
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zipeng Zhang | -- | 5042 | 2024-01-07 03:50:35 | | | |
| 2 | Lindsay Dong | + 16 word(s) | 5058 | 2024-01-08 01:39:05 | | |
Video Upload Options
Lithium slag (LS)’s particle size distribution is comparable to fly ash (FA) and ground granulated blast furnace slag (GGBS), which suggests it can enhance densification and nucleation in concrete. The mechanical treatment of LS promotes early hydration by increasing the solubility of aluminum, lithium, and silicon. LS’s compositional similarity to FA endows it with low-calcium, high-reactivity properties that are suitable for cementitious and geopolymeric applications. Increasing the LS content reduces setting times and flowability while initially enhancing mechanical properties, albeit with diminishing returns beyond a 30% threshold. LS significantly improves chloride ion resistance and impacts drying shrinkage variably.
1. Introduction
2. Physiochemical and Microscopic Analysis of LS
2.1. Physical Properties of Raw LS
2.1.1. Particle Size Distribution
2.1.2. Density, Specific Surface Area, and Moisture Content
2.2. Chemical Properties of LS
2.2.1. Chemical Composition
2.2.2. XRD Results
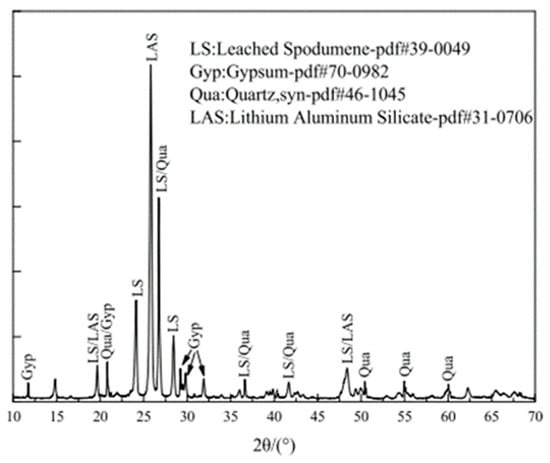
2.3. Microscopic Analysis of LS
2.3.1. SEM-EDS Analysis
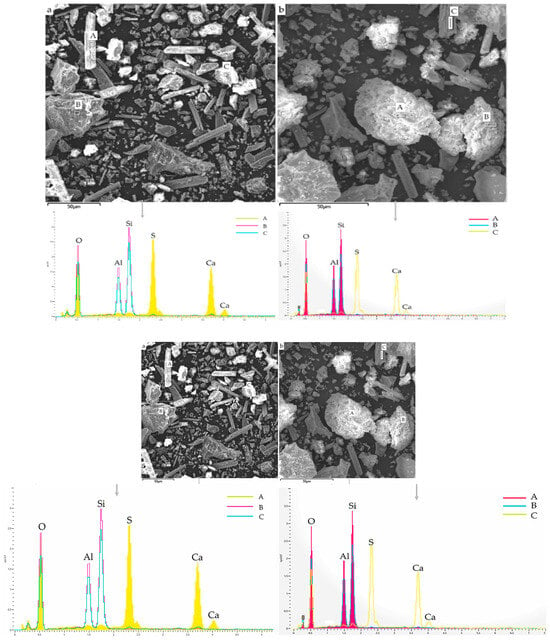
2.3.2. TG-DTG Analysis
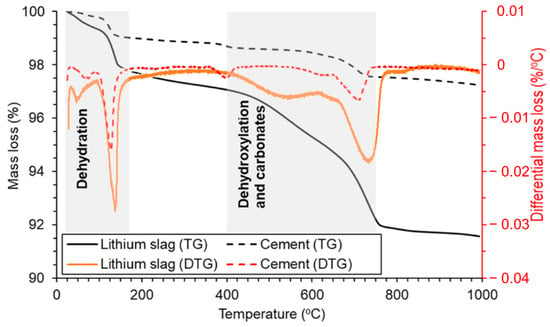
2.3.3. NMR and XPS Analysis
3. Fresh State Properties of Cementitious Composites with LS Incorporation
3.1. Setting Time
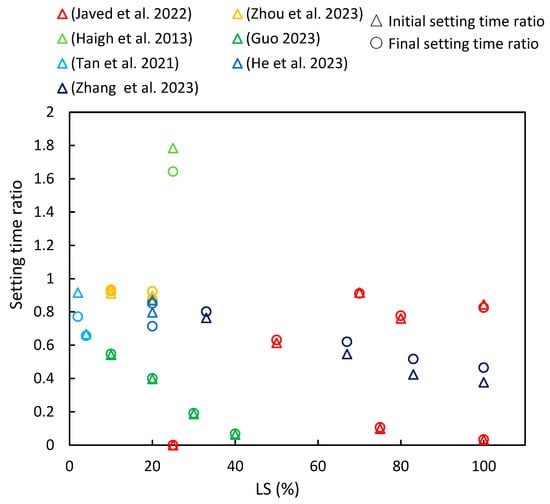
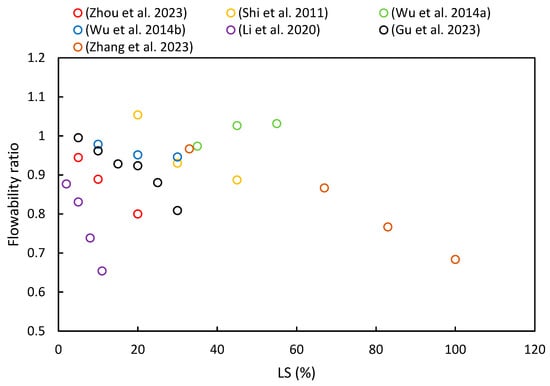
3.2. Flowability
3.3. Rheology
4. Mechanical Properties of Cementitious Composites with LS Incorporation
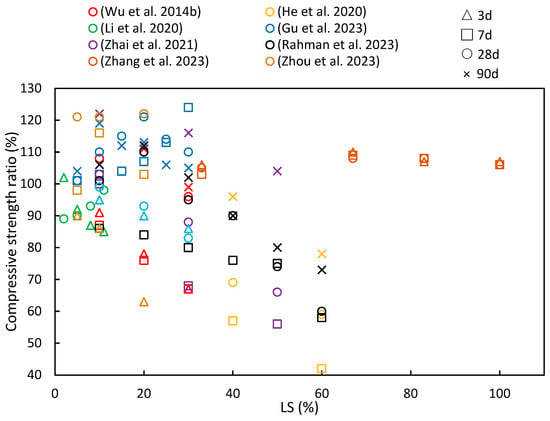
4.1. Compressive Strength
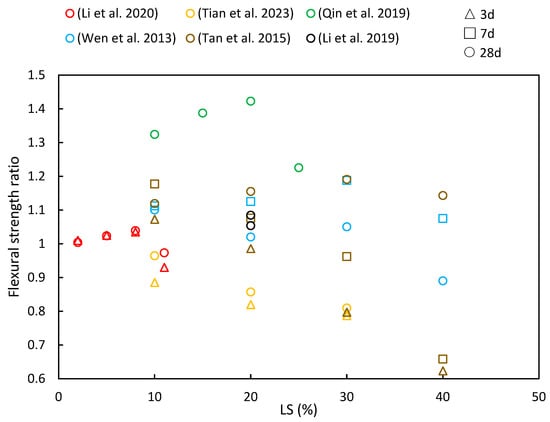
4.2. Flexural Strength
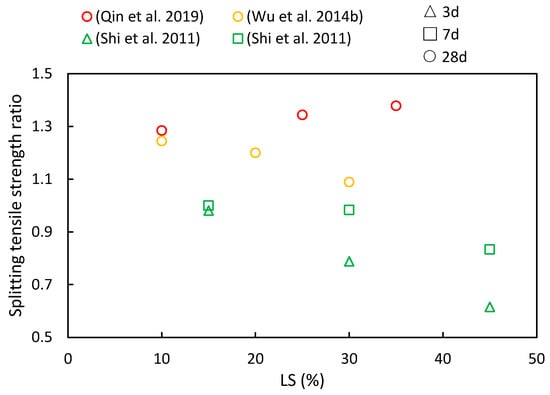
4.3. Splitting Tensile Strength and Elastic Modulus
