Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivan Šoša | -- | 2279 | 2024-01-08 10:23:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 2279 | 2024-01-10 01:41:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Šoša, I. Quetiapine-Related Deaths. Encyclopedia. Available online: https://encyclopedia.pub/entry/53542 (accessed on 08 February 2026).
Šoša I. Quetiapine-Related Deaths. Encyclopedia. Available at: https://encyclopedia.pub/entry/53542. Accessed February 08, 2026.
Šoša, Ivan. "Quetiapine-Related Deaths" Encyclopedia, https://encyclopedia.pub/entry/53542 (accessed February 08, 2026).
Šoša, I. (2024, January 08). Quetiapine-Related Deaths. In Encyclopedia. https://encyclopedia.pub/entry/53542
Šoša, Ivan. "Quetiapine-Related Deaths." Encyclopedia. Web. 08 January, 2024.
Copy Citation
Quetiapine is a second-generation antipsychotic drug available for two and half decades. Due to increased misuse, prescription outside the approved indications, and availability on the black market, it is being encountered in medicolegal autopsies more frequently. For instance, it has been linked to increased mortality rates, most likely due to its adverse effects on the cardiovascular system.
forensic toxicology
quetiapine
tissue modeling
1. Introduction
Quetiapine is an atypical antipsychotic drug (a second-generation antipsychotic drug) used to treat schizophrenia, bipolar, borderline personality, and major depressive disorders; broadly speaking, this treatment has numerous neurocognitive, neuroprotective, and potential off-label indications [1][2]. Developed in 1985, the US approved quetiapine for medical use in 1997; now, it is on the World Health Organization’s List of Essential Medicines [3][4]. Regarding the non-approved uses of approved drugs, the most frequent such use for quetiapine is its wide use as a sleep aid due to its sedating effects [5][6]. The benefits of off-label use do not appear to outweigh the side effects. Nevertheless, it is reported to treat conditions such as Tourette’s syndrome, musical hallucinations, etc. [7][8][9]. Unlike most other antipsychotics, its hypnotic and sedative effects offset any problems with patient compliance.
Quetiapine’ appears to have low dopamine receptor affinity and intense antihistamine activity, which renders it similar to sedating antihistamines [10]. Approximately 90% of serotonin in the human body is stored in the gastrointestinal tract, and quetiapine has a moderate affinity for its receptors [11]. Notwithstanding, quetiapine shows an affinity for various neurotransmitter receptors [12]. Not only does it enhance the serotoninergic transmission, but serotonin, a key neurotransmitter of the brain–gut axis, also plays a vital role in the pathogenesis of emotional distress and gastrointestinal diseases [13]. Specifically, it binds serotonin (5-hydroxytryptamine; 5HT) 5HT2A, adrenergic (α1), muscarinic, and histaminergic receptors, and it has a relatively weak affinity for dopamine D2 receptors [14][15], with an occupancy half-life about twice as long as that for plasma. All of these are cell-surface receptors that intervene in cellular communication.
For quetiapine toxicity to be fatal, it is necessary to combine it with other drugs [16]. Acute overdose typically results in sedation or hypotension and tachycardia, but cardiac arrhythmias, coma, and death have also been reported [17]. For some of them, severe overdosage may result in seizures requiring intubation/mechanical ventilation.
Some cases are hallmarked by cardiac and sinus tachycardia [18][19][20]. Generally, 10–25 mg/L levels are observed in the blood samples obtained from fatal cases during postmortem examinations. Non-toxic levels in postmortem blood extend to around 0.8 mg/kg, but, at the same time, toxic levels in postmortem blood can begin at 0.35 mg/kg. The serum or plasma of quetiapine overdose survivors had concentrations ranging from 1 to 10 mg/L [21][22][23].
