Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eiad Habib | -- | 3016 | 2024-01-05 04:33:38 | | | |
| 2 | Rita Xu | Meta information modification | 3016 | 2024-01-05 04:51:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Habib, E.; Srivasthan, K.; El Masry, H. Sudden Death Risk in Repaired Tetralogy of Fallot. Encyclopedia. Available online: https://encyclopedia.pub/entry/53458 (accessed on 07 February 2026).
Habib E, Srivasthan K, El Masry H. Sudden Death Risk in Repaired Tetralogy of Fallot. Encyclopedia. Available at: https://encyclopedia.pub/entry/53458. Accessed February 07, 2026.
Habib, Eiad, Komandoor Srivasthan, Hicham El Masry. "Sudden Death Risk in Repaired Tetralogy of Fallot" Encyclopedia, https://encyclopedia.pub/entry/53458 (accessed February 07, 2026).
Habib, E., Srivasthan, K., & El Masry, H. (2024, January 05). Sudden Death Risk in Repaired Tetralogy of Fallot. In Encyclopedia. https://encyclopedia.pub/entry/53458
Habib, Eiad, et al. "Sudden Death Risk in Repaired Tetralogy of Fallot." Encyclopedia. Web. 05 January, 2024.
Copy Citation
Although substantial progress has been made to prevent sudden cardiac death in repaired tetralogy of Fallot patients, ventricular arrhythmia and sudden death continue to be major causes of morbidity and mortality in these patients. Greater survival in contemporary cohorts has been attributed to enhanced surgical techniques, more effective management of heart failure, and increased efforts in risk stratification and management of ventricular arrhythmias.
sudden cardiac death
risk stratification
ventricular tachycardia
tetralogy of Fallot
1. Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect [1]. Since the first surgical repair of TOF over 65 years ago, significant advancements in its management have led to increased survival. Advances in surgical interventions combined with enhanced imaging techniques, catheter-based and electrophysiology procedures, heart failure management, and improved surveillance have all contributed to significant progress in long-term outcomes [1]. However, late mortality from sudden cardiac death (SCD) was recognized in the 1970s and has continued to be an area of concern since then [2]. The principal etiologies presumed to contribute to SCD are ventricular arrhythmia and heart failure, though other mechanisms may be involved [3]. Although significant improvement in the detection, treatment, and prevention of such triggers has occurred, SCD remains a leading cause of mortality in this population [4]. Historical cohorts reported up to an 8.3% mortality rate for patients over 35 years [5] (which is 20 times higher than that of the general population); more recent cohorts are showing an annual mortality rate of 1% [6]. As a result, in these patients, researchers have been observing the constant evolution of approaches to enhance the prediction and management of arrhythmia substrates. Efforts have been made to develop comprehensive scoring systems that identify patients at high-risk of SCD and guide the decision for defibrillator implantation [7][8][9].
2. Mechanisms of Ventricular Tachycardia in Repaired TOF
The incidence of ventricular arrythmias in adults with TOF is unknown, but it is likely the dominant mechanism of SCD in this patient population [10]. The leading form of ventricular arrhythmia is monomorphic ventricular tachycardia (VT). A large study evaluating the effectiveness of implantable cardioverter defibrillators (ICDs) for primary and secondary prevention in repaired TOF found that >80% of ICD therapies were targeted specifically at monomorphic VT [11]. Such VTs are usually fast and not well tolerated [12]. Similar to VT related to structural heart disease (including ischemic cardiomyopathy), the leading mechanism of arrhythmia is macro re-entry. The substrate for re-entry in patients with repaired TOF depends on critical anatomic isthmuses (AIs) confined between areas of myocardial fibrosis or surgical patch and anatomic barriers, most commonly the pulmonic or tricuspid annulus [10]. Conduction between those barriers can slow down over time, facilitating re-entry around fixed barriers, most commonly between the ventricular septal defect (VSD) patch or right ventricular outflow tract (RVOT) incision and the pulmonic or tricuspid valve annulus [12][13]. Figure 1 highlights the AIs that may trigger VT. Both presence and geometry of AIs are influenced by variations in original anatomy, type of surgical repair, and presence of concurrent fibrosis [10].
