Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federico Emanuele Pozzi | -- | 2739 | 2024-01-08 09:57:33 | | | |
| 2 | Lindsay Dong | -77 word(s) | 2662 | 2024-01-10 01:31:42 | | | | |
| 3 | Lindsay Dong | -14 word(s) | 2648 | 2024-01-12 09:14:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pozzi, F.E.; Remoli, G.; Tremolizzo, L.; Appollonio, I.; Ferrarese, C.; Cuffaro, L. Brain Health and Cognition in Older Adults. Encyclopedia. Available online: https://encyclopedia.pub/entry/53539 (accessed on 15 January 2026).
Pozzi FE, Remoli G, Tremolizzo L, Appollonio I, Ferrarese C, Cuffaro L. Brain Health and Cognition in Older Adults. Encyclopedia. Available at: https://encyclopedia.pub/entry/53539. Accessed January 15, 2026.
Pozzi, Federico Emanuele, Giulia Remoli, Lucio Tremolizzo, Ildebrando Appollonio, Carlo Ferrarese, Luca Cuffaro. "Brain Health and Cognition in Older Adults" Encyclopedia, https://encyclopedia.pub/entry/53539 (accessed January 15, 2026).
Pozzi, F.E., Remoli, G., Tremolizzo, L., Appollonio, I., Ferrarese, C., & Cuffaro, L. (2024, January 08). Brain Health and Cognition in Older Adults. In Encyclopedia. https://encyclopedia.pub/entry/53539
Pozzi, Federico Emanuele, et al. "Brain Health and Cognition in Older Adults." Encyclopedia. Web. 08 January, 2024.
Copy Citation
subjective cognitive decline
brain health
prevention
Alzheimer’s disease
cognition
1. Introduction
The WHO defines brain health as “the state of brain functioning across cognitive, sensory, social-emotional, behavioral and motor domains, allowing a person to realize their full potential over the life course, irrespective of the presence or absence of disorders” [1]. This is a dynamic definition, as it requires adaptations across the life course in all domains. Other definitions have been proposed, mostly in line with the one endorsed by the WHO [2]. As individuals age, cognition becomes a pivotal determinant of brain health, albeit not the sole factor. Therefore, preventing and addressing cognitive impairment is an imperative objective to supporting brain health, bearing in mind that this process should ideally start at birth and continue throughout the whole life. This should be achieved through a combined implementation of public health policies aimed at reducing factors detrimental to brain health in the general population (such as poverty, lack of education, and racial discrimination [3][4]) and a shift of healthcare services towards early detection of at-risk individuals and personalized prevention strategies [5]. Such new policies should be synergistically developed at a global level to avoid further inequality [6].
2. Brain Health as a New Target
The concept of brain health, defined as the “state of physical, mental, and social well-being”, was first articulated by the WHO in its constitution in the late 1940s. Despite its longstanding presence in health discourse, the lack of a universally accepted definition and the absence of standardized, objective quantitative methods have posed significant challenges, hindering its practical application in real-life settings. This need for a more cohesive understanding and measurement of Brain Health has been emphasized by Hachinski et al. [7].
Recent developments over the past two years have witnessed a notable shift in recognizing brain health as a paramount concern. Various influential associations, including the WHO, the American Heart Association [8], and the European Academy of Neurology [9], have acknowledged and prioritized brain health on their agendas. This growing recognition reflects an increasing understanding of the profound impact of brain well-being on overall health and quality of life.
Epidemiological evidence has further reinforced the importance of brain health, indicating that approximately 90% of all strokes are attributable to a few potentially modifiable risk factors. Therefore, primary prevention is vital to curb the high burden of stroke [10].
During the Brain Health Summit organized by the European Academy of Neurology in Brussels in November 2023, the Brain Health Mission was launched to support the development of National Brain Health plans across Europe (https://www.ean.org/brain-health-mission, accessed on 28 November 2023). The burden of neurological diseases is the highest among non-communicable diseases and in economic society costs [11]. However, achieving brain health is a complex endeavor considering the limited number of breakthrough treatments for many neurological disorders and the limited scientific evidence in preventing neurological disorders [6].