Even though the blockage of histamine-1 receptors produces the soothing effect of quetiapine, arrhythmogenic effects result from the channel inhibition of the ether-a-go-go-related gene (hERG). This may influence the QT interval [24]. The presence of some cardiovascular pathologies, for example, coronary disease, could be the lethal trigger if quetiapine is used, as seen in polydrug intoxications [25][26]. Quetiapine’s deadly effect is governed by whether some medication potentiates this inhibition effect and, if so, to what extent [27][28]. As for respiratory depression, Culebras et al. reported its incidence in three patients on combined antipsychotic–opioid therapy [29]. In randomized clinical trials (RCTs) involving humans, considering the interactions of first-generation antipsychotics and morphine, sedation was scored on a sedation score tool. In eight of the fourteen RCTs, increased sedation scores were reported when morphine and droperidol were combined [19][27]. After the drug’s ingestion and its rapid absorption, it reaches the maximum plasma concentration after 1.5 h, where it binds mostly (83%) to non-specific plasma proteins (human albumin) [13][15][30]. Quetiapine’s bioavailability depends mainly on its first-pass metabolism, which is as poor as 9% [31][32]. Notably, the liver metabolizes many drugs, resulting in the production of water-soluble compounds that can be excreted via the bile [33]. In one stage, this process relies upon the “phase 1 reactions” mediated by cytochrome p450 (CYP). Oxidation, reduction, and hydrolysis reactions are mainly directed by the CYP isozyme CYP3A4. This explains why any drug interaction that modifies quetiapine’s metabolism and pharmacokinetics is more likely to occur with drugs that are inhibitors or inducers of CYP3A4, rather than inhibitors of CYP2D6.
2. Use and Misuse
In a formal sense, issues related to the misuse and abuse potential of quetiapine have not been regarded as a danger. However, those who administer quetiapine should be cautious when prescribing it to individuals with a history of substance abuse (particularly with opioids or anxiolytics). These individuals are “loose cannons” and are at increased risk.
Typically, people whose deaths are related to quetiapine are men in their mid-forties. Their leading causes of death at this age are drug toxicity and natural diseases. Less frequently, however, these deaths are linked to physical assaults [17].
Occasionally, quetiapine is associated with drug misuse, but it has limited potential for misuse [34]. Misuse is most often seen in patients with a history of polysubstance abuse and/or mental illness (especially those who are detained in prisons or secure psychiatric institutions) because the limited access to alternative intoxicants brings quetiapine to the fore.
However, quetiapine has been found to be associated with drug-seeking behavior more than any other atypical antipsychotic. It has standardized street prices and slang terms, such as “Q-ball” (referring to the intravenous injection of quetiapine mixed with cocaine), either alone or in combination with other drugs [16][19].
Quetiapine-Related Fatalities and Fatal Toxicity
Due to increased misuse and availability on the black market, quetiapine-associated deaths are frequently seen in medicolegal practice. Unintentional self-poisoning fatalities are classically related to substance abuse, mental health issues, and physical health problems; quetiapine is no exception. Fatalities—complex suicides and suicide attempts involving antipsychotic or sedative–hypnotic medications are frequently seen [35][36][37]. Poisoning homicides are rare, though they have been described, and quetiapine is used only to incapacitate the victim (as in pediatric homicides) [16][38][39].
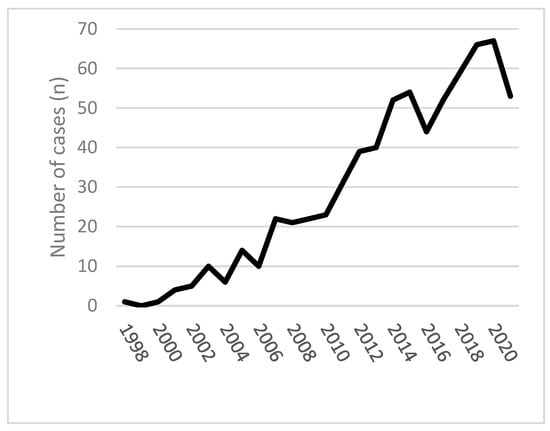
Some reports on quetiapine-related deaths and series lack clinical details or provide only single quetiapine serum concentrations rather than a kinetic course. However, increasing numbers of studies provide more detailed clinical and analytical data on severe overdose cases. Available data from 1998 to 2021 in England and Wales could be a helpful introduction to the field. In Figure 1, six hundred ninety-six deaths involving quetiapine were presented [40].

Figure 1. Quetiapine-related mortality in England and Wales 1998–2021 [40].
3. Quetiapine-Related Deaths
3.1. The Liver and Its Lobar Structure
The liver is a highly vascularized, large (typically weighing around 1.5 kg), and encapsulated organ situated to a large extent in the upper right front portion of the abdomen. It is divided into two major lobes; the smaller left lobe partially overlays the ventricle [41][42].