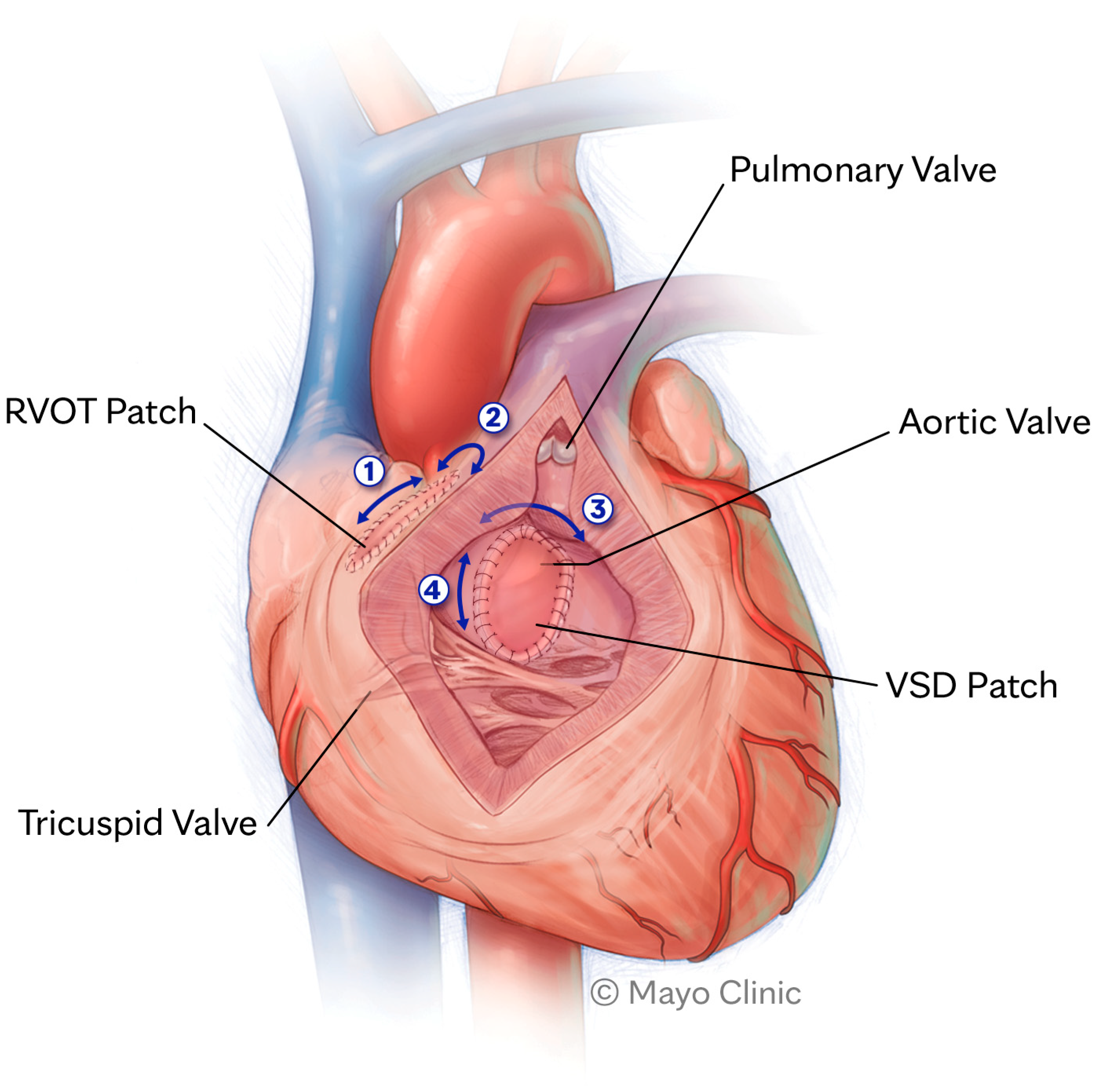
Figure 1. Anatomic isthmuses that may trigger ventricular tachycardia. RVOT, right ventricular outflow tract; VSD, ventricular septal defect.