5. Durability of Cementitious Composites with LS Incorporation
5.1. Chloride Resistance
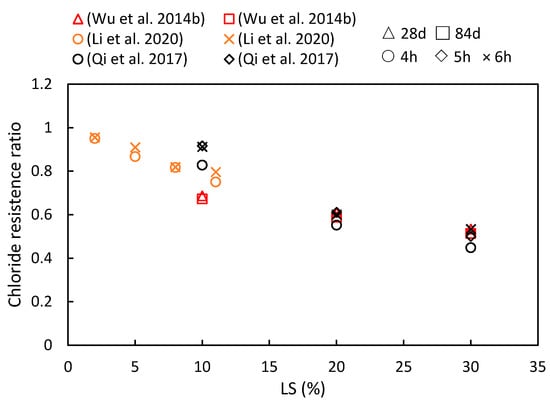
5.2. Shrinkage
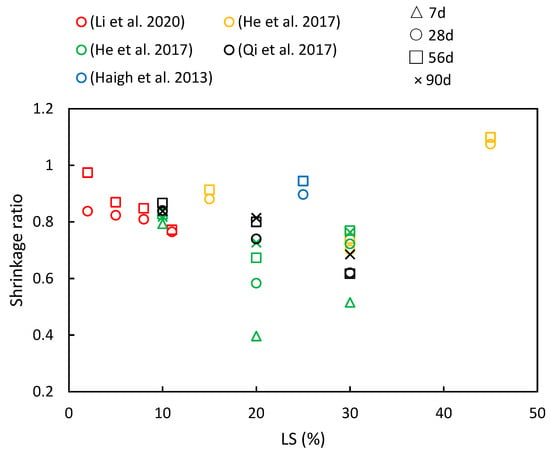
5.3. Sulfate Attack and Carbonation
6. Chemical and Microstructural Investigations of Cementitious Composites with LS Incorporation
6.1. Hydration Heat
6.2. Pore Structure
6.3. XRD Analysis
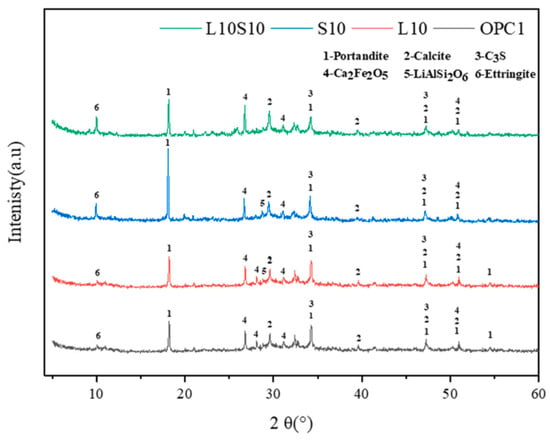
6.4. SEM Analysis

6.5. FTIR and TG Analysis
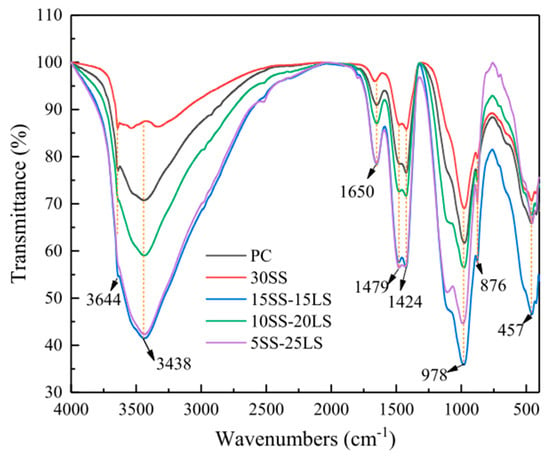
7. Cost, Energy, and Carbon Emission Comparisons
8. Conclusions
(1) The PSD of LS closely resembles that of FA and GGBS. This similarity suggests that LS can exhibit similar effects related to densification and nucleation when integrated into concrete, thus resembling the behavior of FA and other SCMs. Mechanical treatment of LS enhances the dissolution of aluminum, lithium, and silicon in LS, thereby expediting early hydration in LS–cement systems.