The left lobe is smaller and more flattened than the right. Its undersurface presents a gastric impression and omental tuberosity. Brevik et al. prepared a report of seven participants (7/14, 50%) from whom paired samples of liver tissue were obtained (both lobes). A paired t-test of two samples for means established no significant difference regarding quetiapine accumulation in either lobe. The left liver lobe is most likely more susceptible than the right lobe to the postmortem redistribution of zopiclone, and some of its constituents are thinner due to its anatomical proximity to the stomach [43][44]. The documented postmortem redistribution of the drug from the biliary system can also contribute to its apparent accumulation in hepatic tissue [45][46][47]. Classic biliary anatomy includes the left hepatic duct, which emerges from the umbilical fissure along the inferior border of the left lobe. The right hepatic duct drains the right liver lobe and comprises two major branches, the right posterior duct and the right anterior duct [48].
3.2. First-Pass Effect and the Liver
The liver is the body’s primary site for drug metabolism and contains the largest quantity of the critical cytochrome enzyme system, liver alcohol dehydrogenase, and many other enzymes. Like most xenobiotics, including drugs, quetiapine’s pharmacology and toxicology are largely inextricably linked to its metabolism. Due to its significant metabolic potential, central anatomical position, and ability to take away chemicals from the blood, the liver constitutes an organ with a high susceptibility to the effects of xenobiotics. The liver’s involvement is the most obvious in transaminase elevations. These typically occur by the third week of treatment, and levels return to baseline with continued quetiapine administration [46].
The drug’s volume of distribution while it spreads throughout the body is 10 ± 4 Ukg [15][49]. Quetiapine is orally administered as a fumarate salt in the form of tablets. Daily doses in adults range from 150 to 750 mg, and steady-state concentrations are achieved within two days of dosing [50]. No specific plasma proteins that carry quetiapine were identified; however, it is converted into the active proteins and metabolites norquetiapine and 7-hydroxy quetiapine [51][52].
The cytochrome P450 (CYP) system has been observed to extensively metabolize quetiapine in the liver, with less than 5% of the original drug appearing in urine (and minimally in other excretions). Around 73% of 150mg of quetiapine radiolabeled with 100 mCi 14C was recovered in the urine and 21% in the feces within 168 h of administration. The mean terminal half-life of quetiapine is about six hours; in its unchanged form, it accounted for less than 1% of the excreted substance [15][53].
3.3. Liver Tissue from Fresh Cadavers
Resected liver biopsies can be sliced with retained original cellular diversity and in vivo cellular architecture. They can be cultured ex vivo for two weeks [54][55]. Routine toxicology is performed on these tissues using mass spectrometry (GC-MS) or specific high-pressure liquid chromatography (HPLC) [30][56]. Both methods are relatively sensitive, with a limit of quantification for HPLC of μg/L, and the GC-MS method is accurate to 2 μg/L [15].
3.4. Liver Tissue Modeling
Normal hepatocytes, constituting nearly 60% of the total cell population within the liver, along with the HepaRG cell line, are capable of performing the majority of liver functions, including many drug-processing activities at various levels [57]. Transcribing liver-specific genes at high levels without fresh human tissue is challenging, but it can even provide differentiated hepatocyte-like HepaRG cells. In fact, it is more successful than any other liver cell line. As HepaRG cells express most of the drug-processing genes, including major CYPs and UGTs, it should not be surprising that these cell lines have been used as pharmacological and toxicological models [57][58][59].
Even though 2D models are flexible, affordable, and valuable for studies that require large numbers of cells, most cell lines do not have normal liver-specific functions, including those relevant to toxicology. They are genetically abnormal and do not adequately reproduce hepatocyte biology. Meanwhile, 3D cultures offer cell–cell and cell–extracellular matrix interactions, though these methods are often more challenging to translate into high-throughput tactics. Primary hepatocytes in 3D modeling can form spheroids, prolonging the maintenance of hepatic phenotype and function. The ability to transiently proliferate and self-organize is a well-known ability of hepatic cells, and it has also been taken advantage of in forming liver organoids. Organoid models have been developed from various hepatic cell types, and all exhibit various degrees of similarity to human hepatocytes [55][60][61]. Hepatocytes with or without non-parenchymal cells can be spatially patterned in 3D, using, for instance, soft lithography. Combining 3D printing technologies with cytocompatible biological “inks” enables engineers to bioprint tissue models, incorporating parenchymal and stromal cells in spatially patterned arrangements. Unfortunately, such “futuristic” models are challenging to make and maintain.
