Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebecca Yuan | -- | 1412 | 2024-01-05 16:48:45 | | | |
| 2 | Jessie Wu | Meta information modification | 1412 | 2024-01-08 03:24:46 | | | | |
| 3 | Jessie Wu | + 2 word(s) | 1414 | 2024-01-08 03:25:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chatterjee, S.; Yuan, R.; Thapa, S.; Talwar, R. β-1,4-GalT-V and Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/53491 (accessed on 07 February 2026).
Chatterjee S, Yuan R, Thapa S, Talwar R. β-1,4-GalT-V and Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/53491. Accessed February 07, 2026.
Chatterjee, Subroto, Rebecca Yuan, Spriha Thapa, Resham Talwar. "β-1,4-GalT-V and Cancer" Encyclopedia, https://encyclopedia.pub/entry/53491 (accessed February 07, 2026).
Chatterjee, S., Yuan, R., Thapa, S., & Talwar, R. (2024, January 05). β-1,4-GalT-V and Cancer. In Encyclopedia. https://encyclopedia.pub/entry/53491
Chatterjee, Subroto, et al. "β-1,4-GalT-V and Cancer." Encyclopedia. Web. 05 January, 2024.
Copy Citation
β-1,4-GalTs are a family of glycosyltransferases, all having similar properties (i.e., they exclusively transfer galactose residues from a donor UDP-galactose via β-1,4 linkage to acceptor sugars, N-acetyl glucosamine (GlcNAc),glucose (Gl)c, and xylose(Xyl), which can be components of protein or lipids that have different functions).
β-1,4-Galactosyltransferase-V
lactosylceramide
cancer
inflammation
cardiovascular disease
angiogenesis
1. Stem Cells and β-1,4-GalT-V
Aside from the galactosylation of glucosylceramide to form LacCer, β-1,4-GalT-V also galactosylates the β 1to β 6 branch arm of the highly branched N-glycan residues in glycoproteins. Since the malignant transformation of such N-glycan accompanies malignant transformation, it suggests that β-1,4-GalT-V has a pivotal position in stem cell cancers. This tenet is substantiated by reports on breast, prostate, hematopoietic, pancreatic, neck, colorectal, and brain cancers [1], wherein β-1,4-GalT-V is overexpressed compared to healthy subjects. In addition, glioblastomas are known to express 10-fold more of β-1,4-GalT-V than other cancers. This is because of the self-renewal of glioma-initiating stem cells. Conversely, downregulation of β-1,4-GalT-V using corresponding shRNA dose-dependently reduced β-1-4 GalT-V mRNA and protein in a mouse xenograft model of glioblastoma. This was accompanied by reduced cell proliferation in mice bearing gliomas [2]. As glioma-initiating stem cells are characterized by neural stem cell markers, e.g., nestin and CD133, downregulation of β-1,4-GalT-V was also accompanied by reduced mRNA expression of these neural stem cell markers, suggesting that CD133 and nestin may well have β-1-4 galactosylated epitope. And indeed, Western immunoblot assays of xenograft tumor samples from β-1-4 GalT-V shRNA treated mice revealed a decrease in the mass of CD-133 and nestin. In sum, this research revealed that stem cell biomarkers, e.g., CD133 and nestin, may well have a β-1,4-GalT epitope. And that malignant transformation of stem cells may involve galactosylation, resulting in overexpression of these proteins and consequent increase in phenotypes, e.g., proliferation, migration, angiogenesis, and tumor malignancy.
These studies opened the possibility that there may be other phenotypes wherein the β-1,4-GalT epitope may well regulate tumorgenicity. A case in point was Notch1, which was implicated in a demonstration that N-glycosylation regulates cell behavior through regulating membrane protein signaling. Notch1 is a glycoprotein, an N-glycan signaling pathway known to monitor cell differentiation, proliferation, and apoptosis. Studies show that N-glycation of Notch1 can regulate Notch-1 stability. To examine the role of β-1,4-GalT-V in Notch1 galactosylation, β-1,4-GalT-V expression was depleted using shRNA in a primary culture of human brain tumor cells (T698968). Depletion of β-1,4-GalT-V blocked β-1,4-galactosylation of Notch1 associated with a decrease in the migration of Notch1 to the cell surface. It binds to Galectin-3, as Notch1 binding to Galectin is also found to be galactosylation-dependent [3]. Thus, reduced galactosylation of Notch1 resulted in decreased degradation. Since Notch1 antibody immunoprecipitated β-1,4-GalT-V suggests that Notch-1 may be a substrate for β-1,4-GalT-V-mediated galactosylation (Figure 1).