(2) LS exhibits variations in SiO2 + Al2O3 and Ca/(Si + Al) within the ranges of 70.29–80.77% and 0.02–0.14%, respectively. This composition aligns LS with FA, which is characterized by high SiO2 and Al2O3 contents and a low CaO content. This similarity categorizes LS as a low-calcium precursor with chemical reactivity akin to that of FA.
(3) In most of the literature examined, an increase in LS content was shown to lead to a reduction in the initial and final setting times of LS–cement and LS–geopolymer systems. Moreover, the studies determined that flowability decreased with an increase in LS content due to its irregular shape, strong water absorption characteristics, and elevated formation of AFt in the initial stages of hydration.
(4) As LS content increases, the compressive strength, flexural strength, and splitting tensile strength ratios initially increase, with diminishing returns beyond a 30% threshold. This suggests an optimal LS content for achieving favorable mechanical properties. Additionally, with longer curing periods, there is a noticeable upward trend in the compressive strength, flexural strength, and splitting tensile strength ratios.
(5) LS plays a crucial role in enhancing chloride ion migration resistance and reducing shrinkage in cementitious systems. Furthermore, as the composite ages, the resistance of the cementitious system to chloride ions becomes more robust. However, the behavior of drying shrinkage exhibits various trends.
(6) The mechanisms through which LS operates within cementitious composites can be classified into three main categories. Firstly, there is the filling effect: the fine-grained nature of LS improves particle packing, and its fine particles act as pore blockers, thereby reducing interconnectivity between pores and effectively lowering porosity. Secondly, there is the pozzolanic effect: LS reacts with calcium hydroxide to generate additional hydration products, thereby refining large pores and bridging the gap between the paste and aggregates. Thirdly, there is the nucleation effect: LS provides nucleation sites, thereby promoting the preferential production and development of hydration products in these specific locations.
(7) LS not only exhibits similar pozzolanic activity to FA, but it also comes at just one-fifth of the price of FA. This makes LS an economically attractive option for concrete or geopolymer production, thereby significantly reducing manufacturing costs. Moreover, the embodied CO2 and embodied energy of LS are comparable to GGBS, slightly higher than those of FA and SF, and significantly lower than those of MK, LP, and cement. Therefore, LS exhibits the potential for solid waste recycling and sustainable development.
References
- Almeida, O.P.; Etherton-Beer, C.; Sanfilippo, F.; Page, A. Health morbidities associated with the dispensing of lithium to males and females: Cross-sectional analysis of the 10% Pharmaceutical Benefits Scheme sample for 2022. J. Affect. Disord. 2024, 344, 503–509.
- Martín-Alfonso, J.E.; Moreno, G.; Valencia, C.; Sánchez, M.C.; Franco, J.M.; Gallegos, C. Influence of soap/polymer concentration ratio on the rheological properties of lithium lubricating greases modified with virgin LDPE. J. Ind. Eng. Chem. 2009, 15, 687–693.
- Abouhaswa, A.S.; Rabiea, E.A.; Abomostafa, H.M. Structural, optical, and magnetic studies on novel Pr- doped barium lithium fluoroborate glass system. J. Non-Cryst. Solids 2023, 619, 122533.
- Juri, A.Z.; Song, X.-F.; Nakanishi, Y.; Dudley, J.; Jamieson, L.; Yin, L. Surface fractures in pre-crystallized and crystallized zirconia-containing lithium silicate glass-ceramics generated in ultrasonic vibration-assisted machining. J. Mech. Behav. Biomed. Mater. 2023, 147, 106132.
- Ali Shah, S.F.; Chen, B.; Ahmad, M.R.; Haque, M.A. Development of Cleaner One-part geopolymer from lithium slag. J. Clean. Prod. 2021, 291, 125241.
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085.
- Roy, T.; Plante, B.; Benzaazoua, M.; Demers, I. Geochemistry and mineralogy of a spodumene-pegmatite lithium ore at various mineral beneficiation stages. Miner. Eng. 2023, 202, 108312.