Since in vivo quetiapine metabolism pathways generate well-defined metabolite derivatives, this drug was used to explore the consistency of the in vitro metabolic model. Out of many emerging preclinical human-relevant in vitro models used to evaluate toxic injury to the liver, in silico modeling has shown good potential in terms of its affordability and easy maintenance [57]. Mathematical modeling, referred to as physiologically based pharmacokinetic (PBPK) modeling, is basically an in silico technique where mathematical modeling is used to inform and optimize the design in, for instance, forensic toxicology [62].
3.5. Blood
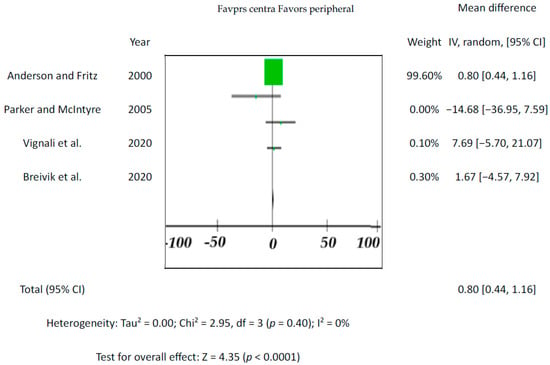
Since the drug’s blood level is the one that affects the individual, blood is the most important tissue for toxicological analysis. This accounts for central (e.g., heart) and peripheral (e.g., femoral) blood. Although there are cases where peripheral vs. central blood concentrations differed significantly, none of the five included studies showed significant differences between the endpoints (p-values varied from 0.18 to 0.59; for overall effect see Figure 2). The results from previous studies indicate that drug concentrations in the central blood are generally higher than in the peripheral blood [63].

3.6. Brain Tissue
Several studies considered brain tissue’s quetiapine, and in the study of Breivik et al. this concentration correlated moderately (positive correlation) with that in the peripheral blood (r = 0.5); unfortunately, the linear regression model was below the level of statistical significance [45]. Skov et al. even claim brain concentrations are about four times those in the blood [65].
3.7. Skeletal Muscle
Breivik et al. even concluded that, in the absence of blood, skeletal muscle may be treated as a preferred matrix for quetiapine concentrations since its concentration in skeletal muscle correlated well with that in peripheral blood.
4. Conclusions
When blood is not available, the analysis of other tissues can provide important information that helps diagnose potential intoxication with quetiapine. The search for an adequate alternative endpoint seems rational, considering the increasing trend of quetiapine misuse and overdoses.
The relatively high concentrations of quetiapine in the liver tissue, and the modest (if any) statistical significance when correlating other endpoints with blood, cast a suspicion on any straightforward recommendation for selecting a relevant matrix. Further investigations and the integration of results obtained in silico and in vitro are needed to improve routine forensic toxicology. Recent endeavors where hair or nails were used as surrogate endpoints point out the advantages of keratin matrices that are much more resistant to post-mortem decomposition than other biological samples.
References
- Soeiro, D.E.S.M.G.; Dias, V.V.; Missio, G.; Balanza-Martinez, V.; Valiengo, L.; Carvalho, A.F.; Moreno, R.A. Role of quetiapine beyond its clinical efficacy in bipolar disorder: From neuroprotection to the treatment of psychiatric disorders (Review). Exp. Ther. Med. 2015, 9, 643–652.
- Morrison, P.; Taylor, D.M.; McGuire, P. Schizophrenia and Related Psychoses. In The Maudsley Prescribing Guidelines in Psychiatry; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–224.
- World Health Organization (WHO). WHO Model List of Essential Medicines-22nd List; WHO: Geneva, Switzerland, 2021.
- Curry, D.E.; Richards, B.L. A Brief Review of Quetiapine. Am. J. Psychiatry Resid. J. 2022, 18, 20–22.
- Lin, C.Y.; Chiang, C.H.; Tseng, M.M.; Tam, K.W.; Loh, E.W. Effects of quetiapine on sleep: A systematic review and meta-analysis of clinical trials. Eur. Neuropsychopharmacol. 2023, 67, 22–36.