3. Preventive Strategies: Who and How
3.1. Who: Subjective Cognitive Decline
According to the definition provided by an international group of experts almost a decade ago, subjective cognitive decline (SCD) is a condition characterized by a self-experienced persistent decline in cognition compared to the previously normal statuses, unrelated to acute events, with normal performances on demographically adjusted standardized cognitive tests used to classify mild cognitive impairment (MCI) [12]. Data from a large meta-analysis show that SCD is associated with a two-fold risk of developing MCI and dementia, but this risk is influenced by several factors, including recruitment source [13]. SCD may represent an early phase of AD cognitive changes (stage 2 of the NIA-AA research framework) [14], and a number of features are proposed that might increase the likelihood of SCD being due to preclinical AD. These features define the so-called SCD-plus and include, but are not limited to, subjective decline in memory, the onset of SCD within the last five years, the feeling of worse performance compared to peers, and the presence of the APOE ε4 genotype [12].
Data on SCD mainly come from large experiences in first-world countries, among which notable examples are given by the SCIENCe cohort in Amsterdam, the Netherlands [15] and the German DELCODE study [16]. These studies have enrolled hundreds of subjects and conducted impressive longitudinal follow-ups, sometimes spanning over a decade. However, one should bear in mind that SCD might have different prevalence and characteristics depending on the specific settings and socio-economics context, as shown by population studies in low-income countries [4].
SCD is a heterogeneous condition that has only recently been studied to find predictors of progression to MCI and dementia. These include also the use of CSF or PET biomarkers. In particular, SCD subjects with positive amyloid biomarkers show a steeper decline in cognition compared to patient with negative CSF biomarkers [17]. In this population a lower plasma Aβ42/Aβ40 ratio is associated with an increased risk of clinical conversion to MCI and brain amyloid deposition at 2-year follow-up [18]. At the same time, a positive brain amyloid-PET scan results in greater cognitive decline and medial temporal atrophy in the following 2 years [19]. These findings might justify the use of biomarkers even in a population otherwise considered “healthy”, as this provides important “short-term” prognostic information.
It may be difficult to differentiate between SCD and MCI, as this depends also on specific cut-offs used to diagnose the latter condition, which may result in different levels of “impairment” allowed in the condition of SCD. Although the current criteria for SCD imply normal performance on standardized neuropsychological tests, a considerable number of subjects may exhibit minor neuropsychological and/or behavioral deficits. These deficits could play a role in the progression to MCI.
3.2. How: Multidomain Personalized Preventive Strategies
According to the 2020 Lancet Commission report, up to 40% of dementia risk may be preventable by acting upon 12 modifiable risk factors [20]. These include diabetes, hypertension, traumatic brain injury, smoking, air pollution, midlife obesity, physical inactivity, depression, alcohol, hearing impairment, social isolation, and poor education. Poor sleep and oral health may be other risk factors, with increasing evidence accumulating in recent years [21][22][23]. The prevention potential and relative impact of these factors may vary based on the specific settings across different regions of the world [24]. Other factors are currently being investigated—such as substance abuse, microbiota and environmental features—although in certain cases, studies may suffer from biases related to reverse causality [25][26][27][28].
Prevention of dementia may take the form of pharmacological and non-pharmacological strategies. Regarding the former, five trials with putative disease-modifying treatments are ongoing in preclinical populations [29], while comparatively more results have been reported for the latter. A few meta-analyses showed that non-pharmacological interventions in older adults with SCD seem to provide small, yet significant benefits on cognition, i.e., cognitive enhancement seems possible even when people appear cognitively normal [30][31]. In older adults (with or without SCD), multidomain personalized interventions may provide significant benefits in terms of both cognition and general health, as shown by the FINGER trial [32]; such interventions are also presumably cost-effective in preventing dementia [33], but further prospective data are warranted. However, other studies with multidimensional interventions have failed to show a clinical benefit, such as the MAPT and preDIVA trials [34][35].
Multidomain interventions are mostly aimed at maintaining and improving cardiovascular fitness, including healthy diet, physical activity, and reduction of cardiovascular risk factors. A key mechanism through which such interventions could improve brain health and prevent neurodegenerative diseases is a positive effect on the neurovascular unit [5]. This is not surprising given the centrality of the neurovasculome to brain health and neurodegeneration [36]. The addition of sleep improvement could theoretically improve waste product clearance within the brain by enhancing the glymphatic system activity [23].
