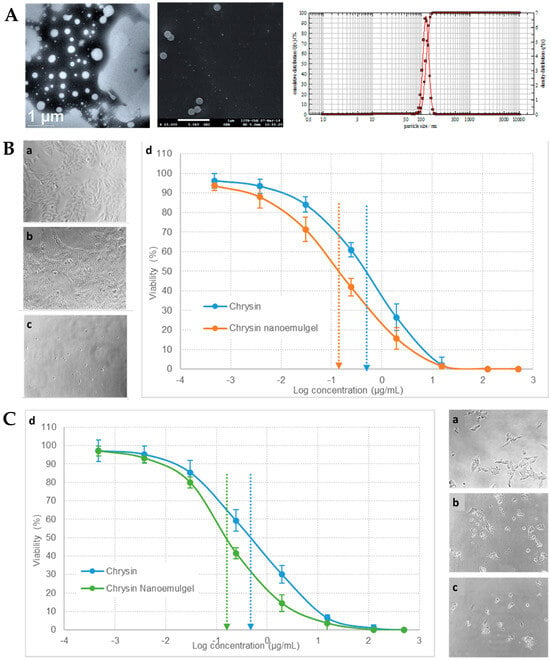
® HP was proven to be the best combination for highest solubilization. The physicochemical characterization of the nanoemulsifying drug delivery system showed its small particle size (
Figure 8A), of less than 300 nm, a good homogeneity, with a PDI value around 0.26, indicating narrow droplet size distribution, and a negative zeta potential value, of about −15 mV, indicative of reasonable formulation stabilization potential. Moreover, the formulation was further stabilized by the incorporation of the oil droplets in a semisolid gel base. The chrysin-nanoemulsifying preconcentrate was added to water containing the gelling agent, Pluronic
® F-127, at 10 °C, for easier mixture and overall nanoemulgel preparation, due to this polymer’s thermosensitive properties. In addition, the droplet size and PDI values of the system did not change significantly after a 3-month storage period, hence ensuring its good stability under the studied conditions. Regarding the results of the performed ex vivo permeation studies (rat abdominal skin), while a chrysin conventional Pluronic
® gel base, used as control, showed poor penetration through the skin, the developed nanoemulgel exhibited a superior performance due to the presence of nanosized droplets, which resulted in better and faster skin permeation. In vitro cytotoxicity studies of the developed chrysin nanoemulgel were performed on various skin cancer cell lines, including A375 (human melanoma,
Figure 8B), A375.S2 (human melanoma), SK-MEL-2 (human melanoma,
Figure 8C), B16-F1 (murine melanoma), and A431 (human epidermoid carcinoma). Results showed that chrysin had matrix metalloproteinase-2 inhibitory activity, leading to a strong antiproliferative effect, with cell mobility and migration inhibition, with the developed nanoemulgel having a better performance than the control formulation, leading to deeper changes in cancer cell morphology. Furthermore, biocompatibility tests were also carried out, on L929 cells (noncancerous murine cells), which showed no changes in cell growth, therefore making the developed formulation safe for topical application. In conclusion, the incorporation of chrysin into a topical nanoemulgel formulation resulted in a significant enhancement of its therapeutic response in cytotoxicity studies, with the developed drug delivery platform technology exhibiting substantial advantages when compared to conventional formulations, such as its versatility, extended skin permeation, and retention, and the ability to potentially reduce systemic drug absorption.
Figure 8. (
A) Developed chrysin nanoformulation’s droplet size and size distribution, with transmission electron microscopy image (left), scanning electron microscopy image (middle), and photon cross-correlation spectroscopy results (right); (
B) A375 cells’ morphological observation and growth inhibition after no treatment (control cells, a), treatment with chrysin solution (b), or treatment with the developed chrysin nanoemulgel (c), and respective in vitro cytotoxicity profile (cell viability %) (d); (
C) SK-MEL-2 cells’ morphological observation and growth inhibition after no treatment (control cells, a), treatment with chrysin solution (b), or treatment with the developed chrysin nanoemulgel (c), and respective in vitro cytotoxicity profile (cell viability %) (d); adapted from Nagaraja et al.