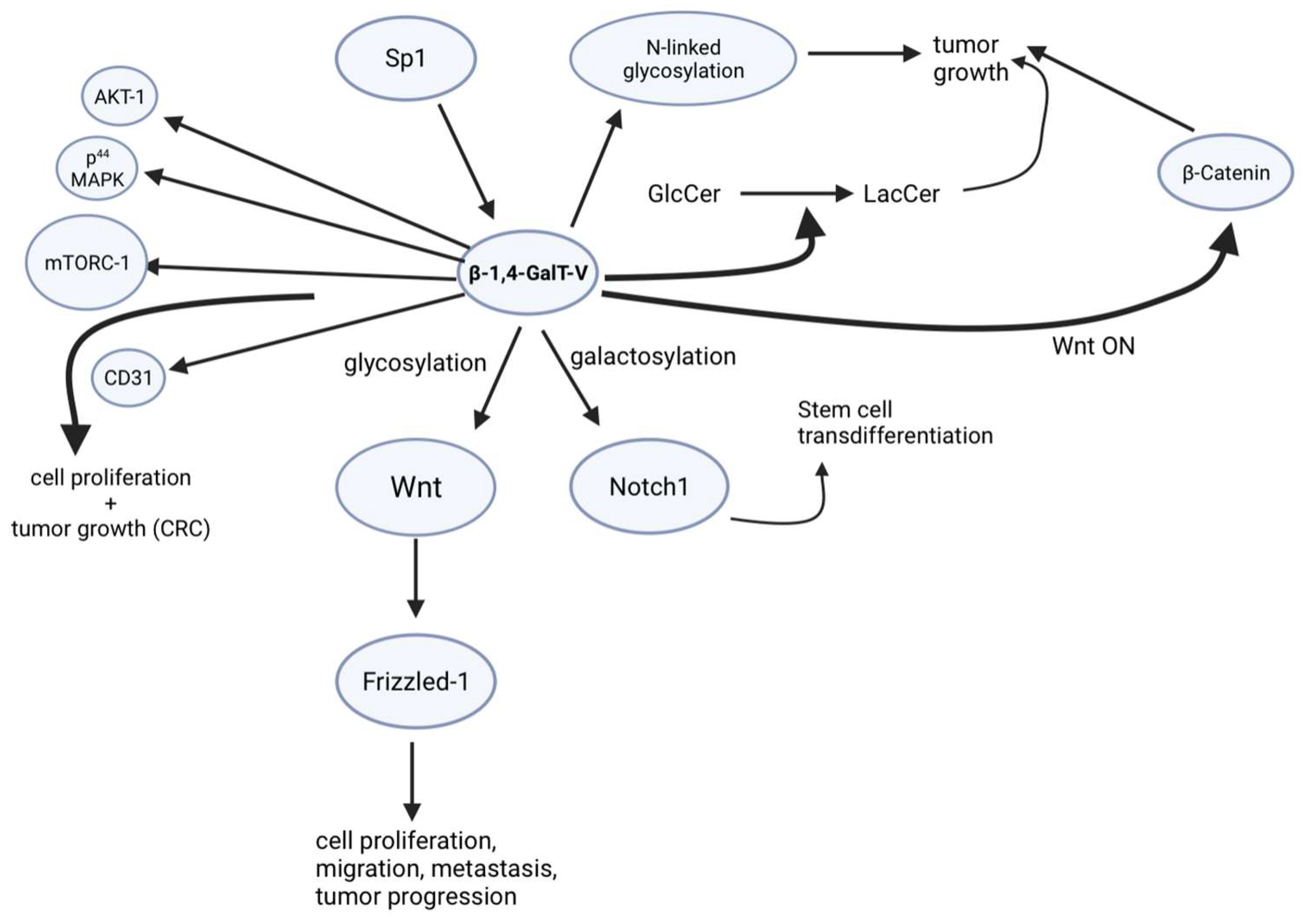
Figure 1. Signaling pathways by which β-1,4-GalT-V regulate various effector molecules in colorectal cancer (CRC).