On the other hand, brain health also involves the ability to adapt to and function despite increasing damage, a concept that partially overlaps with cognitive reserve [37]. While cognitive reserve may be primarily built through education and cognitive activities in the first part of life, higher education does not necessarily protect against late life cognitive decline [38]. On the other hand, brain resilience may be increased through cognitive training and socialization even in late adulthood [5]. Indeed, it has been shown that cognitive activities over the whole lifespan, including leisure activities in late life, may delay the onset of cognitive decline independent of early life education [39][40][41].
3.3. Beyond High-Risk: Universal Preventive Approach in Mid- and Latelife Cognitively Unimpaired
While subjects with SCD present a higher risk of developing cognitive impairment and are therefore ideal targets of tailored preventive approaches, cognitively unimpaired middle-aged adults should not be ignored either. Indeed, observational evidence has showed that healthier lifestyle in late midlife is associated with overall better cognitive performance [42]. Multidomain intervention trials including cognitively unimpaired adults at midlife are ongoing [43]. A notable example of these is the Barcelona Brain Health Initiative, a prospective cohort studies enrolling hundreds of cognitively unimpaired middle-aged participants to evaluate and promote determinants of brain health [44].
Since it might be impossible to implement personalized prevention in the whole population, brain health should be achieved through a universal preventive approach included in a public brain health agenda. This should include societal and political changes aiming at increasing physical activity, social integration, education and lifelong learning, cognitive activity, adopting a healthy diet, stopping smoking and reducing alcohol intake, and—with respect to elderly people—reducing the burden of chronic conditions and anticholinergic medications [45]. In middle-aged individuals, poor sleep may be an additional factor to target, as this is associated with lower brain health [46]. It is imperative that these changes are implemented at a global level, thus making brain health a worldwide priority with strategic and substantial investments [47]. Social and socioeconomic factors need also to be addressed to achieve an equitable approach to risk reduction since those are known to be associated with lifestyle risk factors [48][49].
Taken singularly, some of the aforementioned interventions also have the potential to modulate key biological aspects related to brain health. For instance, optimal intakes of vitamin B12, folates, omega-3 fatty acids, and antioxidants provided by adherence to the Mediterranean diet seem to reduce amyloid accumulation and decrease white matter hyperintensities [50][51].
4. Brain Health Services and the Monza Experience
Preventive strategies are now implemented mostly in isolated and uncoordinated experiences, often in the context of clinical trials. While general recommendations are probably easy to disseminate, there is still a lack of a structured and rational approach to tailored prevention, both within memory clinics and at a population level.
It must be acknowledged that preventive lifestyle changes are not easy to sustain even when proposed within a specialized preventive center. Therefore, systemic socio-political changes at a population level should make healthy choices easier and drive positive changes also in low-risk individuals who are not likely to come to medical attention [5]. The implementation of BHS, pursuing a “high-risk approach”, needs to be accompanied by a “population-wide approach” if scholars want to see meaningful changes and maintain equity [9]. Moreover, the reductions in a few relevant risk factors—such as education or air pollution [52]—greatly depend on governmental policies and cannot be addressed even by extremely specialized neurological services. For instance, the Norwegian approach to brain health—combining primordial, primary, and secondary prevention—has demonstrated the potential to reduce the incidence of dementia, stroke and ischemic heart disease (the so-called “triple threat”) [53][54][55].
Advocacy by neurologists for brain health with political entities and decision-makers is crucial, encouraging them to address and prioritize these aspects and setting policy agendas [56]. It should also be noted that efforts for brain health should not be limited to resource-rich countries but rather part of global actions pursuing what has been proposed as a “brain healthy diplomacy” [57][58].
BHS have been recently proposed by the European Task Force for Brain Health Services panel of experts. A six-part user manual for BHS was published in 2021, including papers on dementia risk profiling, risk communication and reduction, and cognitive enhancement [59][60][61][62][63][64]. This was followed this year by a paper by Frisoni et al., tracing the roadmap of such services [65].
BHS substantially differ from memory clinics, and have distinct concepts. Instead of a diagnostic process, users undergo a risk assessment; instead of treatment, subjects are offered strategies to mitigate risk and possibly cognitive enhancement in the future. General practitioners may lack the expertise to provide risk estimates and tailored interventions beyond general recommendations; on the other hand, memory clinics are not currently designed for these subjects and have little to offer them beyond reassurance and, again, general recommendations. BHS will ideally fill this gap [65].