- Anderson, S.L.; Vande Griend, J.P. Quetiapine for insomnia: A review of the literature. Am. J. Health Syst. Pharm. 2014, 71, 394–402.
- Kreys, T.J.; Phan, S.V. A literature review of quetiapine for generalized anxiety disorder. Pharmacotherapy 2015, 35, 175–188.
- Sacks, O. Musicophilia-La Musique, le Cerveau et Nous; Média Diffusion: Paris, France, 2018.
- Kalari, V.K.; Morrison, P.E.; Budman, C.L. Atypical antipsychotics for treatment of Tourette syndrome. In International Review of Movement Disorders; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4, pp. 203–235.
- Fischer, B.A.; Boggs, D.L. The role of antihistaminic effects in the misuse of quetiapine: A case report and review of the literature. Neurosci. Biobehav. Rev. 2010, 34, 555–558.
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342.
- Moreines, J.L.; Owrutsky, Z.L.; Gagnon, K.G.; Grace, A.A. Divergent effects of acute and repeated quetiapine treatment on dopamine neuron activity in normal vs. chronic mild stress induced hypodopaminergic states. Transl. Psychiatry 2017, 7, 1275.
- Barandouzi, Z.A.; Lee, J.; Del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648.
- Mlambo, R.; Liu, J.; Wang, Q.; Tan, S.; Chen, C. Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders. Pharmaceuticals 2023, 16, 603.
- DeVane, C.L.; Nemeroff, C.B. Clinical pharmacokinetics of quetiapine: An atypical antipsychotic. Clin. Pharmacokinet. 2001, 40, 509–522.
- Pilgrim, J.L.; Drummer, O.H. The toxicology and comorbidities of fatal cases involving quetiapine. Forensic Sci. Med. Pathol. 2013, 9, 170–176.
- Gibiino, S.; Trappoli, A.; Balzarro, B.; Atti, A.R.; De Ronchi, D. Coma After Quetiapine Fumarate Intentional Overdose in a 71-year-old Man: A Case Report. Drug Saf. Case Rep. 2015, 2, 3.
- Parker, D.R.; McIntyre, I.M. Case studies of postmortem quetiapine: Therapeutic or toxic concentrations? J. Anal. Toxicol. 2005, 29, 407–412.
- Andersen, F.D.; Simonsen, U.; Andersen, C.U. Quetiapine and other antipsychotics combined with opioids in legal autopsy cases: A random finding or cause of fatal outcome? Basic Clin. Pharmacol. Toxicol. 2021, 128, 66–79.
- Wu, C.S.; Tsai, Y.T.; Tsai, H.J. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: A nation-wide case-crossover study. J. Am. Heart Assoc. 2015, 4, e001568.
- Saito, T.; Tsuji, T.; Namera, A.; Morita, S.; Nakagawa, Y. Comparison of serum and whole blood concentrations in quetiapine overdose cases. Forensic Toxicol. 2022, 40, 403–406.
- Ostad Haji, E.; Wagner, S.; Fric, M.; Laux, G.; Pittermann, P.; Roschke, J.; Hiemke, C. Quetiapine and norquetiapine serum concentrations and clinical effects in depressed patients under augmentation therapy with quetiapine. Ther. Drug Monit. 2013, 35, 539–545.
- Balit, C.R.; Isbister, G.K.; Hackett, L.P.; Whyte, I.M. Quetiapine poisoning: A case series. Ann. Emerg. Med. 2003, 42, 751–758.
- Cubeddu, L.X. Iatrogenic QT Abnormalities and Fatal Arrhythmias: Mechanisms and Clinical Significance. Curr. Cardiol. Rev. 2009, 5, 166–176.
- El Mazloum, R.; Snenghi, R.; Zorzi, A.; Zilio, F.; Dorigo, A.; Montisci, R.; Corrado, D.; Montisci, M. Out-of-hospital cardiac arrest after acute cocaine intoxication associated with Brugada ECG patterns: Insights into physiopathologic mechanisms and implications for therapy. Int. J. Cardiol. 2015, 195, 245–249.
- Montisci, M.; Thiene, G.; Ferrara, S.D.; Basso, C. Cannabis and cocaine: A lethal cocktail triggering coronary sudden death. Cardiovasc. Pathol. 2008, 17, 344–346.