Slow conduction (defined as conduction velocity < 0.5 m/s) across any pathway of interstitial fibrosis surrounded by inexcitable borders is shown to be a sensitive and specific marker for the presence of an arrhythmogenic isthmus that can sustain VT. Detailed electroanatomic mapping studies have demonstrated that lack of any slowly conducting anatomic isthmus in the RVOT of such patients correlates with lack of VT inducibility during electrophysiologic provocation, and absence of clinical VT on 2-year follow-up. On the other hand, the presence of a slowly conducting AI correlates with VT inducibility in 93% of patients [13].
Thus, the pathogenesis of VT in repaired TOF is related to an initial substrate due to the congenital anomaly and subsequent surgical corrections. Persistent hemodynamic insults, including volume/pressure overload, dilation, hypertrophy, and aging, lead to progressive fibrosis and slow conduction [10]. Triggers (such as PVCs and non-sustained ventricular tachycardia (NSVT)) that are facilitated by diffuse fibrosis coupled with susceptible substrate allow the generation of sustained VT [10].
In a minority of patients, VT substrates may be remote from the defined AIs, for instance, in relation to the duration of right ventricular ischemia during surgery or postcardiotomy fibrosis in others, and may create substrate changes in non-RVOT locations [10]. These patients are also at an increased risk of bundle branch re-entrant VT, especially with underlying conduction abnormalities and commonly present with right bundle-branch block [13]. Polymorphic VT or ventricular fibrillation are more frequently encountered in patients with suboptimal hemodynamics and worsening ventricular function. The relationship between these arrhythmias and the AIs remains unclear.
Bradyarrhythmia and SCD in Repaired TOF
While most sudden deaths in repaired TOF patients are assumed to be related to ventricular tachyarrhythmias and heart failure, a strong association between late-onset high-grade atrioventricular (AV) block and SCD exists and is well documented in the literature [14][15]. A large epidemiologic analysis noted an incidence of 1% in this population, with 0.6% undergoing permanent pacemaker implantation [16]. Patients typically present with syncope or cardiac arrest, often in the absence of a known trigger or electrocardiogram (ECG) changes [17]. As with degenerative conduction disease, AV block risk factors include a prolonged PR interval, left fascicular disease, and bifascicular block [18]. Additionally, delayed recovery of postoperative AV block following initial surgical repair (occurring beyond the third day) has been independently linked to a six-fold increase in risk of SCD over a 30-year follow-up [18]. However, it is important to note that these results pertain to a cohort who underwent a historic surgical repair approach, and various other risk factors may have contributed to their risk for SCD. To date, no prospective studies have examined the role of screening strategies in risk stratification for late AV block in repaired TOF patients. Whether extended invasive or noninvasive rhythm monitoring can detect occult conduction disease before SCD is yet to be seen. Finally, the utility of invasive electrophysiology evaluation to assess conduction intervals and, ultimately, its role in risk stratification of these patients remains unclear [19].
3. Risk Stratification
3.1. Risk Factors
Although SCD is among the leading causes of mortality in adults with TOF, recent studies have shown that the annual risk of SCD in adult patients with repaired TOF is 0.2% [20]. Since this number is far too low to warrant invasive risk stratification or implantation of ICDs for every patient, significant interest has been shown in identifying individuals at higher risk for SCD. To date, no single risk factor has sufficiently stratified the risk of SCD in repaired TOF patients. However, various risk factors allow for a more precise prediction of risk within this population [5]. Early multicenter studies demonstrated that the most common risk factors for SCD were left or right ventricular (RV) systolic dysfunction, prolonged QRS duration (≥180 milliseconds), late age of repair, and transannular patch repair [5]. A recently published meta-analysis of over 7000 patients with repaired TOF further noted the consistent impact of age, QRS duration, older age of repair, previous palliative shunts, atrial arrhythmias, and RV or left ventricular (LV) dysfunction on the risk of SCD in these patients [21].
