
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Bransfield | -- | 4577 | 2024-01-08 03:36:41 | | | |
| 2 | Jason Zhu | Meta information modification | 4577 | 2024-01-08 06:25:51 | | |
Video Upload Options
Microbes are associated with a number of mental disorders, including autism, schizophrenia, bipolar disorder, depressive disorders, and anxiety disorders, as well as suicidality and aggressive or violent behaviors. Specific microbes that have been associated or potentially associated with at least one of these conditions include Aspergillus, Babesia, Bartonella, Borna disease virus, Borrelia burgdorferi (Lyme disease), Candida, Chlamydia, coronaviruses (e.g., SARS-CoV-2), Cryptococcus neoformans, cytomegalovirus, enteroviruses, Epstein–Barr virus, hepatitis C, herpes simplex virus, human endogenous retroviruses, human immunodeficiency virus, human herpesvirus-6 (HHV-6), human T-cell lymphotropic virus type 1, influenza viruses, measles virus, Mycoplasma, Plasmodium, rubella virus, Group A Streptococcus (PANDAS), Taenia solium, Toxoplasma gondii, Treponema pallidum (syphilis), Trypanosoma, and West Nile virus.
1. Introduction
2. Definitions of Relevant Terms
2.1. Microbes and Related Terms
2.2. Health
2.3. Illness
2.4. Mental Health
2.5. Mental Illness
2.6. Acute vs. Chronic Illness
3. Models for Understanding Disease
3.1. A Multisystem Approach to Understanding the Cause of Disease
3.2. An Evolutionary or Darwinian Approach to Understanding the Cause of Disease
3.3. An Organismal Approach to Understanding Disease
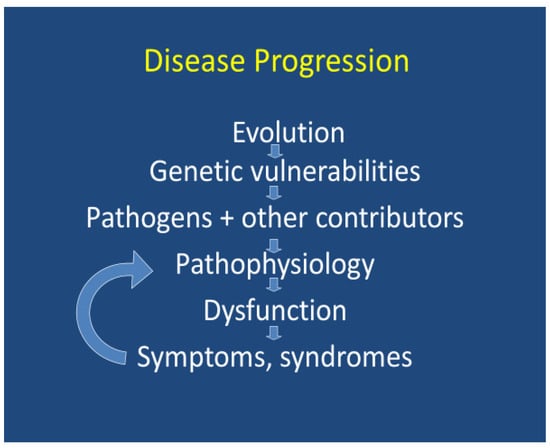
3.4. Infections Associated with Mental Illness
3.5. What Mental Illnesses Are Associated with Specific Microbes?
3.6. Disease Models
-
Acute vs. chronic infections
-
Complex interactive infections
-
Total load theory
4. Pathophysiology
4.1. Is Trauma from Infection or from the Host’s Immune Reaction to the Infection?
4.2. Psychoneuroimmunology or Psychoimmunology
4.3. Clinical Presentation Variability
4.4. Infections Early in Life with Later Consequences
5. Clinical Considerations: Assessment
5.1. Clinical Assessment
5.2. Laboratory and Other Diagnostic Testing
6. Treatment Options
References
- Human Microbiome Data Portal Project. Available online: https://portal.hmpdacc.org (accessed on 22 August 2022).
- O’Connor, S.M.; Taylor, C.E.; Hughes, J.M. Emerging infectious determinants of chronic diseases. Emerg. Infect. Dis. 2006, 12, 1051–1057.
- Knobler, S.L.; O’Connor, S.; Lemon, S.M.; Najafi, M. (Eds.) The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary; National Academies Press: Washington, DC, USA, 2004.
- Munjal, S.; Ferrando, S.J.; Freyberg, Z. Neuropsychiatric Aspects of Infectious Diseases: An Update. Crit. Care Clin. 2017, 33, 681–712.