[49], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
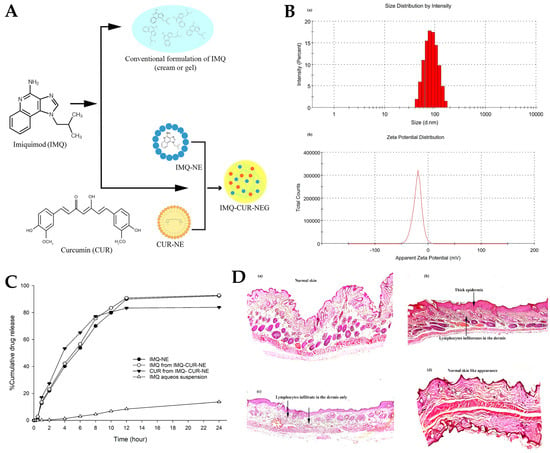
A different study, by Algahtani et al.
[53], aimed to develop a novel formulation that combined the immunomodulatory effects of imiquimod (IMQ) and the anti-inflammatory properties of curcumin (CUR), for skin cancer treatment, using a nanoemulgel delivery system (
Figure 9A). IMQ is a commonly used chemotherapeutic agent for skin cancer
[54][55]. On the other hand, the combination with CUR has been shown to improve the therapeutic effectiveness of various chemotherapeutics, with this molecule also having intrinsic anticancer properties
[56][57]. Nevertheless, the topical delivery of IMQ and CUR can be difficult due to their poor solubility and low skin penetration properties, and IMQ has been shown to lead to psoriasis-like lesions when applied topically
[58][59]. Hence, the purpose of the incorporation of both IMQ and CUR into a topical nanoemulgel was to not only enhance drug permeation and provide sustained release of these drugs, but also reduce IMQ’s topical side effects, leading to improved therapeutic outcomes for skin cancer patients. For the selection of the most adequate excipients, IMQ solubility studies were performed at room temperature, and results showed that this drug’s solubility was maximized in oleic acid, in the oil category, which best mixed with Tween
® 20, in the surfactant category, and Transcutol
® HP, in the cosurfactant/cosolvent category, to form a stable nanoemulsion system through spontaneous emulsification, an advantageous low-energy method. Different ratio mixtures of oil and surfactant/cosurfactant were tested, and the different nanoemulsions were formed by addition of the aqueous phase. The preparation procedure included the accurate weighting of the necessary quantity of CUR and dissolving it completely in a homogeneous mixture of oil, surfactant, and cosurfactant, with the help of vortex mixing. Then, the aqueous phase was added, and the mixture was vortexed again. Clear, easily flowable, and transparent formulations were selected for further studies and characterized for relevant parameters. The analysis showed that the optically clear nanoemulsion formulations were composed of fine dispersed droplets in nanosized dimensions, ranging between 91.07 and 98.88 nm (
Figure 9B). An increase in oil droplet size was correlated with an increase in oleic acid concentration and a decrease in surfactant/cosurfactant concentration. The zeta potential of the droplet surface was negative, ranging from −10.9 to −35.8 mV (
Figure 9B), due to the presence of anionic groups in the fatty acids and glycols present in the nanoemulsions’ composition. This could be a potential advantage, as nanoemulsion droplets with a negative zeta potential tend to be more able to penetrate deeper in the skin. For the thermodynamic stability studies, the nanoemulsion formulations were subjected to stress conditions, namely, centrifugation, and heating–cooling and freeze–thaw cycles. Results confirmed their stability, since they did not show any physical changes such as phase separation, creaming, cracking, or coalescence. The final selected preliminary nanoemulsion formula, with a drug strength of 15 mg/mL, had a high percentage of drug content of 99.26%, a narrow droplet size distribution (10.57 nm) with a PDI of 0.094, a negative zeta potential of −18.7 mV, and a viscosity of 125.48 cP. The in vitro drug release for the optimized nanoemulsion was also performed (
Figure 9C) using the dialysis bag technique. The release of IMQ from an aqueous suspension (control) was just 11% at 24 h, while the release from the preliminary nanoemulsion, containing IMQ only, was quite high, being around 92%. The incorporation of CUR into the nanoemulsion did not affect the release of IMQ, since the drug release from the formulation combining both drugs was 92.84% for IMQ and 83.94% for CUR. The selected nanoemulsion was then transformed into a nanoemulgel by incorporating Carbopol
