
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Allison B. Reiss | -- | 1997 | 2024-01-07 03:19:56 | | | |
| 2 | Jason Zhu | Meta information modification | 1997 | 2024-01-08 06:27:00 | | |
Video Upload Options
Prostate cancer is the second leading cause of cancer death in men in the United States. Androgen deprivation therapy (ADT) is currently the primary treatment for metastatic prostate cancer, and some studies have shown that the use of anti-androgen drugs is related to a reduction in cognitive function, mood changes, diminished quality of life, dementia, and possibly Alzheimer’s disease. ADT has potential physiological effects such as a reduction in white matter integrity and a negative impact on hypothalamic functions due to the lowering of testosterone levels or the blockade of downstream androgen receptor signaling by first- and second-generation anti-androgen drugs.
1. Introduction
2. Indications for ADT
3. ADT Medications and Their Mechanism of Action
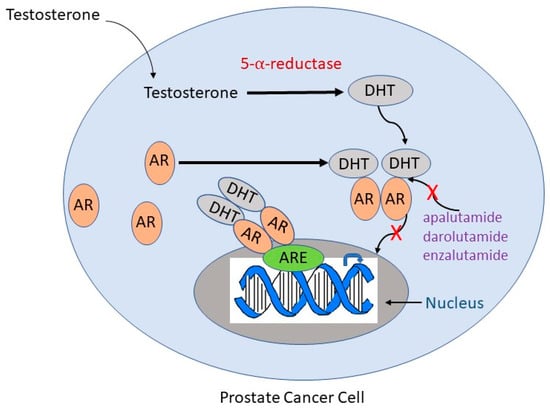
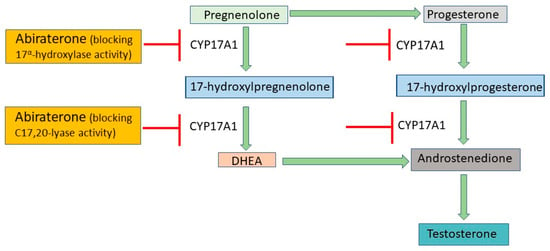
4. ADT Risks and Side Effects
4.1. Overview of Side Effects
4.2. Hot Flushes
4.3. Sexual Dysfunction
4.4. Bone Density
4.5. Cardiovascular Effects of ADT
4.6. ADT and Cognition
Those with prostate cancer are generally older, with most above age 65 and are therefore in the age category more likely to be diagnosed with dementia from Alzheimer's disease and other etiologies. In addition, there is more and more evidence supporting a relationship between ADT and cognitive issues while prostate cancer itself does not impair cognition. Common complaints of those experiencing cognitive issues on ADT include difficulty with memory, concentration and focus. The causality of this association has not been established, but the field is moving forward and the problem merits attention because it has a profound effect on quality of life. There is a need for treatment to address the cognitive complaints of those living with prostate cancer and receiving ADT that is prompting research into new approaches.
References
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens Health 2018, 12, 1807–1823.
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044.
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836.
- Zitzmann, M. Testosterone, mood, behaviour and quality of life. Andrology 2020, 8, 1598–1605.
- Bennett, C.L.; Tosteson, T.D.; Schmitt, B.; Weinberg, P.D.; Ernstoff, M.S.; Ross, S.D. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: A meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer Prostatic Dis. 1999, 2, 4–8.
- Mandel, P.; Hoeh, B.; Wenzel, M.; Preisser, F.; Tian, Z.; Tilki, D.; Steuber, T.; Karakiewicz, P.I.; Chun, F.K.H. Triplet or Doublet Therapy in Metastatic Hormone-sensitive Prostate Cancer Patients: A Systematic Review and Network Meta-analysis. Eur. Urol. Focus 2023, 9, 96–105.
- Hall, M.E.; Huelster, H.L.; Luckenbaugh, A.N.; Laviana, A.A.; Keegan, K.A.; Klaassen, Z.; Moses, K.A.; Wallis, C.J.D. Metastatic hormone-sensitive prostate cancer: Current perspective on the evolving therapeutic landscape. Onco. Targets Ther. 2020, 13, 3571–3581.