One study also showed that β-1,4-GalT-V depletion blocked the trans-differentiation of glioma stem cells into endothelial cells and possibly angiogenesis [4]. Inactivation of this pathway with y-secretase or Notch1 knockdown leads to inhibition of glioma stem-like cells into endothelial cells. Recently, it was found that glioma stem-like cells transdifferentiate into tumor vascular endothelial cells, which was indicated as a new mechanism for VEGF-independent angiogenesis in malignant glioblastoma. N-glycans play a large role in regulating the activation of Notch signaling by manipulating the Notch receptor–ligand interaction. A study explores the role of β-1,4-GalT-V in gliomas (its trans-differentiation of glioma stem-like cells into endothelial cells) and the mechanism of Notch to develop better therapeutic drugs for this aggressive human cancer. It is important because it was shown, for the first time, that β-1,4-GalT-V can regulate trans-differentiation of glioma stem-like cells into endothelial cells, and depleting β-1,4-GalT-V was a key step in improving the survival of mice with glioblastoma tumors. N-glycan in tumor angiogenesis has been accumulating evidence as playing a critical role in tumor angiogenesis. β-1,4-GalT-V was also found to regulate Notch1 signaling in the trans-differentiation process of glioma stem-like cells into vascular endothelial cells (Figure 1); by decreasing the expression of β-1,4-GalT-V, Notch1 cleavage was reduced. Although the mechanism is currently unknown, the connection between β-1,4-GalT-V and Notch1 was discovered in this research, which is crucial to tumor angiogenesis, leading to possible cancer therapies [4].
2. Cell Migration and β-1,4-GalT-V
Members of the Frizzled family of integral membrane proteins are implicated in many developmental events, including specifying cell fate, orienting cell/planar polarity, and directing cell migration. Members of the Frizzled family function as cell surface receptors for secreted Wnt proteins. β-1,4-GalT-V glycosylates Frizzled1 (Figure 1), which plays a crucial role in promoting cell migration. Secreted Frizzled-related protein 1 (SFRP1) is a member of the SFRP family that modulates the Wnt signal transduction pathway. Downregulation of SFRP1 expression has been observed in CRC. Studies have shown that SFRP1 also suppresses cell proliferation, migration, and invasion and promotes apoptosis in CRC cells. Changes in the N-glycan structure of glycoproteins in glioma cells affect cell migration and tumor malignancy. GnT-V activates EGF-mediated signaling and, in part, promotes cell migration through the modification of N-glycans on receptor protein tyrosine phosphatase kappa (RPTPκ). Studies have shown that membrane-proximal N-glycosylation on integrin β1 positively regulates cell migration by promoting β1 activation. This suggests a novel regulatory mechanism wherein N-glycosylation near the cell membrane on β1 may serve as a platform that facilitates its complex formation on the cell membrane, thereby affecting integrin-mediated functions [5].