Further users of BHS might be people with functional cognitive disorders and the so called “worried wells”. The former are subjects usually presenting with attentional cognitive symptoms mostly in the context of anxiety, depression, or chronic pain [66].
An important strength of BHS will be providing scientifically sound recommendations to a population that is increasingly recognizing the importance of prevention. This is important to counterbalance the promotion of pseudoscience or questionable products that feed on this need for prevention and brain health, making billions in the process [67][68].
In order to calculate the risk of dementia, BHS operators need to comprehensively assess risk factors and protective factors, including the twelve factors listed by the Lancet Commission [20] as well as other emerging ones as detailed previously. Risk profiling should take all of these factors into account, possibly with the aid of multidomain clinical risk scores such as CAIDE, BSDI, or ANU-ADRI [69][70][71]. It is crucial that these risk scores are used in the population in which they have been validated; for instance, CAIDE may be used for people aged 39–64, while BDSI is indicated in people over 64. They also provide different predictions, the CAIDE being at 20 years, and the BDSI up to 5 years. While such risk models are certainly useful, it is important to understand their limitations and imperfect accuracy, around 70–80% [60].
Risk communication in BHS should ideally follow established protocols developed from evidence gathered from memory clinics, clinical trials, and research registries. In this sense, it has been shown that disclosure of APOE status or amyloid-PET results to cognitively unimpaired individuals might be a sort of preventive intervention itself, as it seems to increase the propensity of high-risk subjects to adopt a healthier lifestyle. At the same time, this did not lead to increased anxiety or depression [72][73].
After risk profiling in the context of BHS, subjects at high risk should be proposed personalized multidomain interventions targeting several risk factors at once with sufficient intensity [62]. To improve adherence, a few considerations need to made: firstly, smaller changes introduced gradually may help sustain long-term adoption of a healthier lifestyle.
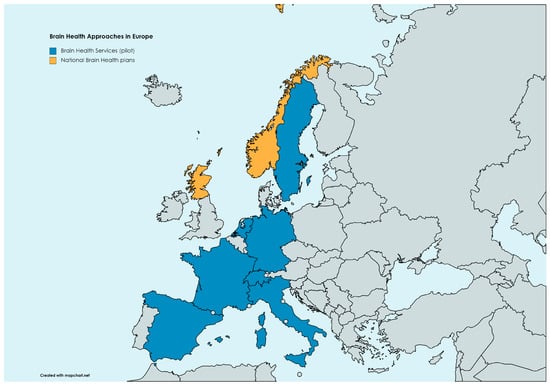
As of today, only a few experiences worldwide have been implemented to shift care standards in neurology from brain diseases to brain health. Even before the proposal of BHS, a similar service has been detailed within the Center for Brain Health at NorthShore Neurological Institute in Illinois, US [74]. Another recent proposal is the Scottish Model for Brain Health Services, which probably represents the first nationwide approach to prevention, substantially overlapping with the BHS outlined by the European Task Force [75]. Other pilot experiences with BHS are ongoing in different European countries, including those at Karolinska Institut in Sweden, at the Uniklinik Köln in Germany, at BarcelonaBeta in Spain, at the Amsterdam UMC in the Netherlands, at Paris La Pitié-Salpetrière in France, and at Geneva HUG in Switzerland. A map of brain health approaches in Europe is shown in Figure 1.

Figure 1. Brain health approaches in Europe as of today. Brain health services are limited to pilot experiences in Amsterdam (the Netherlands), Barcelona (Spain), Geneva (Switzerland), Köln (Germany), Monza (Italy), Paris (France), and Stockholm (Sweden). National brain health plans are ongoing in Scotland and Norway.
References
- Optimizing Brain Health across the Life Course; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240054561.
- Chen, Y.; Demnitz, N.; Yamamoto, S.; Yaffe, K.; Lawlor, B.; Leroi, I. Defining brain health: A concept analysis. Int. J. Geriatr. Psychiatry 2022, 37, 1–13.
- Simons, R.L.; Ong, M.L.; Beach, S.R.H.; Lei, M.K.; Philibert, R.; Mielke, M.M. Direct and indirect effects of socioeconomic status and discrimination on subjective cognitive decline: A longitudinal study of African American women. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2023, 78, 799–808.