- Huarcaya-Victoria, J.; Alarcon-Ruiz, C.A.; Barzola-Farfán, W.; Cruzalegui-Bazán, C.; Cabrejos-Espinoza, M.; Aspilcueta-Montoya, G.; Cornero-Quispe, F.; Salazar-Bellido, J.; Villarreal, B. One-year follow-up of depression, anxiety, and quality of life of Peruvian patients who survived COVID-19. Qual. Life Res. 2023, 8, 1–11.
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. 2021, 72, e1–e48.
- Bransfield, R.C.; Cook, M.J.; Bransfield, D.R. Proposed Lyme Disease Guidelines and Psychiatric Illnesses. Healthcare 2019, 7, 105.
- Maxwell, S.P.; Brooks, C.; McNeely, C.L.; Thomas, K.C. Neurological Pain, Psychological Symptoms, and Diagnostic Struggles among Patients with Tick-Borne Diseases. Healthcare 2022, 10, 1178.
- Bransfield, R.C. Neuropsychiatric Lyme Borreliosis: An Overview with a Focus on a Specialty Psychiatrist’s Clinical Practice. Healthcare 2018, 6, 104.
- Bransfield, R.C.; Aidlen, D.M.; Cook, M.J.; Javia, S. A Clinical Diagnostic System for Late-Stage Neuropsychiatric Lyme Borreliosis Based upon an Analysis of 100 Patients. Healthcare 2020, 8, 13.
- Fallon, B.A.; Madsen, T.; Erlangsen, A.; Benros, M.E. Lyme Borreliosis and Associations with Mental Disorders and Suicidal Behavior: A Nationwide Danish Cohort Study. Am. J. Psychiatry 2021, 178, 921–931.
- Bransfield, R.C. The psychoimmunology of lyme/tick-borne diseases and its association with neuropsychiatric symptoms. Open Neurol. J. 2012, 6, 88–93.
- Fallon, B.A.; Nields, J.A.; Burrascano, J.J.; Liegner, K.; DelBene, D.; Liebowitz, M.R. The neuropsychiatric manifestations of Lyme borreliosis. Psychiatr. Q. 1992, 63, 95–117.
- Huber, M.; Knottnerus, J.A.; Green, L.; van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; van der Meer, J.W.; et al. How should we define health? BMJ 2011, 343, d4163.
- Kleinman, A.; Eisenberg, L.; Good, B. Culture, illness and care. Clinical lessons from anthropologic and cross-cultural research. Ann. Intern. Med. 1978, 88, 251–258.
- Kleinman, A. The meaning of symptoms and disorders. In The Illness Narratives; Basic Books: New York, NY, USA, 1988 1988; p. 3.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Publishing: Arlington, VA, USA, 2022.
- Galderisi, S.; Heinz, A.; Kastrup, M.; Beezhold, J.; Sartorius, N. Toward a new definition of mental health. World Psychiatry 2015, 14, 231–233.
- CDC. Centers for Disease Control and Prevention, National Center for Chronic Diseases and Health Promotion (NCCDPHP). About Chronic Diseases. Available online: https://www.cdc.gov/chronicdisease/about/index.htm (accessed on 26 August 2022).
- Ewald, P.W. Evolution of virulence. Infect. Dis. Clin. N. Am. 2004, 18, 1–15.
- Nesse, R.M. Darwinian Medicine. Encyclopedia Britannica. 2019. Available online: https://www.britannica.com/science/Darwinian-medicine (accessed on 13 August 2022).
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298.
- Ewald, P.W. The evolution of virulence and emerging diseases. J. Urban Health 1998, 75, 480–491.
- Robinson-Agramonte, M.d.l.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033.
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2015, 2, 258–270.
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav. Immun. 2017, 62, 46–52.
- Marrie, R.A.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Patten, S.B.; Singer, A.; Lix, L.M.; Hitchon, C.A.; El-Gabalawy, R.; et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J. Psychosom. Res. 2017, 101, 17–23.
- Müller, N.; Schwarz, M.J. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000.
- Furtado, M.; Katzman, M.A. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015, 229, 37–48.