- Kundu, T.; Rath, S.S.; Das, S.K.; Parhi, P.K.; Angadi, S.I. Recovery of lithium from spodumene-bearing pegmatites: A comprehensive review on geological reserves, beneficiation, and extraction. Powder Technol. 2023, 415, 118142.
- Lemougna, P.N.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Recycling lithium mine tailings in the production of low temperature (700–900 °C) ceramics: Effect of ladle slag and sodium compounds on the processing and final properties. Constr. Build. Mater. 2019, 221, 332–344.
- Rahman, S.A.; Shaikh, F.U.A.; Sarker, P.K. A comprehensive review of properties of concrete containing lithium refinery residue as partial replacement of cement. Constr. Build. Mater. 2022, 328, 127053.
- Australian Lithium Production to Grow by 24.5% in 2022 as Capacity Expands. 2022. Available online: https://www.mining-technology.com/comment/australian-lithium-production/?cf-view (accessed on 27 July 2022).
- Liu, Z.; Wang, J.; Jiang, Q.; Cheng, G.; Li, L.; Kang, Y.; Wang, D. A green route to sustainable alkali-activated materials by heat and chemical activation of lithium slag. J. Clean. Prod. 2019, 225, 1184–1193.
- Lu, J.; Yu, Z.; Zhu, Y.; Huang, S.; Luo, Q.; Zhang, S. Effect of Lithium-Slag in the Performance of Slag Cement Mortar Based on Least-Squares Support Vector Machine Prediction. Materials 2019, 12, 1652.
- Tan, H.; Li, X.; He, C.; Ma, B.; Bai, Y.; Luo, Z. Utilization of lithium slag as an admixture in blended cements: Physico-mechanical and hydration characteristics. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 129–133.
- Li, M.; Liu, H.; Duan, P.; Ruan, S.; Zhang, Z.; Ge, W. The effects of lithium slag on microstructure and mechanical performance of metakaolin-based geopolymers designed by response surface method (RSM). Constr. Build. Mater. 2021, 299, 123950.
- Guo, C.; Wang, R. Utilizing lithium slag to improve the physical-chemical properties of alkali-activated metakaolin-slag pastes: Cost and energy analysis. Constr. Build. Mater. 2023, 403, 133164.
- Jayalath, A.; San Nicolas, R.; Sofi, M.; Shanks, R.; Ngo, T.; Aye, L.; Mendis, P. Properties of cementitious mortar and concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 2016, 120, 408–417.
- Rupasinghe, M.; San Nicolas, R.; Mendis, P.; Sofi, M.; Ngo, T. Investigation of strength and hydration characteristics in nano-silica incorporated cement paste. Cem. Concr. Compos. 2017, 80, 17–30.
- Rahman, S.; Dodd, A.; Khair, S.; Shaikh, F.; Sarker, P.; Hosan, M.A. Assessment of lithium slag as a supplementary cementitious material: Pozzolanic activity and microstructure development. Cem. Concr. Compos. 2023, 143, 105262.
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. A comprehensive micro-nano investigative approach to study the development of aluminosilicate gel in binary blends of lithium slag geopolymer. Cem. Concr. Compos. 2024, 145, 105338.
- Safari, A.; Lim, H. The benefit of delithiated beta spodumene to reduce the carbon footprint of cemented paste backfill. In Proceedings of the Paste 2023: 25th International Conference on Paste, Thickened and Filtered Tailings, University of Alberta, Edmonton, and Australian Centre for Geomechanics, Perth, Banff, AB, Canada, 29 April–3 May 2023; Wilson, G.W., Beier, N.A., Sego, D.C., Fourie, A.B., Reid, D., Eds.; University of Alberta, Edmonton, and Australian Centre for Geomechanics, Perth: Edmonton, NW, Canada, 2023; pp. 82–97.
- Argın, G.; Uzal, B. Enhancement of pozzolanic activity of calcined clays by limestone powder addition. Constr. Build. Mater. 2021, 284, 122789.
- Haigh, M.; Dumitru, I.; Munn, B.; Papworth, F. Development of new high performance supplementary cementitious material—A lithium production by-product. In Proceedings of the CIA Binnual Conference, Queensland, Australia, 13 October 2013.