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282.
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, 1616–1622.
- Mell, L.K.; Pugh, S.L.; Jones, C.U.; Nelson, T.J.; Zakeri, K.; Rose, B.S.; Zeitzer, K.L.; Gore, E.M.; Bahary, J.P.; Souhami, L.; et al. Effects of Androgen Deprivation Therapy on Prostate Cancer Outcomes According to Competing Event Risk: Secondary Analysis of a Phase 3 Randomised Trial. Eur. Urol. 2023, in press.
- Tobiansky, D.J.; Wallin-Miller, K.G.; Floresco, S.B.; Wood, R.I.; Soma, K.K. Androgen Regulation of the Mesocorticolimbic System and Executive Function. Front. Endocrinol. 2018, 9, 279.
- Giatti, S.; Garcia-Segura, L.M.; Barreto, G.E.; Melcangi, R.C. Neuroactive steroids, neurosteroidogenesis and sex. Prog. Neurobiol. 2019, 176, 1–17.
- Janowsky, J.S. The role of androgens in cognition and brain aging in men. Neuroscience 2006, 138, 1015–1020.
- Resnick, S.M.; Matsumoto, A.M.; Stephens-Shields, A.J.; Ellenberg, S.S.; Gill, T.M.; Shumaker, S.A.; Pleasants, D.D.; Barrett-Connor, E.; Bhasin, S.; Cauley, J.A.; et al. Testosterone Treatment and Cognitive Function in Older Men With Low Testosterone and Age-Associated Memory Impairment. JAMA 2017, 317, 717–727.
- Nieschlag, E.; Nieschlag, S. Endocrine history: The history of discovery, synthesis and development of testosterone for clinical use. Eur. J. Endocrinol. 2019, 180, R201–R212.
- Ng, K.; Smith, S.; Shamash, J. Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): Advances and Treatment Strategies in the First-Line Setting. Oncol. Ther. 2020, 8, 209–230.
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801.
- Gim, H.J.; Park, J.; Jung, M.E.; Houk, K.N. Conformational dynamics of androgen receptors bound to agonists and antagonists. Sci. Rep. 2021, 11, 15887.
- Rehman, Y.; Rosenberg, J.E. Abiraterone acetate: Oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des. Devel. Ther. 2012, 6, 13–18.
- Guo, C.; Yeh, S.; Niu, Y.; Li, G.; Zheng, J.; Li, L.; Chang, C. Targeting androgen receptor versus targeting androgens to suppress castration resistant prostate cancer. Cancer Lett. 2017, 397, 133–143.
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373.
- Mitsiades, N.; Kaochar, S. Androgen receptor signaling inhibitors: Post-chemotherapy, pre-chemotherapy and now in castration-sensitive prostate cancer. Endocr. Relat. Cancer 2021, 28, T19–T38.
- Corona, G.; Filippi, S.; Comelio, P.; Bianchi, N.; Frizza, F.; Dicuio, M.; Rastrelli, G.; Concetti, S.; Sforza, A.; Vignozzi, L.; et al. Sexual function in men undergoing androgen deprivation therapy. Int. J. Impot. Res. 2021, 33, 439–447.
- Russell, N.; Hoermann, R.; Cheung, A.S.; Zajac, J.D.; Grossmann, M. Effects of oestradiol treatment on hot flushes in men undergoing androgen deprivation therapy for prostate cancer: A randomised placebo-controlled trial. Eur. J. Endocrinol. 2022, 187, 617–627.
- Bargiota, A.; Oeconomou, A.; Zachos, I.; Samarinas, M.L.; Pisters, L.; Tzortzis, V. Adverse effects of androgen deprivation therapy in patients with prostate cancer: Focus on muscle and bone health. J. BUON 2020, 25, 1286–1294.
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251.
- Kaplan, M.; Mahon, S.M.; Lubejko, B.G.; Ginex, P.K. Hot Flashes: Clinical Summary of the ONS Guidelines™ for Cancer Treatment-Related Hot Flashes in Women With Breast Cancer and Men With Prostate Cancer. Clin. J. Oncol. Nurs. 2020, 24, 430–433.