- Jansson, S.; Malham, M.; Wewer, V.; Rask, C.U. Psychiatric comorbidity in childhood onset immune-mediated diseases—A systematic review and meta-analysis. Acta Paediatr. 2022, 111, 490–499.
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 816.
- Brown, T.M.; Boyle, M.F. Delirium. BMJ 2002, 325, 644–647.
- Loewenstein, R.J.; Sharfstein, S.S. Neuropsychiatric aspects of acquired immune deficiency syndrome. Int. J. Psychiatry Med. 1984, 13, 255–260.
- Anthony, S.J.; Epstein, J.H.; Murray, K.A.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Ali Khan, S.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13.
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect. Dis. 2017, 10, 1178633617732296.
- Bransfield, R.C. Did Infections Caused by World War I Contribute to Causing World War II? ContagionLive. 5 January 2018. Available online: https://www.contagionlive.com/view/did-infections-caused-by-world-war-i-contribute-to-causing-world-war-ii (accessed on 18 December 2022).
- Kuodi, P.; Gorelik, Y.; Edelstein, M. Characterisation of the long-term physical and mental health consequences of SARS-CoV-2 infection: A systematic review and meta-analysis protocol. PLoS ONE 2022, 17, e0266232.
- Embers, M.E.; Ramamoorthy, R.; Philipp, M.T. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. 2004, 6, 312–318.
- Rogovskyy, A.S.; Gillis, D.C.; Ionov, Y.; Gerasimov, E.; Zelikovsky, A. Antibody Response to Lyme Disease Spirochetes in the Context of VlsE-Mediated Immune Evasion. Infect. Immun. 2016, 85, e00890-16.
- Berndtson, K. Review of evidence for immune evasion and persistent infection in Lyme disease. Int. J. Gen. Med. 2013, 6, 291–306.
- Elsner, R.A.; Hastey, C.J.; Olsen, K.J.; Baumgarth, N. Suppression of Long-Lived Humoral Immunity Following Borrelia burgdorferi Infection. PLoS Pathog. 2015, 11, e1004976.
- Ford, L.; Tufts, D.M. Lyme Neuroborreliosis: Mechanisms of B. burgdorferi Infection of the Nervous System. Brain Sci. 2021, 11, 789.
- Cabello, F.C.; Godfrey, H.P.; Newman, S.A. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 2007, 15, 350–354.
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134.
- Virolainen, S.J.; VonHandorf, A.; Viel, K.C.; Weirauch, M.T.; Kottyan, L.C. Gene–environment interactions and their impact on human health. Genes. Immun. 2023, 24, 1–11.
- Yolken, R.; Torrey, E.F.; Dickerson, F. Neuropsychiatric Consequences of Viral Infections-Focus on SARS2 and Other Coronaviruses. Available online: https://neuroimmune.org/wp-content/uploads/2022/05/yolken.pdf (accessed on 26 August 2022).
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial Nature of Tick-Borne Diseases. mBio 2019, 10, e02055-19.
- Berghoff, W. Chronic Lyme Disease and Co-infections: Differential Diagnosis. Open Neurol. J. 2012, 6, 158–178.
- Smith, A.; Oertle, J.; Warren, D.; Prato, D. Chronic Lyme Disease Complex and Its Commonly Undiagnosed Primary and Secondary Co-Infections. Open J. Med. Microbiol. 2015, 5, 143–158.
- Nicolson Nicolson Haier Chronic fatigue syndrome patients subsequently diagnosed with Lyme disease Borrelia burgdorferi: Evidence for Mycoplasma species coinfections. J. Chronic Fatigue Syndr. 2008, 14, 5–17.
- Grab, D.J.; Nyarko, E.; Barat, N.C.; Nikolskaia, O.V.; Dumler, J.S. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin. Vaccine Immunol. 2007, 14, 1420–1424.