- He, Z.-h.; Du, S.-g.; Chen, D. Microstructure of ultra high performance concrete containing lithium slag. J. Hazard. Mater. 2018, 353, 35–43.
- He, Y.; Kang, Q.; Lan, M.; Peng, H. Mechanism and assessment of the pozzolanic activity of melting-quenching lithium slag modified with MgO. Constr. Build. Mater. 2023, 363, 129692.
- Liu, Z.; Wang, J.-X.; Li, L.; Wang, D.-M. Characteristics of alkali-activated lithium slag at early reaction age. J. Mater. Civ. Eng. 2019, 31, 4019312.
- Javed, U.; Uddin Ahmed Shaikh, F.; Kumar Sarker, P. Microstructural investigation of thermo-mechanically processed lithium slag for geopolymer precursor using various characterization techniques. Constr. Build. Mater. 2022, 342, 127952.
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive utilization of waste residue from lithium extraction process of spodumene. Miner. Eng. 2021, 170, 106986.
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. Microstructural investigation of lithium slag geopolymer pastes containing silica fume and fly ash as additive chemical modifiers. Cem. Concr. Compos. 2022, 134, 104736.
- Zhai, M.; Zhao, J.; Wang, D.; Wang, Y.; Wang, Q. Hydration properties and kinetic characteristics of blended cement containing lithium slag powder. J. Build. Eng. 2021, 39, 102287.
- Karrech, A.; Dong, M.; Elchalakani, M.; Shahin, M. Sustainable geopolymer using lithium concentrate residues. Constr. Build. Mater. 2019, 228, 116740.
- Li, J.; Lian, P.; Huang, S.; Huang, L. Recycling of lithium slag extracted from lithium mica by preparing white Portland cement. J. Environ. Manag. 2020, 265, 110551.
- Tan, H.; Li, M.; He, X.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2020, 250, 119528.
- Yiren, W.; Dongmin, W.; Yong, C.; Dapeng, Z.; Ze, L. Micro-morphology and phase composition of lithium slag from lithium carbonate production by sulphuric acid process. Constr. Build. Mater. 2019, 203, 304–313.
- Li, B.; Cao, R.; You, N.; Chen, C.; Zhang, Y. Products and properties of steam cured cement mortar containing lithium slag under partial immersion in sulfate solution. Constr. Build. Mater. 2019, 220, 596–606.
- Tan, H.; Zhang, X.; He, X.; Guo, Y.; Deng, X.; Su, Y.; Yang, J.; Wang, Y. Utilization of lithium slag by wet-grinding process to improve the early strength of sulphoaluminate cement paste. J. Clean. Prod. 2018, 205, 536–551.
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J. Environ. Manag. 2020, 276, 111274.
- Li, J.; Huang, S. Recycling of lithium slag as a green admixture for white reactive powder concrete. J. Mater. Cycles Waste Manag. 2020, 22, 1818–1827.
- He, Z.-h.; Li, L.-y.; Du, S.-g. Mechanical properties, drying shrinkage, and creep of concrete containing lithium slag. Constr. Build. Mater. 2017, 147, 296–304.
- Qin, Y.; Chen, J.; Li, Z.; Zhang, Y. The Mechanical Properties of Recycled Coarse Aggregate Concrete with Lithium Slag. Adv. Mater. Sci. Eng. 2019, 2019, 8974625.
- Wu, F.F.; Shi, K.B.; Dong, S.K. Influence of concrete with lithium-slag and steel slag by early curing conditions. Key Eng. Mater. 2014, 599, 52–55.
- Shi, K.; Zhang, S. Ring method test on the early-age anti-cracking capability of high-performance lithium slag concrete. Appl. Mech. Mater. 2011, 94–96, 782–785.
- Qin, Y.; Li, Y.W.; Lan, X.Z.; Su, Y.S.; Wang, X.Y.; Wu, Y.D. Structural behavior of the stiffened double-skin profiled composite walls under compression. Steel Compos. Struct. 2019, 31, 1–12.
- Celik, I.B. The effects of particle size distribution and surface area upon cement strength development. Powder Technol. 2009, 188, 272–276.