- Khan, A.; Lewis, R.; Hughes, S. Managing Hot Flushes in Men Receiving Androgen Deprivation Therapy for Prostate Cancer. Trends Urol. Men’s Health 2014, 5, 31–33.
- Wibowo, E.; Schellhammer, P.; Wassersug, R.J. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J. Urol. 2011, 185, 17–23.
- Beer, T.M.; Schellhammer, P.F.; Corman, J.M.; Glodé, L.M.; Hall, S.J.; Whitmore, J.B.; Frohlich, M.W.; Penson, D.F. Quality of life after sipuleucel-T therapy: Results from a randomized, double-blind study in patients with androgen-dependent prostate cancer. Urology 2013, 82, 410–415.
- Kaplan, I.; Bubley, G.J.; Bhatt, R.S.; Taplin, M.E.; Dowling, S.; Mahoney, K.; Werner, E.; Nguyen, P. Enzalutamide With Radiation Therapy for Intermediate-Risk Prostate Cancer: A Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1416–1422.
- Hussain, A.; Jiang, S.; Varghese, D.; Appukkuttan, S.; Kebede, N.; Gnanasakthy, K.; Macahilig, C.; Waldeck, R.; Corman, S. Real-world burden of adverse events for apalutamide- or enzalutamide-treated non-metastatic castration-resistant prostate cancer patients in the United States. BMC Cancer 2022, 22, 304.
- Allan, C.A.; Collins, V.R.; Frydenberg, M.; McLachlan, R.I.; Matthiesson, K.L. Androgen deprivation therapy complications. Endocr. Relat. Cancer 2014, 21, T119–T129.
- Fisher, W.I.; Johnson, A.K.; Elkins, G.R.; Otte, J.L.; Burns, D.S.; Yu, M.; Carpenter, J.S. Risk factors, pathophysiology, and treatment of hot flashes in cancer. CA Cancer J. Clin. 2013, 63, 167–192.
- Russell, N.; Hoermann, R.; Cheung, A.S.; Zajac, J.d.; Handelsman, D.J.; Grossman, M. Short-term effects of transdermal estradiol in men undergoing androgen deprivation therapy for prostate cancer: A randomized placebo-controlled trial. Eur. J. Endocrinol. 2018, 178, 565–576.
- Kouriefs, C.; Georgiou, M.; Ravi, R. Hot flushes and prostate cancer: Pathogenesis and treatment. BJU Int. 2002, 89, 379–383.
- Qan’ir, Y.; DeDeaux, D.; Godley, P.A.; Mayer, D.K.; Song, L. Management of Androgen Deprivation Therapy-Associated Hot Flashes in Men With Prostate Cancer. Oncol. Nurs. Forum. 2019, 46, E107–E118.
- Crabb, S.; Morgan, A.; Hunter, M.S.; Stefanopoulou, E.; Griffiths, G.; Richardson, A.; Fenlon, D.; Fleure, L.; Raftery, J.; Boxall, C.; et al. A multicentre randomised controlled trial of a guided self-help cognitive behavioural therapy to MANage the impact of hot flushes and night sweats in patients with prostate CANcer undergoing androgen deprivation therapy (MANCAN2). Trials 2023, 24, 450.
- Gryzinski, G.M.; Fustok, J.; Raheem, O.M.; Bernie, H.L. Sexual Function in Men Undergoing Androgen Deprivation Therapy. Androg. Clin. Res. Ther. 2022, 3, 149–158.
- Corona, G.; Rastrelli, G.; Morgentaler, A.; Sforza, A.; Mannucci, E.; Maggi, M. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur. Urol. 2017, 72, 1000–1011.
- Lewis, R.W.; Mills, T.M. Effect of androgens on penile tissue. Endocrine 2004, 23, 101–105.
- Rizk, P.J.; Kohn, T.P.; Pastuszak, A.W.; Khera, M. Testosterone therapy improves erectile function and libido in hypogonadal men. Curr. Opin. Urol. 2017, 27, 511–515.
- Hotta, Y.; Kataoka, T.; Kimura, K. Testosterone Deficiency and Endothelial Dysfunction: Nitric Oxide, Asymmetric Dimethylarginine, and Endothelial Progenitor Cells. Sex. Med. Rev. 2019, 7, 661–668.