- Jarefors, S.; Karlsson, M.; Forsberg, P.; Eliasson, I.; Ernerudh, J.; Ekerfelt, C. Reduced number of interleukin-12 secreting cells in patients with Lyme borreliosis previously exposed to Anaplasma phagocytophilum. Clin. Exp. Immunol. 2006, 143, 322–328.
- Djokic, V.; Akoolo, L.; Primus, S.; Schlachter, S.; Kelly, K.; Bhanot, P.; Parveen, N. Protozoan Parasite Babesia microti Subverts Adaptive Immunity and Enhances Lyme Disease Severity. Front. Microbiol. 2019, 10, 1596.
- Bransfield, R.C. A Tale of Two Pandemics: Lyme and COVID-19. In Proceedings of the 2021 ILADS Scientific Conference, Orlando, FL, USA, 16 October 2021.
- Szewczyk-Dąbrowska, A.; Budziar, W.; Harhala, M.; Baniecki, K.; Pikies, A.; Jędruchniewicz, N.; Kaźmierczak, Z.; Gembara, K.; Klimek, T.; Witkiewicz, W.; et al. Correlation between COVID-19 severity and previous exposure of patients to Borrelia spp. Sci. Rep. 2022, 12, 15944.
- Goswami, A.; Wendt, F.R.; Pathak, G.A.; Tylee, D.S.; De Angelis, F.; De Lillo, A.; Polimanti, R. Role of microbes in the pathogenesis of neuropsychiatric disorders. Front. Neuroendocrinol. 2021, 62, 100917.
- Morton, J.T.; Jin, D.M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217.
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22.
- Imai, D.M.; Feng, S.; Hodzic, E.; Barthold, S.W. Dynamics of connective-tissue localization during chronic Borrelia burgdorferi infection. Lab. Investig. 2013, 93, 900–910.
- Bockenstedt, L.K.; Gonzalez, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Investig. 2012, 122, 2652–2660.
- Eisenstein, M. The skin microbiome and its relationship with the human body explained. Nature 2020, 588, S210–S211.
- Scholthof, K.B. The disease triangle: Pathogens, the environment and society. Nat. Rev. Microbiol. 2007, 5, 152–156.
- Dorfman, K. The Total Load Theory: Why So Many Children Have Developmental Problems. Available online: https://epidemicanswers.org/total-load-theory-why-so-many-children-have-developmental-problems/ (accessed on 18 December 2022).
- Bransfield, R.C. Relationship of Inflammation and Autoimmunity to Psychiatric Sequelae in Lyme Disease. Psychiatr. Ann. 2012, 42, 337–341.
- Fallon, B.A.; Levin, E.S.; Schweitzer, P.J.; Hardesty, D. Inflammation and central nervous system Lyme disease. Neurobiol. Dis. 2010, 37, 534–541.
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763.
- Burke, H.M.; Davis, M.C.; Otte, C.; Mohr, D.C. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005, 30, 846–856.
- Somani, A.; Singh, A.K.; Gupta, B.; Nagarkoti, S.; Dalal, P.K.; Dikshit, M. Oxidative and Nitrosative Stress in Major Depressive Disorder: A Case Control Study. Brain Sci. 2022, 12, 144.
- Halperin, J.J.; Heyes, M.P. Neuroactive kynurenines in Lyme borreliosis. Neurology 1992, 42, 43–50.
- Gasse, T.; Murr, C.; Meyersbach, P.; Schmutzhard, E.; Wachter, H.; Fuchs, D. Neopterin production and tryptophan degradation in acute Lyme neuroborreliosis versus late Lyme encephalopathy. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 685–689.
- Gasse, T.; Widner, B.; Baier-Bitterlich, G.; Sperner-Unterweger, B.; Leblhuber, F.; Wachter, H.; Fuchs, D. Abnormal tryptophan metabolism, neurologic/psychiatric disturbances and its relationship to immune activation. In Neurochemical Markers of Degenerative Nervous Diseases and Drug Addiction, Progress in HPLC-HPCE, 7th ed.; Qureshi, G.A., Parvez, H., Caudy, P., Parvez, S., Eds.; Ridderprint BV: Ridderkerk, The Netherlands, 1998.