® 934 as the gelling agent. The nanoemulgel had a mean droplet size of 78.39 nm and PDI of 0.254, which were still considered adequate for system stability and topical application. In spreadability studies, the spreading factors of the placebo nanoemulgel, the IMQ-loaded nanoemulgel, and the IMQ-CUR-loaded nanoemulgel were found to be equivalent, being equal to 0.82 cm
2/m, 0.85 cm
2/m, and 0.87 cm
2/m, respectively. Hence, all formulations showed good extrudability potential from the container tube, adequate for patient-friendly applications. The overall drug content in the final nanoemulgel formulation was quite high, being around 99%, and the measured pH was 5.5, adequate in order to minimize skin irritation, since the ideal value is between 5 and 6. Ex vivo skin permeation and deposition studies were then performed in rat skin using Franz diffusion cells. Again, different formulations were compared, namely, the placebo nanoemulgel, the IMQ-loaded nanoemulgel, and the IMQ-CUR-loaded nanoemulgel. Formulations were introduced in the donor compartment, and the aliquots were taken at specified periods of time, filtered, and analyzed using UV-visible spectrophotometry. The tape stripping technique was used to determine the amount of drug deposited on the skin layer. The skin deposition of IMQ from the IMQ-nanoemulgel was 1205.2 μg/cm
2, and 1367 μg/cm
2 from the IMQ-CUR-nanoemulgel, which was 5 times higher than that obtained from a conventional gel formulation (control). CUR skin deposition was also around 9 times higher (5178 μg/cm
2) in the developed IMQ-CUR-nanoemulgel than in the conventional gel formulation (570 μg/cm
2). The percutaneous IMQ drug flux was also determined, being equal to 0.042 µg/cm
2.h for the IMQ-nanoemulgel, and 0.071 µg/cm
2.h for the IMQ-CUR-nanoemulgel, which was around 18 times higher than for the conventional gel formulation (0.004 µg/cm
2.h). Hence, the developed nanoemulgel showed an improved permeation profile when compared to a conventional gel formulation, with the nanoemulgel containing both IMQ and CUR displaying apparent synergistic effects. In vivo studies were also performed, on mice, and skin pathological changes after 10 days of topical application of different formulations were monitored. Psoriasis-like symptoms started to appear on mice treated with the IMQ conventional gel formulation from the 2nd day of application and worsened until the 10th day. On the other hand, mice treated with the IMQ-nanoemulgel exhibited a delayed appearance of these symptoms, which was possibly correlated with a more controlled drug release from the formulation. Fortunately, the application of the IMQ-CUR-nanoemulgel did not lead to the appearance of psoriasis-like symptoms, which was connected with the antipsoriatic activity of CUR. Additionally, on the 11th day after the application of the formulations on the skin, the skin was collected for histopathology analysis (
Figure 9D). While untreated skin showed regular epidermis and dermis, skin treated with the conventional gel showed hyperkeratosis, parakeratosis, acanthosis, and epidermal infiltrates. The IMQ-nanoemulgel treated skin showed comparable results, but less thickening of the epidermis layer, but the skin treated with the IMQ-CUR-nanoemulgel showed similar characteristics to untreated normal skin, with only a reduced number of infiltrates being observed. Hence, a stable nanoemulgel with optimal rheological properties, allowing easy spreadability on the skin, exhibiting high encapsulation efficiency for both IMQ and CUR, with sustained release profiles and enhanced permeation across the skin barrier, was successfully developed, while reducing psoriasis-like lesions in an animal model.