- Borelli, W.V.; Zimmer, E.R.; Bieger, A.; Coelho, B.; Pascoal, T.A.; Chaves, M.L.F.; Amariglio, R.; Castilhos, R.M. Subjective cognitive decline in Brazil: Prevalence and association with dementia modifiable risk factors in a population-based study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12368.
- Sabayan, B.; Doyle, S.; Rost, N.S.; Sorond, F.A.; Lakshminarayan, K.; Launer, L.J. The role of population-level preventive care for brain health in ageing. Lancet Healthy Longev. 2023, 4, e274–e283.
- Owolabi, M.O.; Leonardi, M.; Bassetti, C.; Jaarsma, J.; Hawrot, T.; Makanjuola, A.I.; Dhamija, R.K.; Feng, W.; Straub, V.; Camaradou, J.; et al. Global synergistic actions to improve brain health for human development. Nat. Rev. Neurol. 2023, 19, 371–383.
- Hachinski, V.; Avan, A.; Gillilands, J.; Oveisgharan, S. A new definition of brain health. Lancet Neurol. 2021, 20, 335–336.
- Lazar, R.M.; Howard, V.J.; Kernan, W.N.; Aparicio, H.J.; Levine, D.A.; Viera, A.J.; Jordan, L.C.; Nyenhuis, D.L.; Possin, K.L.; Sorond, F.A.; et al. A primary care agenda for brain health: A scientific statement from the American Heart Association. Stroke 2021, 52, E295–E308.
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One brain, one life, one approach. Eur. J. Neurol. 2022, 29, 2559–2566.
- Global prevention of stroke: Focus on the individual? Lancet Neurol. 2019, 18, 903.
- “GBD 2019 Mental Disorders Collaborators” Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150.
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852.
- Pike, K.E.; Cavuoto, M.G.; Li, L.; Wright, B.J.; Kinsella, G.J. Subjective cognitive decline: Level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychol. Rev. 2022, 32, 703–735.
- Jack, C.R.J.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562.
- Slot, R.E.R.; Verfaillie, S.C.J.; Overbeek, J.M.; Timmers, T.; Wesselman, L.M.P.; Teunissen, C.E.; Dols, A.; Bouwman, F.H.; Prins, N.D.; Barkhof, F.; et al. Subjective Cognitive Impairment Cohort (SCIENCe): Study design and first results. Alzheimer’s Res. Ther. 2018, 10, 76.
- Jessen, F.; Spottke, A.; Boecker, H.; Brosseron, F.; Buerger, K.; Catak, C.; Fliessbach, K.; Franke, C.; Fuentes, M.; Heneka, M.T.; et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimer’s Res. Ther. 2018, 10, 15.
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.P.; Verberk, I.M.W.; Verfaillie, S.C.J.; Slot, R.E.R.; Van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; Van Den Bosch, K.A.; et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020, 95, 46–58.
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer’s disease in individuals with subjective cognitive decline. Alzheimer’s Res. Ther. 2023, 15, 2.
- Hong, Y.J.; Ho, S.H.; Jeong, J.H.; Park, K.H.; Kim, S.Y.; Wang, M.J.; Choi, S.H.; Yang, D.W. Impacts of baseline biomarkers on cognitive trajectories in subjective cognitive decline: The CoSCo prospective cohort study. Alzheimer’s Res. Ther. 2023, 15, 132.
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446.
- Gottesman, R.F.; Seshadri, S. Risk factors, lifestyle behaviors, and vascular brain health. Stroke 2022, 53, 394–403.
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709.
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868.
- Mukadam, N.; Sommerlad, A.; Huntley, J.; Livingston, G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: An analysis using cross-sectional survey data. Lancet Glob. Health 2019, 7, e596–e603.
- Besser, L.M.; Brenowitz, W.D.; Meyer, O.L.; Hoermann, S.; Renne, J. Methods to address self-selection and reverse causation in studies of neighborhood environments and brain health. Int. J. Environ. Res. Public Health 2021, 18, 6484.
- Maasakkers, C.M.; Weijs, R.W.J.; Dekkers, C.; Gardiner, P.A.; Ottens, R.; Olde Rikkert, M.G.M.; Melis, R.J.F.; Thijssen, D.H.J.; Claassen, J.A.H.R. Sedentary behaviour and brain health in middle-aged and older adults: A systematic review. Neurosci. Biobehav. Rev. 2022, 140, 104802.