- Wirleitner, B.; Neurauter, G.; Schröcksnadel, K.; Frick, B.; Fuchs, D. Interferon-gamma-induced conversion of tryptophan: Immunologic and neuropsychiatric aspects. Curr. Med. Chem. 2003, 10, 1581–1591.
- Myint, A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012, 279, 1375–1385.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530.
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 678164.
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377.
- Hemmer, B.; Gran, B.; Zhao, Y.; Marques, A.; Pascal, J.; Tzou, A.; Kondo, T.; Cortese, I.; Bielekova, B.; Straus, S.E.; et al. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat. Med. 1999, 5, 1375–1382.
- Soulas, P.; Woods, A.; Jaulhac, B.; Knapp, A.M.; Pasquali, J.L.; Martin, T.; Korganow, A.S. Autoantigen, innate immunity, and T cells cooperate to break B cell tolerance during bacterial infection. J. Clin. Investig. 2005, 115, 2257–2267.
- Sigal, L.H. Immunologic mechanisms in Lyme neuroborreliosis: The potential role of autoimmunity and molecular mimicry. Semin. Neurol. 1997, 17, 63–68.
- Kreye, J.; Reincke, S.M.; Prüss, H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat. Rev. Immunol. 2020, 20, 645–646.
- Sherbet, S.G.S. Bacterial Infections and the Pathogenesis of Autoimmune Conditions. Br. J. Med. Pract. 2009, 2, 6–13.
- Tausk, F.; Elenkov, I.; Moynihan, J. Psychoneuroimmunology. Dermatol. Ther. 2008, 21, 22–31.
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011, 17, 3729–3740.
- Klein, H.C.; de Witte, L.; Bransfield, R.; De Deyn, P.P. PET and SPECT in Neurology; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Leenders, K.L., Eds.; Springer: Cham, Switzerland, 2020.
- Wichers, M.C.; Koek, G.H.; Robaeys, G.; Verkerk, R.; Scharpé, S.; Maes, M. IDO and interferon-alpha-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 2005, 10, 538–544.
- Saikarthik, J.; Saraswathi, I.; Alarifi, A.; Al-Atram, A.A.; Mickeymaray, S.; Paramasivam, A.; Shaikh, S.; Jeraud, M.; Alothaim, A.S. Role of neuroinflammation mediated potential alterations in adult neurogenesis as a factor for neuropsychiatric symptoms in Post-Acute COVID-19 syndrome-A narrative review. PeerJ 2022, 10, e14227.
- Casey, B.J.; Heller, A.S.; Gee, D.G.; Cohen, A.O. Development of the emotional brain. Neurosci. Lett. 2019, 693, 29–34.
- Cattarinussi, G.; Miola, A.; Trevisan, N.; Valeggia, S.; Tramarin, E.; Mucignat, C.; Morra, F.; Minerva, M.; Librizzi, G.; Bordin, A.; et al. Altered brain regional homogeneity is associated with depressive symptoms in COVID-19. J. Affect. Disord. 2022, 313, 36–42.
- Zhuang, X.; Zhan, B.; Jia, Y.; Li, C.; Wu, N.; Zhao, M.; Chen, N.; Guo, Y.; Du, Y.; Zhang, Y.; et al. IL-33 in the basolateral amygdala integrates neuroinflammation into anxiogenic circuits via modulating BDNF expression. Brain Behav. Immun. 2022, 102, 98–109.
- Prell, T.; Dirks, M.; Arvanitis, D.; Braun, D.; Peschel, T.; Worthmann, H.; Schuppner, R.; Raab, P.; Grosskreutz, J.; Weissenborn, K. Cerebral patterns of neuropsychological disturbances in hepatitis C patients. J. Neurovirol. 2019, 25, 229–238.