Figure 9. (
A) Schematic representation of the developed IMQ-CUR-nanoemulgel; (
B) droplet size (a) and zeta potential (b) distribution of the developed preliminary nanoemulsion; (
C) in vitro IMQ and CUR drug release profiles from different formulations, including an IMQ-nanoemulsion (IMQ-NE, with no CUR), IMQ-CUR-nanoemulsion (with both IMQ and CUR), and an IMQ aqueous suspension (control); (
D) histopathology images of the mice’s skin after the ten days of topical treatment, with either the IMQ gel (b), the IMQ-nanoemulgel (c), the IMQ-CUR-nanoemulgel (d), or no treatment (a); CUR—curcumin; IMQ—imiquimod; adapted from Algahtani et al.
[53], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).
4. Skin Inflammatory Diseases
Psoriasis is a chronic inflammatory skin disorder affecting millions of individuals worldwide, being characterized by abnormal keratinocyte proliferation and inflammation. Current treatment options have limitations; hence, there arises the need for the development of novel and effective therapies
[60][61][62]. In this context, another study, by Pund et al.
[63], investigated the transcutaneous delivery of leflunomide, an immunomodulatory drug, using a nanoemulgel formulation for the treatment of both melanoma and psoriasis
[64][65][66]. Based on leflunomide solubility studies, the excipients selected to form the preliminary O/W nanoemulsion were Capryol
® 90, Cremophor
® EL, and Transcutol
® HP, as oil base, surfactant, and cosolvent, respectively. These components were found to have good miscibility with each other, and the formed preliminary nanoemulsion was transformed into a nanoemulgel by incorporating it into a gel matrix made of Pluronic
® F-127. The preliminary nanoemulsions were evaluated for droplet size, which was found to be in the range of 98.7 to 280.92 nm, with a PDI between 0.2 and 0.3, indicative of narrow size distribution, and a slightly negative zeta potential of −7,8 mV. These characterization parameters were also measured in the nanoemulgel, with no significant changes being observed. Regarding the nanoemulgel’s viscosity, the shear thinning nature of the poloxamer gel was demonstrated by the decrease in viscosity at higher shear rates, which allows for effortless dispensing of the product from the container and smooth application on the skin. Additionally, the nanoemulgel demonstrated potent antipsoriatic activity by inhibiting the proliferation of human keratinocytes (HaCaT cell line) and reducing proinflammatory cytokine levels, such as interleukin-6 and tumor necrosis factor-alpha. The suppression of these proinflammatory cytokines is crucial in mitigating the inflammatory response associated with psoriasis. Moreover, the developed formulations also exhibited antipsoriatic activity by effect on leukocyte infiltration and keratinocyte proliferation. Furthermore, the nanoemulgel displayed significant antimelanoma activity by inducing apoptosis in A375 and SK-MEL-2 melanoma cells and inhibiting tumor cell proliferation. These effects were attributed to the cytotoxicity of leflunomide, which can target multiple signaling pathways involved in cancer growth and survival. Safety assays involved systemic biocompatibility assessment, by hemolytic toxicity evaluation, with results showing that the nanoemulgel had a minimal 0.25% hemolytic toxicity compared to the positive control (Triton-X), suggesting its compatibility with blood cells and potential for reducing the risk of adverse effects. In conclusion, the development and characterization of a novel leflunomide-loaded nanoemulgel was successful, with the results indicating that the developed formulation possesses favorable physicomechanical characteristics for transcutaneous delivery and exhibiting promising in vitro antipsoriatic and antimelanoma activity, suggesting its potential as a novel therapeutic approach for the treatment of these diseases.
In another study, by Shehata et al.