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80.
- Testai, F.D.; Gorelick, P.B.; Aparicio, H.J.; Filbey, F.M.; Gonzalez, R.; Gottesman, R.F.; Melis, M.; Piano, M.R.; Rubino, T.; Song, S.Y. Use of marijuana: Effect on brain health: A scientific statement from the American Heart Association. Stroke 2022, 53, 176–187.
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385.
- Smart, C.M.; Karr, J.E.; Areshenkoff, C.N.; Rabin, L.A.; Hudon, C.; Gates, N.; Ali, J.I.; Arenaza-Urquijo, E.M.; Buckley, R.F.; Chetelat, G.; et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: Systematic review, meta-analysis, and preliminary recommendations. Neuropsychol. Rev. 2017, 27, 245–257.
- Sheng, C.; Yang, K.; Wang, X.; Li, H.; Li, T.; Lin, L.; Liu, Y.; Yang, Q.; Wang, X.; Wang, X.; et al. Advances in non-pharmacological interventions for subjective cognitive decline: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2020, 77, 903–920.
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263.
- Wimo, A.; Handels, R.; Antikainen, R.; Eriksdotter, M.; Jönsson, L.; Knapp, M.; Kulmala, J.; Laatikainen, T.; Lehtisalo, J.; Peltonen, M.; et al. Dementia prevention: The potential long-term cost-effectiveness of the FINGER prevention program. Alzheimer’s Dement. 2022, 1–10.
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.M.-N.; Dantoine, T.; Dartigues, J.-F.J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389.
- van Charante, E.P.M.; Richard, E.; Eurelings, L.S.; van Dalen, J.W.; Ligthart, S.A.; van Bussel, E.F.; Hoevenaar-Blom, M.P.; Vermeulen, M.; van Gool, W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet 2016, 388, 797–805.
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; Van Veluw, S.J.; et al. The neurovasculome: Key roles in brain health and cognitive impairment: A scientific statement From the American Heart Association/American Stroke Association. Stroke 2023, 54, E251–E271.
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012.
- Mungas, D.; Early, D.R.; Glymour, M.M.; Zeki Al Hazzouri, A.; Haan, M.N. Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology 2018, 32, 77–88.
- Hall, C.B.; Lipton, R.B.; Sliwinski, M.; Katz, M.J.; Derby, C.A.; Verghese, J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 2009, 73, 356–361.
- Lee, A.T.C.; Richards, M.; Chan, W.C.; Chiu, H.F.K.; Lee, R.S.Y.; Lam, L.C.W. Association of daily intellectual activities with lower risk of incident dementia among older Chinese adults. JAMA Psychiatry 2018, 75, 697–703.
- Hotz, I.; Deschwanden, P.F.; Mérillat, S.; Jäncke, L. Entorhinal cortex thinning is related to white matter hyperintensity growth, memory decline, and leisure activity in cognitively healthy older adults. Neuroimage 2023, 284, 120461.
- Cody, K.A.; Koscik, R.L.; Erickson, C.M.; Berman, S.E.; Jonaitis, E.M.; Williams, V.J.; Mueller, K.D.; Christian, B.T.; Chin, N.A.; Clark, L.R.; et al. Associations of the Lifestyle for Brain Health index with longitudinal cognition and brain amyloid beta in clinically unimpaired older adults: Findings from the Wisconsin Registry for Alzheimer’s Prevention. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12351.
- Chang, Y.-K.; Erickson, K.I.; Aghjayan, S.L.; Chen, F.-T.; Li, R.-H.; Shih, J.-R.; Chang, S.-H.; Huang, C.-M.; Chu, C.-H. The multi-domain exercise intervention for memory and brain function in late middle-aged and older adults at risk for Alzheimer’s disease: A protocol for Western–Eastern Brain Fitness Integration Training trial. Front. Aging Neurosci. 2022, 14, 929789.
- Cattaneo, G.; Bartrés-Faz, D.; Morris, T.P.; Sánchez, J.S.; Macià, D.; Tarrero, C.; Tormos, J.M.; Pascual-Leone, A. The Barcelona brain health initiative: A cohort study to define and promote determinants of brain health. Front. Aging Neurosci. 2018, 10, 321.