- Denton, A.R.; Samaranayake, S.A.; Kirchner, K.N.; Roscoe, R.F., Jr.; Berger, S.N.; Harrod, S.B.; Mactutus, C.F.; Hashemi, P.; Booze, R.M. Selective monoaminergic and histaminergic circuit dysregulation following long-term HIV-1 protein exposure. J. Neurovirol. 2019, 25, 540–550.
- Kamat, R.; Brown, G.G.; Bolden, K.; Fennema-Notestein, C.; Archibald, S.; Marcotte, T.D.; Letendre, S.L.; Ellis, R.J.; Woods, S.P.; Grant, I.; et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J. Clin. Exp. Neuropsychol. 2014, 36, 854–866.
- Bransfield, R.C. Preventable cases of autism: Relationship between chronic infectious diseases and neurological outcome. Pediatr. Health 2009, 3, 125–140.
- Green, M.J.; Watkeys, O.J.; Whitten, T.; Thomas, C.; Karluki, M.; Dean, K.; Laurens, K.R.; Harris, F.; Carr, V.J. Increased incidence of childhood mental disorders following exposure to early life infection. Brain Behav. Immun. 2021, 97, 376–382.
- Petersen, L.; Gasse, C.; Mortensen, P.B.; Dalsgaard, S.; Yolken, R.H.; Mors, O.; Benros, M.E. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry 2019, 76, 271–279.
- Sabourin, K.R.; Reynolds, A.; Schendel, D.; Rosenberg, S.; Croen, L.A.; Pinto-Martin, J.A.; Schieve, L.A.; Newschaffer, C.; Lee, L.C.; Di Guiseppi, C. Infections in children with autism spectrum disorder: Study to Explore Early Development (SEED). Autism Res. 2019, 12, 136–146.
- Boyd, C.M.; Darer, J.; Boult, C.; Fried, L.P.; Boult, L.; Wu, A.W. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA 2005, 294, 716–724.
- Bransfield, R.C.; Friedman, K.J. Differentiating Psychosomatic, Somatopsychic, Multisystem Illnesses, and Medical Uncertainty. Healthcare 2019, 7, 114.
- Citera, M.; Freeman, P.R.; Horowitz, R.I. Empirical validation of the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire for suspected Lyme disease. Int. J. Gen. Med. 2017, 10, 249–273.
- Shroff, G.; Hopf-Seidel, P. A Novel Scoring System Approach to Assess Patients with Lyme Disease (Nutech Functional Score). J. Glob. Infect. Dis. 2018, 10, 3–6.
- Fallon, B.A.; Zubcevik, N.; Bennett, C.; Doshi, S.; Rebman, A.W.; Kishon, R.; Moeller, J.R.; Octavien, N.R.; Aucott, J.N. The General Symptom Questionnaire-30 (GSQ-30): A Brief Measure of Multi-System Symptom Burden in Lyme Disease. Front. Med. 2019, 6, 283.
- Centers for Disease Control and Prevention. National Healthcare Safety Network: Surveillance vs. Clinical. Frequently Asked Questions (FAQs) Frequently Asked Questions (FAQs). Available online: https://www.cdc.gov/nhsn/faqs/faqs-miscellaneous.html (accessed on 7 June 2023).
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric Acute-onset Neuropsychiatric Syndrome and Mycoplasma Pneumoniae Infection: A Case Report Analysis with a Metabolomics Approach. Curr. Pediatr. Rev. 2020, 16, 183–193.
- Weinstein, E.R.; Rebman, A.W.; Aucott, J.N.; Johnson-Greene, D.; Bechtold, K.T. Sleep quality in well-defined Lyme disease: A clinical cohort study in Maryland. Sleep 2018, 41, zsy035.
- Modolfsy, H. Sleep and the immune system. Int. J. Immunopharmacol. 1995, 17, 649–654.
- Kelley, K.W. The role of growth hormone in modulation of the immune response. Ann. N. Y. Acad. Sci. 1990, 594, 95–103.