- Zhang, Y.; Zhang, L.; Wang, Q.; Han, D.; Li, Z. Iron ore tailings, phosphate slags, and lithium slags as ternary supplementary cementitious materials for concrete: Study on compression strength and microstructure. Mater. Today Commun. 2023, 36, 106644.
- Rahman, S.M.A.; Mahmood, A.H.; Shaikh, F.U.A.; Sarker, P.K. Fresh state and hydration properties of high-volume lithium slag cement composites. Mater. Struct. 2023, 56, 91.
- Odler, I. The BET-specific surface area of hydrated Portland cement and related materials. Cem. Concr. Res. 2003, 33, 2049–2056.
- Sarbak, Z.; Stańczyk, A.; Kramer-Wachowiak, M. Characterisation of surface properties of various fly ashes. Powder Technol. 2004, 145, 82–87.
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399.
- Qin, Y.; Chen, J.; Liu, K.; Lu, Y. Durability properties of recycled concrete with lithium slag under freeze-thaw cycles. Mater. Technol. 2021, 55, 171–181.
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151.
- Gao, W.; Jian, S.; Li, X.; Tan, H.; Li, B.; Lv, Y.; Huang, J. The use of contaminated soil and lithium slag for the production of sustainable lightweight aggregate. J. Clean. Prod. 2022, 348, 131361.
- He, Y.; Zhang, G.; He, S.; Liu, S.; Jiang, M. Effect of C-S-H-PCE and TEA on performances of lithium slag-cement binder. J. Build. Eng. 2023, 78, 107659.
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019, 138, 161–167.
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.-P.; Fabritius, T. α→γ→β-phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60.
- Maslyk, M.; Dallos, Z.; Koziol, M.; Seiffert, S.; Hieke, T.; Petrović, K.; Kolb, U.; Mondeshki, M.; Tremel, W. A Fast and Sustainable Route to Bassanite Nanocrystals from Gypsum. Adv. Funct. Mater. 2022, 32, 2111852.
- Bernal, S.A.; Juenger, M.C.; Ke, X.; Matthes, W.; Lothenbach, B.; De Belie, N.; Provis, J.L. Characterization of supplementary cementitious materials by thermal analysis. Mater. Struct. 2017, 50, 1–13.
- Khandelwal, S.; Rhee, K.Y. Evaluation of pozzolanic activity, heterogeneous nucleation, and microstructure of cement composites with modified bentonite clays. Constr. Build. Mater. 2022, 323, 126617.
- Burris, L.E.; Juenger, M.C. Effect of calcination on the reactivity of natural clinoptilolite zeolites used as supplementary cementitious materials. Constr. Build. Mater. 2020, 258, 119988.
- Zhang, Y.; Yang, B.; Gu, X.; Han, D.; Wang, Q. Improving the performance of ultra-high performance concrete containing lithium slag by incorporating limestone powder. J. Build. Eng. 2023, 72, 106610.
- Zhou, M.; Yan, J.; Fan, J.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Lu, Z. Insight to workability, compressive strength and microstructure of lithium slag-steel slag based cement under standard condition. J. Build. Eng. 2023, 75, 107076.
- Tan, H.; Li, M.; He, X.; Su, Y.; Yang, J.; Zhao, H. Effect of wet grinded lithium slag on compressive strength and hydration of sulphoaluminate cement system. Constr. Build. Mater. 2021, 267, 120465.
- Noor, L.; Tuinukuafe, A.; Ideker, J.H. A critical review of the role of ettringite in binders composed of CAC–PC–C and CSA–PC–C. J. Am. Ceram. Soc. 2023, 106, 3303–3328.
- Matalkah, F.; Salem, T.; Shaafaey, M.; Soroushian, P. Drying shrinkage of alkali activated binders cured at room temperature. Constr. Build. Mater. 2019, 201, 563–570.
- Luo, Q.; Liu, Y.; Dong, B.; Ren, J.; He, Y.; Wu, K.; Wang, Y. Lithium slag-based geopolymer synthesized with hybrid solid activators. Constr. Build. Mater. 2023, 365, 130070.
- Wu, F.F.; Shi, K.B.; Dong, S.K. Properties and Microstructure of HPC with Lithium-Slag and Fly Ash. Key Eng. Mater. 2014, 599, 70–73.