- Hussenoeder, F.S.; Riedel-Heller, S.G. Primary prevention of dementia: From modifiable risk factors to a public brain health agenda? Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1289–1301.
- Namsrai, T.; Ambikairajah, A.; Cherbuin, N. Poorer sleep impairs brain health at midlife. Sci. Rep. 2023, 13, 1874.
- Kolappa, K.; Seeher, K.; Dua, T. Brain health as a global priority. J. Neurol. Sci. 2022, 439, 120326.
- Röhr, S.; Pabst, A.; Baber, R.; Engel, C.; Glaesmer, H.; Hinz, A.; Schroeter, M.L.; Witte, A.V.; Zeynalova, S.; Villringer, A.; et al. Social determinants and lifestyle factors for brain health: Implications for risk reduction of cognitive decline and dementia. Sci. Rep. 2022, 12, 12965.
- Röhr, S.; Rodriguez, F.S.; Siemensmeyer, R.; Müller, F.; Romero-Ortuno, R.; Riedel-Heller, S.G. How can urban environments support dementia risk reduction? A qualitative study. Int. J. Geriatr. Psychiatry 2022, 37.
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary patterns and cognitive health in older adults: A systematic review. J. Alzheimer’s Dis. 2019, 67, 583–619.
- Smith, P.J.; Blumenthal, J.A. Dietary factors and cognitive decline. J. Prev. Alzheimer’s Dis. 2015, 3, 53–64.
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. Neurotoxicology 2012, 33, 972–984.
- Avan, A.; Aamodt, A.H.; Selbæk, G.; Bovim, G.; Bassetti, C.L.A.; Boon, P.; Grisold, W.; Hachinski, V. Decreasing incidence of stroke, ischaemic heart disease and dementia in Norway, 1990–2019, a Global Burden of Disease study: An opportunity. Eur. J. Neurol. 2023, 30, 2267–2277.
- Owolabi, M.O. Improving brain and vascular health at the population level: Lessons from the Norwegian success story. Eur. J. Neurol. 2023, 30, 2141–2143.
- Helse-og Omsorgsdepartementet. National Brain Health Strategy (2018–2024). 2017. Available online: https://www.braincouncil.eu/wp-content/uploads/2018/04/Annex-Full-Norwegian-Brain-Health-Strategy.pdf (accessed on 28 November 2023).
- Castellani, B.; Bartington, S.; Wistow, J.; Heckels, N.; Ellison, A.; Van Tongeren, M.; Arnold, S.R.; Barbrook-Johnson, P.; Bicket, M.; Pope, F.D.; et al. Mitigating the impact of air pollution on dementia and brain health: Setting the policy agenda. Environ. Res. 2022, 215, 114362.
- Ternes, K.; Iyengar, V.; Lavretsky, H.; Dawson, W.D.; Booi, L.; Ibanez, A.; Vahia, I.; Reynolds, C.; DeKosky, S.; Cummings, J.; et al. Brain health INnovation Diplomacy: A model binding diverse disciplines to manage the promise and perils of technological innovation. Int. Psychogeriatr. 2020, 32, 955–979.
- Ibáñez, A.; Pina-Escudero, S.D.; Possin, K.L.; Quiroz, Y.T.; Peres, F.A.; Slachevsky, A.; Sosa, A.L.; Brucki, S.M.D.; Miller, B.L. Dementia caregiving across Latin America and the Caribbean and brain health diplomacy. Lancet Healthy Longev. 2021, 2, e222–e231.
- Altomare, D.; Molinuevo, J.L.; Ritchie, C.; Ribaldi, F.; Carrera, E.; Dubois, B.; Jessen, F.; McWhirter, L.; Scheltens, P.; van der Flier, W.M.; et al. Brain Health Services: Organization, structure, and challenges for implementation. A user manual for Brain Health Services—Part 1 of 6. Alzheimer’s Res. Ther. 2021, 13, 168.
- Ranson, J.M.; Rittman, T.; Hayat, S.; Brayne, C.; Jessen, F.; Blennow, K.; van Duijn, C.; Barkhof, F.; Tang, E.; Mummery, C.J.; et al. Modifiable risk factors for dementia and dementia risk profiling. A user manual for Brain Health Services—Part 2 of 6. Alzheimer’s Res. Ther. 2021, 13, 169.