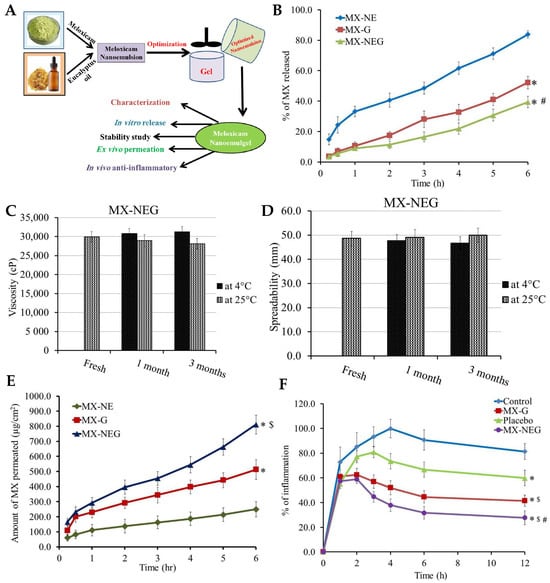
[67], the authors presented the development, characterization, and optimization of a novel eucalyptus-oil-based nanoemulgel loaded with meloxicam (
Figure 10A) aimed at enhancing the anti-inflammatory efficacy of the drug for topical application. Nonsteroidal anti-inflammatory drugs, such as meloxicam (MX), have been widely used to alleviate inflammatory conditions; however, their application is often limited by issues like systemic toxicity and poor drug delivery to the targeted site
[68][69]. To overcome these limitations, a nanoemulgel formulation was designed to facilitate controlled drug release and improve local drug concentration, as chronic inflammation is a critical pathophysiological process associated with numerous dermatological disorders with a strong inflammatory basis, hence necessitating effective and targeted therapeutic interventions. Therefore, in their research, several preliminary nanoemulsion formulations were developed by carefully blending specific ingredients. To create the oily phase, 1% (
w/
w) of MX was combined with a precise amount of eucalyptus oil and a cosolvent/cosurfactant, Transcutol
® P. For the aqueous phase, varying quantities of Tween
® 80 (as a surfactant) and PEG 400 (as a cosurfactant) were mixed with distilled water. The two phases were then meticulously merged, but achieving a well-mixed and stable nanoemulsion required high shear homogenization, so the formulation’s homogeneity was further enhanced by sonication. Additionally, the high solubility of meloxicam in the various components further confirmed their suitability as part of the formulation’s composition, with solubility values of 216 ± 11 mg/mL in eucalyptus oil, 130 ± 10 mg/mL in Tween
® 80, 68 ± 6 mg/mL in Transcutol
® P, and 146 ± 5 mg/mL in PEG 400. In the pursuit of optimal formulations, several nanoemulsions incorporating MX were developed, with different excipient proportions. Remarkably, all formulations exhibited excellent stability at room temperature, showing no signs of phase separation. The developed nanoemulsions displayed particle sizes within the nanometric range, ranging from 139 ± 2.31 to 257 ± 3.61 nm. Predictably, an increase in oil concentration led to larger particle sizes, likely due to a corresponding increase in the dispersed phase. Conversely, when the surfactant concentration was increased, while keeping the oil concentration constant, the formulation’s particle size decreased, a finding that aligned with previous studies, which reported an inverse relationship between nanoemulsion particle size and surfactant concentration. The results of the in vitro drug release studies (
Figure 10B) performed on the preliminary nanoemulsions showed that the percentage of MX released from the NE formulations varied between 55.0 ± 2.8% and 87.6 ± 3.9% after 6 h, hence exhibiting a controlled release profile. Notably, increasing the oil concentration in the preparation led to a decrease in MX release, likely due to the larger particle size, resulting in a smaller surface area available for drug release. The optimized nanoemulsion formulation was then transformed into a nanoemulgel by integrating HPMC as the gelling agent. A conventional HPMC gel formulation, containing MX, was also developed, for comparison purposes. Both the MX-loaded nanoemulgel and the MX-gel formulation appeared smooth, homogenous, and physically stable, demonstrating drug distributions above 99.3%, indicating a uniform dispersion of the drug. Additionally, both formulations, and especially the MX-nanoemulgel formulation, showed suitable viscosity for easy skin application (
Figure 10C) and satisfactory spreadability (
Figure 10D). Moreover, stability assessment, over 1 and 3 months, showed nonsignificant variations in the MX-nanoemulgel formulation’s characterization parameters. In vitro drug release comparison revealed that the MX-nanoemulgel exhibited a lower percentage of drug release (39.4 ± 3.7%) compared to the MX-gel (52.1 ± 4.2%) and the optimized preliminary nanoemulsion (83.9 ± 2.41%), hence exhibiting a more controlled release pattern. This could be attributed to the incorporation of HPMC into the formulation’s external phase, resulting in a slower drug release due to increased viscosity. Nevertheless, the results of the ex vivo skin permeation study (