- Andersen, F.D.; Joca, S.; Hvingelby, V.; Arjmand, S.; Pinilla, E.; Steffensen, S.C.; Simonsen, U.; Andersen, C.U. Combined effects of quetiapine and opioids: A study of autopsy cases, drug users and sedation in rats. Addict. Biol. 2022, 27, e13214.
- Khokhar, M.A.; Rathbone, J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst. Rev. 2016, 12, CD002830.
- Culebras, X.; Corpataux, J.B.; Gaggero, G.; Tramer, M.R. The antiemetic efficacy of droperidol added to morphine patient-controlled analgesia: A randomized, controlled, multicenter dose-finding study. Anesth. Analg. 2003, 97, 816–821.
- Zargar, S.; Wani, T.A.; Alsaif, N.A.; Khayyat, A.I.A. A Comprehensive Investigation of Interactions between Antipsychotic Drug Quetiapine and Human Serum Albumin Using Multi-Spectroscopic, Biochemical, and Molecular Modeling Approaches. Molecules 2022, 27, 2589.
- Narala, A.; Veerabrahma, K. Preparation, Characterization and Evaluation of Quetiapine Fumarate Solid Lipid Nanoparticles to Improve the Oral Bioavailability. J. Pharm. 2013, 2013, 265741.
- Junior, E.; Duarte, L.; Suenaga, E.; de Carvalho Cruz, A.; Nakaie, C. Comparative bioavailability of two quetiapine formulations in healthy volunteers after a single dose administration. J. Bioequiv Availab. 2011, 3, 178–181.
- Schonborn, J.L.; Gwinnutt, C. The Role of the Liver in Drug Metabolism Anaesthesia Tutorial of the Week 179 17th May 2010. ATOTW 2010. Available online: https://resources.wfsahq.org/atotw/the-role-of-the-liver-in-drug-metabolism/ (accessed on 20 November 2023).
- Waal, H.; Vold, J.H.; Skurtveit, S.O. Quetiapine abuse—Myth or reality? Tidsskr. Nor. Laegeforen 2020, 140, 1228–1230.
- Ybanez, L.; Spiller, H.A.; Badeti, J.; Casavant, M.J.; Rine, N.; Michaels, N.L.; Zhu, M.; Smith, G.A. Suspected suicides and suicide attempts involving antipsychotic or sedative-hypnotic medications reported to America’s Poison Centers, 2000–2021. Clin. Toxicol. 2023, 61, 294–304.
- Cetin, N.; Konuk, N. Suicide attempt with a very high dose of quetiapine. Klin. Psikofarmakol. Bul.-Bull. Clin. Psychopharmacol. 2011, 21, 67–69.
- Kinoshita, H.; Tanaka, N.; Kumihashi, M.; Jamal, G.; Ito, A.; Yamashita, T.; Ozawa, Y.; Ameno, K. An autopsy case of drowning under the influence of multiple psychotropic drugs. Arch. Med. Sadowej Kryminol. 2019, 69, 222–227.
- Burke, M.P.; Path, D.F.; Alamad, S.; Dip, G.; Opeskin, K. Death by smothering following forced quetiapine administration in an infant. Am. J. Forensic Med. Pathol. 2004, 25, 243–245.
- Bertol, E.; Vaiano, F.; Argo, A.; Zerbo, S.; Trignano, C.; Protani, S.; Favretto, D. Overdose of Quetiapine-A Case Report with QT Prolongation. Toxics 2021, 9, 339.
- Office for National Statistics (UK). Number of Drug-Related Deaths Due to Quetiapine Use in England and Wales from 1998 to 2021. Available online: https://www.ons.gov.uk/file?uri=%2fpeoplepopulationandcommunity%2fbirthsdeathsandmarriages%2fdeaths%2fdatasets%2fdeathsrelatedtodrugpoisoningbyselectedsubstances%2f2019registrations/2019pivot3.xlsx (accessed on 25 October 2023).
- Vaja, R.; Rana, M. Drugs and the liver. Anaesth. Intensive Care Med. 2020, 21, 517–523.
- Jones, G.R.; Singer, P.P. Drugs-of-Abuse in Liver. Drug Test. Altern. Biol. Specim. 2008, 139–156.
- Fuke, C.; Berry, C.L.; Pounder, D.J. Postmortem diffusion of ingested and aspirated paint thinner. Forensic Sci. Int. 1996, 78, 199–207.