3. Upregulation of β-1,4-GalT-V in Cell Proliferation and Tumor Growth
In addition to aberrant glycosylation, it has been discovered that β-1,4-GalT-V also participates in cellular processes as an oncogenic signal transducer. This was demonstrated in CRC, where β-1,4-GalT-V exhibits increased expression levels (approximately 6.5-fold) as compared to normal tissues. There is also an increase in its enzymatic activity and its product LacCer in CRC [6]. As a matter of fact, while studies in many human cancer cell lines have shown β-1,4-GalT transferases to have little change in enzymatic activity upon malignant transformation, the same studies show β-1,4-GalT-V to have a 2-3-fold increase in cancer cells such as NIG 3T3 and MTAg. Furthermore, similar studies found that human cancer cells showed up to 5 times more levels of increase of β-1,4-GalT-V. In a human colorectal cancer cell line (HCT-116), the gene/protein expression of β-1,4-GalT-V, LacCer mass, and cell proliferation has a cell density-dependent increase, which was concomitantly mitigated by treatment with D-PDMP. In addition, another study revealed that the mRNA levels of β-1,4GalT-V were specifically increased in human colorectal cancer, contributing to tumor growth and metastasis. There was also an upregulation of both β-1,4-GalT-V activity and mass, as well as a most noticeable increase in the level of LacCer shown in human colorectal cancer tissues [6]. Thus, an overexpression of β-1,4-GalT-V was shown to increase tumor growth, while suppression by gene ablation diminished tumor growth. Furthermore, β-1,4-GalT-V upregulation could also lead to the increased activity of several other CRC gene biomarkers, which is crucial to the diagnosis and treatment of CRC. This could also be generalized to other forms of cancer as well [7].
β-1,4-GalT-V was also revealed to be highly enriched in vascular tissues—especially in single layers of endothelial cells, which take up most of the surface of the blood capillaries and are directly exposed to circulating blood. Large amounts of β-1,4-GalT-V are also associated with the cytoplasm in human colorectal cancer cells. When a non-coding DNA strand of β-1,4-GalT-V was introduced into cancer cells in an animal model, the tumor development was suppressed. Patients with inflammatory bowel disease (IBD) who have a greater risk of developing CRC have an elevated count of β-1,4-GalT-V. It can become an important biomarker for patients [8]. As shown in previous studies, VEGF was shown to stimulate β-1,4-GalT-V and to generate LacCer, eventually leading to angiogenesis using an “oxygen sensitive signaling” pathway. Tumors need VEGF to facilitate angiogenesis in many tumor types. One study connected the findings of recent studies to tumor growth as well as cell proliferation to investigate whether inhibiting glycosphingolipid synthesis would mitigate these phenotypes. It was shown that inhibiting LacCer synthase activity and reducing LacCer may reduce renal cancer in mice. Moreover, from these results, it was shown that β-1,4-GalT-V activity leading to LacCer production is central to signaling events involved in angiogenesis and cell proliferation, which led to decreased renal cancer tumors.
References
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807.
- Wei, Y.; Zhou, F.; Ge, Y.; Chen, H.; Cui, C.; Liu, D.; Yang, Z.; Wu, G.; Shen, J.; Gu, J.; et al. Regulation of the β1,4-Galactosyltransferase I promoter by E2F1. J. Biochem. 2010, 148, 263–271.
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Harazono, Y.; Gao, X.; Hogan, V.; Raz, A. Galectin-3 inhibits osteoblast differentiation through notch signaling. Neoplasia 2014, 16, 939–949.
- Cui, C.; Chen, X.; Liu, Y.; Cao, B.; Xing, Y.; Liu, C.; Yang, F.; Li, Y.; Yang, T.; Hua, L.; et al. β1,4-Galactosyltransferase V activates Notch1 signaling in glioma stem-like cells and promotes their trans-differentiation into endothelial cells. J. Biol. Chem. 2018, 293, 2219–2230.
- Hou, S.; Hang, Q.; Isaji, T.; Lu, J.; Fukda, T.; Gu, J. Importance of membrane-proximal N-glycosylation on integrin β1 in its activation and complex formation. FASEB J. 2016, 30, 4120–4131.
- Chatterjee, S.B.; Hou, J.; Bandaru, V.V.; Pezhouh, M.K.; Syed Rifat Mannan, A.A.; Sharma, R. Lactosylceramide synthase β-1,4-galt-V: A novel target for the diagnosis and therapy of human colorectal cancer. Biochem. Biophys. Res. Commun. 2019, 508, 380–386.
- Cavenee, W.K. Accumulation of genetic defects during astrocytoma progression. Cancer 1992, 70, 1788–1793.
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
519
Revisions:
3 times
(View History)
Update Date:
08 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