- Al-Kheetan, M.J.; Rahman, M.M.; Balakrishna, M.N.; Chamberlain, D.A. Performance enhancement of self-compacting concrete in saline environment by hydrophobic surface protection. Can. J. Civ. Eng. 2019, 46, 677–686.
- Biricik, Ö.; Mardani, A. Parameters affecting thixotropic behavior of self compacting concrete and 3D printable concrete; A state-of-the-art review. Constr. Build. Mater. 2022, 339, 127688.
- Souza, M.T.; Ferreira, I.M.; de Moraes, E.G.; Senff, L.; de Oliveira, A.P.N. 3D printed concrete for large-scale buildings: An overview of rheology, printing parameters, chemical admixtures, reinforcements, and economic and environmental prospects. J. Build. Eng. 2020, 32, 101833.
- Luo, Q.; Wang, Y.; Hong, S.; Xing, F.; Dong, B. Properties and microstructure of lithium-slag-based geopolymer by one-part mixing method. Constr. Build. Mater. 2021, 273, 121723.
- He, Z.; Chang, J.; Du, S.; Liang, C.; Liu, B. Hydration and microstructure of concrete containing high volume lithium slag. Mater. Express 2020, 10, 430–436.
- Gu, X.; Wang, H.; Zhu, Z.; Liu, J.; Xu, X.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768.
- Wen, H. Property research of green concrete mixed with lithium slag and limestone flour. Adv. Mater. Res. 2013, 765–767, 3120–3124.
- Tian, Y.; Xie, Z.; Yuan, Q.; Jamaa, G.M.; Yang, C.; Zhu, X. Improving the rheological behavior of alkali-activated slag pastes by using low surface free energy mineral admixtures. Constr. Build. Mater. 2023, 392, 131879.
- Qi, L.; Huang, S.; Zhou, Y.; Li, J.; Peng, W.; Wen, Y. Influence of lithium slag from lepidolite on the durability of concrete. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12151.
- He, Z.; Wu, R.; Yu, Y.; Gao, Y. Effect of Lithium Slag on Drying Shrinkage of Concrete with Manufactured-sand. J. Residuals Sci. Technol. 2017, 14, 171–176.
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Sofi, M.; Sabri, Y. Reaction mechanism of alkali-activated brick clay mill residues. Constr. Build. Mater. 2022, 341, 127817.
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823.
- Dorn, T.; Blask, O.; Stephan, D. Acceleration of cement hydration—A review of the working mechanisms, effects on setting time, and compressive strength development of accelerating admixtures. Constr. Build. Mater. 2022, 323, 126554.
- He, Y.; Liu, S.; Hooton, R.D.; Zhang, X.; He, S. Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Constr. Build. Mater. 2022, 318, 125757.
- Haustein, E.; Kuryłowicz-Cudowska, A. The effect of fly ash microspheres on the pore structure of concrete. Minerals 2020, 10, 58.
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679.
- Miah, M.J.; Patoary, M.M.H.; Paul, S.C.; Babafemi, A.J.; Panda, B. Enhancement of mechanical properties and porosity of concrete using steel slag coarse aggregate. Materials 2020, 13, 2865.
- Schovanz, D.; Tiecher, F.; Hasparyk, N.P.; Kuperman, S.; Lermen, R.T. Evaluation of delayed ettringite formation through physical, mechanical, and microstructural assays. ACI Mater. J. 2021, 118, 101–109.
- Flores-Ledesma, A.; Tejeda-Cruz, A.; Bucio, L.; Wintergerst, A.M.; Rodríguez-Chávez, J.A.; Moreno-Vargas, Y.A.; Arenas-Alatorre, J.A. Hydration products and bioactivity of an experimental MTA-like cement modified with wollastonite and bioactive glass. Ceram. Int. 2020, 46, 15963–15971.
- Karim, M.R.; Chowdhury, F.I.; Zabed, H.; Saidur, M.R. Effect of elevated temperatures on compressive strength and microstructure of cement paste containing palm oil clinker powder. Constr. Build. Mater. 2018, 183, 376–383.
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean Prod. 2020, 263, 121477.