- Pounder, D.J.; Davies, J.I. Zopiclone poisoning: Tissue distribution and potential for postmortem diffusion. Forensic Sci. Int. 1994, 65, 177–183.
- Breivik, H.; Frost, J.; Lokken, T.N.; Slordal, L. Post mortem tissue distribution of quetiapine in forensic autopsies. Forensic Sci. Int. 2020, 315, 110413.
- LiverTox, L. Clinical and Research Information on Drug-Induced Liver Injury Bethesda; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Abdel-Misih, S.R.; Bloomston, M. Liver anatomy. Surg. Clin. N. Am. 2010, 90, 643–653.
- Aguiar, J.A.; Riaz, A.; Thornburg, B. Biliary Anatomy. Semin. Interv. Radiol. 2021, 38, 251–254.
- Anderson, D.T.; Fritz, K.L. Quetiapine (Seroquel) concentrations in seven postmortem cases. J. Anal. Toxicol. 2000, 24, 300–304.
- Hamsa, A.; Karumandampalayam Shanmugaramasamy, K.; Kariyarambath, P.; Kathirvel, S. Quetiapine Fumarate: A Review of Analytical Methods. J. Chromatogr. Sci. 2022, 61, bmac100.
- Liu, T.L.; Fang, L.S.; Liou, J.R.; Dai, J.S.; Chen, Y.L. Determination of quetiapine and its metabolites in plasma by field-enhanced sample stacking. J. Food Drug Anal. 2021, 29, 709–716.
- Lee, H.J.; Choi, J.S.; Choi, B.H.; Hahn, S.J. Effects of norquetiapine, the active metabolite of quetiapine, on cloned hERG potassium channels. Neurosci. Lett. 2018, 664, 66–73.
- Ortega-Ruiz, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Abad-Santos, F.; Zubiaur, P. The Pharmacogenetics of Treatment with Quetiapine. Future Pharmacol. 2022, 2, 276–286.
- Kartasheva-Ebertz, D.; Gaston, J.; Lair-Mehiri, L.; Massault, P.P.; Scatton, O.; Vaillant, J.C.; Morozov, V.A.; Pol, S.; Lagaye, S. Adult human liver slice cultures: Modelling of liver fibrosis and evaluation of new anti-fibrotic drugs. World J. Hepatol. 2021, 13, 187–217.
- Saxton, S.H.; Stevens, K.R. 2D and 3D liver models. J. Hepatol. 2023, 78, 873–875.
- Hernández-Mesa, M.; Moreno-González, D. Current Role of Mass Spectrometry in the Determination of Pesticide Residues in Food. Separations 2022, 9, 148.
- Poloznikov, A.; Gazaryan, I.; Shkurnikov, M.; Nikulin, S.; Drapkina, O.; Baranova, A.; Tonevitsky, A. In vitro and in silico liver models: Current trends, challenges and opportunities. ALTEX 2018, 35, 397–412.
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.C.; et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2021, 70, 567–574.
- Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80.
- De Siervi, S.; Turato, C. Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. Int. J. Mol. Sci. 2023, 24, 4529.
- Lee, J.H.; Ho, K.L.; Fan, S.K. Liver microsystems in vitro for drug response. J. Biomed. Sci. 2019, 26, 88.
- Dede, E.; Tindall, M.J.; Cherrie, J.W.; Hankin, S.; Collins, C. Physiologically-based pharmacokinetic and toxicokinetic models for estimating human exposure to five toxic elements through oral ingestion. Environ. Toxicol. Pharmacol. 2018, 57, 104–114.
- Jones, A.W.; Holmgren, A.; Ahlner, J. Post-mortem concentrations of drugs determined in femoral blood in single-drug fatalities compared with multi-drug poisoning deaths. Forensic Sci. Int. 2016, 267, 96–103.
- Vignali, C.; Freni, F.; Magnani, C.; Moretti, M.; Siodambro, C.; Groppi, A.; Osculati, A.M.M.; Morini, L. Distribution of quetiapine and metabolites in biological fluids and tissues. Forensic Sci. Int. 2020, 307, 110108.
- Skov, L.; Johansen, S.S.; Linnet, K. Postmortem Quetiapine Reference Concentrations in Brain and Blood. J. Anal. Toxicol. 2015, 39, 557–561.
More
Information
Subjects:
Pathology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
10 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