Figure 10E), across rat skin, supported the superiority of the developed nanoemulgel when compared to the other formulations, since it was observed that after 6 h a significantly greater amount of MX permeated from the nanoemulgel formulation, with a steady-state transdermal flux value of 141.28 ± 9.17 μg/cm
2, compared to the permeation from the MX-gel formulation, which showed a value of 84.28 ± 10.83 μg/cm
2. The permeation of the MX-nanoemulgel was found to be enhanced by 1.68-fold when compared to the conventional MX-gel. This increased permeation of the drug from the nanoemulgel formulation can be attributed to its small particle size, leading to a larger surface area than the conventional gel formulation, since by incorporating the drug into nanosized globules its permeation through the skin layer is facilitated. Furthermore, the presence of Tween
® 80, PEG 400, and Transcutol
® P in the formulation also played a vital role in enhancing MX permeation from the nanoemulgel, due to these surfactants/cosurfactants’ capacity for increasing drug permeation through biological barriers. Moreover, according to the skin irritation tests performed on animals treated with the developed topical MX-nanoemulgel to ensure the safety of the formulation, no irritation, erythema, or edema were observed, indicating its safety. In addition, an in vivo anti-inflammatory study (
Figure 10F), in which edema was induced in the rats’ hind-paw, was also performed. The thickness of the edema directly correlated with the percentage of inflammation. After 12 h, inflammation percentages were 81.3 ± 6.4%, 59.8 ± 6.4%, 41.5 ± 4.6%, and 27.8 ± 5.7% for the control group, placebo (nanoemulgel vehicle, with no drug), MX-gel, and MX-nanoemulgel groups, respectively. The placebo group’s reduced inflammation percentage, when compared to the control group, confirmed the anti-inflammatory effects of eucalyptus oil. Furthermore, the MX-nanoemulgel-treated group demonstrated a significantly higher anti-inflammatory effect when compared to the MX-gel group, suggesting the nanoemulgel’s greater anti-inflammatory effect due to improved skin permeability. Hence, the study provided evidence of eucalyptus oil’s anti-inflammatory effects, and its synergistic action with MX, making the developed nanoemulgel a promising option for treating inflammation-related skin conditions. In conclusion, an MX-loaded nanoemulgel was successfully developed and optimized, offering a promising nanocarrier topical formulation for enhanced drug delivery, good adhesion, and easy application onto the skin.
Figure 10. (
A) Schematic representation of the developed MX and eucalyptus oil nanoemulgel, including performed physicochemical and efficacy characterization studies; (
B) in vitro drug release profiles of the developed preliminary MX-nanoemulsion (MX-NE), conventional MX-gel (MX-G), and MX-nanoemulgel (MX-NEG), with *
p < 0.05 compared to the preliminary MX-nanoemulsion, and #
p < 0.05 compared to the conventional MX-gel; (
C,
D) stability profiles of the developed MX-nanoemulgel formulation, after 1 and 3 months, under storage at 4 °C and 25 °C, in what concerns viscosity (C) and spreadability (D); (
E) ex vivo drug permeation profiles of the developed preliminary MX-nanoemulsion (MX-NE), conventional MX-gel (M-G), and MX-nanoemulgel (MG-NEG), with *
p < 0.05 compared to the preliminary MX-nanoemulsion, and $
p < 0.05 compared to the conventional MX-gel; (
F) anti-inflammatory effects of various formulations on rat hind-paw edema, including the developed MX-nanoemulgel (MG-NEG), a conventional MX-gel (M-G), a placebo formulation (nanoemulgel vehicle with no drug), and a control group (no treatment), with *
p < 0.05 compared to the control group, $
p < 0.05 compared to the placebo group, and #
p < 0.05 compared to the conventional MX-gel group; MX—meloxicam; adapted from Shehata et al.
[67], reproduced with permission from MDPI (Creative Commons CC BY 4.0 470 license).