- Cunningham, G.R.; Stephens-Shields, A.J.; Rosen, R.C.; Wang, C.; Bhasin, S.; Matsumoto, A.M.; Parsons, J.K.; Gill, T.M.; Molitch, M.E.; Farrar, J.T.; et al. Testosterone Treatment and Sexual Function in Older Men With Low Testosterone Levels. J. Clin. Endocrinol. Metab. 2016, 10, 3096–3104.
- Wassersug, R.J. Maintaining intimacy for prostate cancer patients on androgen deprivation therapy. Curr. Opin. Support. Palliat. Care. 2016, 10, 55–65.
- Vitolins, M.Z.; Griffin, L.; Tomlinson, W.V.; Vuky, J.; Adams, P.T.; Moose, D.; Frizzell, B.; Lesser, G.J.; Naughton, M.; Radford, J.E.; et al. Randomized trial to assess the impact of venlafaxine and soy protein on hot flashes and quality of life in men with prostate cancer. J. Clin. Oncol. 2013, 31, 4092–4098.
- Kim, D.K.; Lee, H.S.; Park, J.Y.; Kim, J.W.; Ahn, H.K.; Ha, J.S.; Cho, K.S. Androgen-Deprivation Therapy and the Risk of Newly Developed Fractures in Patients With Prostate Cancer: A Nationwide Cohort Study in Korea. Sci. Rep. 2021, 11, 10057.
- Lin, J.K.; Parikh, R.B. Bone Health in Prostate Cancer Survivors: Recent Lessons and Opportunities for Improvement. Eur. Urol. Focus. 2023, 9, 422–424.
- Chin, K.Y.; Ima-Nirwana, S. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66.
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324.
- Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Testosterone and Bone Health in Men: A Narrative Review. J. Clin. Med. 2021, 10, 530.
- Hussain, A.; Tripathi, A.; Pieczonka, C.; Cope, D.; McNatty, A.; Logothetis, C.; Guise, T. Bone health effects of androgen-deprivation therapy and androgen receptor inhibitors in patients with nonmetastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 290–300.
- Zhu, X.; Wu, S. Increased Risk of Hypertension with Enzalutamide in Prostate Cancer: A Meta-Analysis. Cancer Investig. 2019, 37, 478–488.
- Gheorghe, G.S.; Hodorogea, A.S.; Ciobanu, A.; Nanea, I.T.; Gheorghe, A.C.D. Androgen Deprivation Therapy, Hypogonadism and Cardiovascular Toxicity in Men with Advanced Prostate Cancer. Curr. Oncol. 2021, 28, 3331–3346.
- Agarwal, M.; Canan, T.; Glover, G.; Thareja, N.; Akhondi, A.; Rosenberg, J. Cardiovascular effects of androgen deprivation therapy in prostate cancer. Curr. Oncol. Rep. 2019, 21, 91.
- Mitsuzuka, K.; Arai, Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int. J. Urol. 2018, 25, 45–53.
- Ketchandji, M.; Kuo, Y.F.; Shahinian, V.B.; Goodwin, J.S. Cause of death in older men after the diagnosis of prostate cancer. J. Am. Geriatr. Soc. 2009, 57, 24–30.
- Ng, C.T.; Bonilla, H.M.G.; Bryce, A.H.; Singh, P.; Herrmann, J. Approaches to Prevent and Manage Cardiovascular Disease in Patients Receiving Therapy for Prostate Cancer. Curr. Cardiol. Rep. 2023, 25, 889–899.
- Challa, A.A.; Calaway, A.C.; Cullen, J.; Garcia, J.; Desai, N.; Weintraub, N.L.; Deswal, A.; Kutty, S.; Vallakati, A.; Addison, D.; et al. Cardiovascular Toxicities of Androgen Deprivation Therapy. Curr. Treat. Options Oncol. 2021, 22, 47.
- Kakkat, S.; Pramanik, P.; Singh, S.; Singh, A.P.; Sarkar, C.; Chakroborty, D. Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections. Int. J. Mol. Sci. 2023, 24, 6984.