3.2. Age
Older patient age, late age of initial repair, and late age at pulmonary valve replacement (PVR) have all been linked to an increased risk of ventricular arrhythmia in TOF patients. A large multicenter observational study noted age at initial repair to be an independent predictor of VT in univariate analyses, in addition to being a predictor of SCD in multivariate analyses [5]. Multiple prospective and retrospective multicenter registries demonstrated an association between older age at PVR and risk of VT, SCD or appropriate ICD therapies [21][22]. Yet, examining the influence of age on surgical repair independently from the surgical technique is challenging, as advancements in surgical techniques have occurred simultaneously with the push for early surgical repair. Patients undergoing repair at an older age are more likely to have increased myocardial fibrosis in light of multiple surgical interventions (hence surgical scars), worsened hemodynamics, and increased RV remodeling and hypertrophy.
3.3. Clinical Risk Factors
The presence of arrhythmia-like symptoms, including palpitations, pre-syncope, or syncope, has been linked with a higher incidence of malignant arrhythmias and SCD [23][24]. Khairy et al. [25] demonstrated that a reported history of syncope prior to the electrophysiological study was predictive of a higher risk of inducible monomorphic or polymorphic sustained VT (OR 4.9, p-value < 0.0001). Moreover, heart failure symptoms, including dyspnea, orthopnea, and edema (particularly NYHA class II or higher), have been independently associated with higher rates of SCD and ventricular arrhythmia [9][24][26].
Cardiopulmonary exercise testing is typically utilized to assess the extent of exercise intolerance in congenital heart disease (CHD) patients. A study by Müller et al. [27] revealed that predicted peak oxygen uptake (VO2) that was ≤65% or ventilatory efficiency (expressed as V E/V CO2 slope) ≥31 were independent predictors of sustained VT and mortality. A similar trend was observed in another prospective trial, demonstrating that a peak VO2 ≤ 17 mL/kg/m2 was associated with ventricular arrhythmias and all-cause mortality [9].
3.4. Surgical Risk Factors
As mentioned above, one of the proposed risk factors for SCD in repaired TOF patients is the effect of the surgical era in which the repairs occurred. Mechanisms for SCD that have been postulated in such patients include prolonged cyanosis and extensive right ventriculostomy, which have been shown to increase the incidence of ventricular arrhythmias [28]. The location and extent of ventriculotomy in these patients influence the dimensions of an AI that can propagate VT. A multicenter analysis indicated that the increasing complexity of defects in repaired TOF patients independently confers a higher risk of SCD [7]. Complex repairs, including patients with pulmonary atresia or a double outlet RV, resulted in a four-fold increased risk of SCD compared with conventional anatomy [7]. Another multicenter cohort study demonstrated that patients who underwent repair with a conduit associated with RV remodeling/hypertrophy had a higher mortality rate than patients with native outflow tracts [29]. In addition, palliative shunts before definite repair have been associated with ventricular arrhythmias [23]. Conversely, TOF patients who undergo transannular patch repair or who have an intact ventricular septum, even with significant RVOT obstruction, are at low risk of VT and SCD. In these cases, the repair circumvents the creation of an AI [23].
3.5. QRS Duration
Most patients with repaired TOF have a complete RBBB. Additional hemodynamic alterations (such as increased right ventricular pressure or volume overload) can induce ventricular fibrosis, further slowing down conduction and causing prolongation of the QRS complex [30]. A landmark study identified a strong association between QRS duration of ≥180 milliseconds and VT/SCD [5]. However, this older study examined patients from a different surgical era, while contemporary surgical techniques correlate with narrower QRS durations. As such, QRS duration must be evaluated within the context of the surgical era, and in current populations, a lower QRS duration (150 milliseconds) may be sufficient to indicate an AI and risk of VT [30]. More recent cohorts have confirmed these associations and noted that the risk association is continuous instead of dichotomous [23][31].