- Visser, L.N.C.; Minguillon, C.; Sánchez-Benavides, G.; Abramowicz, M.; Altomare, D.; Fauria, K.; Frisoni, G.B.; Georges, J.; Ribaldi, F.; Scheltens, P.; et al. Dementia risk communication. A user manual for Brain Health Services—Part 3 of 6. Alzheimer’s Res. Ther. 2021, 13, 170.
- Solomon, A.; Stephen, R.; Altomare, D.; Carrera, E.; Frisoni, G.B.; Kulmala, J.; Molinuevo, J.L.; Nilsson, P.; Ngandu, T.; Ribaldi, F.; et al. Multidomain interventions: State-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services—Part 4 of 6. Alzheimer’s Res. Ther. 2021, 13, 171.
- Brioschi Guevara, A.; Bieler, M.; Altomare, D.; Berthier, M.; Csajka, C.; Dautricourt, S.; Démonet, J.F.; Dodich, A.; Frisoni, G.B.; Miniussi, C.; et al. Protocols for cognitive enhancement. A user manual for Brain Health Services—Part 5 of 6. Alzheimer’s Res. Ther. 2021, 13, 172.
- Milne, R.; Altomare, D.; Ribaldi, F.; Molinuevo, J.L.; Frisoni, G.B.; Brayne, C. Societal and equity challenges for Brain Health Services. A user manual for Brain Health Services—Part 6 of 6. Alzheimer’s Res. Ther. 2021, 13, 173.
- Frisoni, G.B.; Altomare, D.; Ribaldi, F.; Villain, N.; Brayne, C.; Mukadam, N.; Abramowicz, M.; Barkhof, F.; Berthier, M.; Bieler-Aeschlimann, M.; et al. Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. Lancet Reg. Health Eur. 2023, 26, 100576.
- McWhirter, L.; Ritchie, C.; Stone, J.; Carson, A. Functional cognitive disorders: A systematic review. Lancet Psychiatry 2020, 7, 191–207.
- Crawford, C.; Boyd, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Deuster, P.A. A public health issue: Dietary supplements promoted for brain health and cognitive performance. J. Altern. Complement. Med. 2020, 26, 265–272.
- Hellmuth, J.; Rabinovici, G.D.; Miller, B.L. The rise of pseudomedicine for dementia and brain health. JAMA 2019, 321, 543.
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741.
- Barnes, D.E.; Beiser, A.S.; Lee, A.; Langa, K.M.; Koyama, A.; Preis, S.R.; Neuhaus, J.; McCammon, R.J.; Yaffe, K.; Seshadri, S.; et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimer’s Dement. 2014, 10, 656–665.e1.
- Anstey, K.J.; Cherbuin, N.; Herath, P.M. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev. Sci. 2013, 14, 411–421.
- Bemelmans, S.A.S.A.; Tromp, K.; Bunnik, E.M.; Milne, R.J.; Badger, S.; Brayne, C.; Schermer, M.H.; Richard, E. Psychological, behavioral and social effects of disclosing Alzheimer’s disease biomarkers to research participants: A systematic review. Alzheimer’s Res. Ther. 2016, 8, 46.
- Largent, E.A.; Harkins, K.; Van Dyck, C.H.; Hachey, S.; Sankar, P.; Karlawish, J. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS ONE 2020, 15, e0229137.
- Fosnacht, A.M.; Patel, S.; Yucus, C.; Pham, A.; Rasmussen, E.; Frigerio, R.; Walters, S.; Maraganore, D. From brain disease to brain health: Primary prevention of Alzheimer’S disease and related disorders in a health system using an electronic medical record-based approach. J. Prev. Alzheimer’s Dis. 2017, 4, 157–164.
- Ritchie, C.W.; Waymont, J.M.J.; Pennington, C.; Draper, K.; Borthwick, A.; Fullerton, N.; Chantler, M.; Porteous, M.E.; Danso, S.O.; Green, A.; et al. The Scottish Brain Health Service model: Rationale and scientific basis for a national care pathway of Brain Health Services in Scotland. J. Prev. Alzheimer’s Dis. 2022, 9, 348–358.
More
Information
Subjects:
Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
633
Revisions:
3 times
(View History)
Update Date:
12 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