3.6. QRS Fragmentation
Fragmentation of the QRS complex (fQRS) is defined by the presence of three or more notches of the widened QRS in two or more contiguous leads. It has been associated with myocardial fibrosis and increased risk of SCD [32][33]. fQRS is present in up to 40% of patients with repaired TOF [34][35], and is more commonly found in patients with RV dysfunction, dyssynchrony, or fibrosis [34][36]. A prospective, multicenter study evaluated the influence of fQRS on mortality in patients with repaired TOF. The extent of fQRS was superior in predicting mortality compared to QRS duration, and fQRS was also predictive of ventricular arrythmias [34]. Another long-term follow-up study of repaired TOF patients who had implanted ICDs noted that QRS fragmentation was the sole independent predictor of appropriate ICD therapies in patients with devices inserted for primary prevention [37].
3.7. Cardiac Imaging
Echocardiography and cardiovascular magnetic resonance (CMR) can identify subclinical features that can recognize TOF patients at increased risk for SCD. Multiple studies have shown an association between RV remodeling (RV size and hypertrophy) and ventricular arrhythmias [29][38][39]. In addition, ventricular dyssynchrony and abnormalities in global longitudinal strain are implicated [40][41][42]. The presence and extent of late gadolinium enhancement (LGE) on CMR have been associated with spontaneous and inducible VT [39][43]. A study by Khairy et al. [11] demonstrated that elevated LV diastolic pressures were associated with a higher risk of ventricular arrhythmias. However, the thresholds for LV/RV dysfunction that translate to a significant risk of VT/SCD remain unknown.
3.8. Genetic Syndromes
Patients with genetic syndromes, especially DiGeorge syndrome (22q11 microdeletion syndrome) associated with pulmonary atresia and repaired TOF, have a higher mortality rate and as much as five times higher risk of SCD [44]. However, these patients have higher rates of LV systolic dysfunction and more deleterious effects resulting from extra-cardiac lesions that may be confounding factors for higher rates of mortality compared to TOF patients without genetic syndromes [45].
4. Risk Scores
4.1. Khairy Score
In a landmark analysis published in 2004 [25], Khairy et al. showed that inducible monomorphic or polymorphic VT following rigorous programmed ventricular stimulation increased the likelihood of SCD five-fold compared to established noninvasive risk factors alone. Conversely, noninducibility was shown to have a favorable prognosis with 89% 15-year survival. Extrapolating from results, the Khairy score [11] was published in 2008 to better predict the need for ICD implantation for primary prevention in this patient population. Although widespread adoption and implementation of this risk score have provided significant benefits, clinicians should be aware of some important pitfalls when using this score in contemporary management. Firstly, calculating the score requires the derivation of LV end-diastolic pressure in addition to results of an invasive electrophysiology study (EPS). This limits its usefulness as a screening tool. An approach that is commonly used is to refer patients with multiple noninvasive risk factors to have an invasive EPS to further stratify their risk of arrhythmia and SCD [10]. Secondly, patients with complex defects (such as pulmonary atresia, double outlet RV, or Rastelli repair) were excluded from the study cohorts, which may underestimate the risk of SCD within these specific groups of patients [7]. Finally, patients included in the initial cohort had multiple comorbidities by contemporary standards, including a late age of repair (average age 4.5 years), 50% requiring palliative shunts before definitive repair, 17% with documented sustained VT, and 25% with a history of syncope [25]. A reanalysis of the Khairy score that was recently published highlighted a suboptimal C-index of 0.6 in predicting SCD [46]. As discussed previously, it is plausible that older surgical techniques, along with longer durations of cyanotic circulation during childhood, may have resulted in more significant myocardial fibrosis. This, in turn, lead to a larger substrate for ventricular arrhythmia and a greater risk of SCD that may not be appreciated within a contemporary cohort.
To enhance the versatility of the Khairy score while recognizing the robust and comprehensive disease discrimination that CMR can provide in patients with repaired TOF, Bokma and colleagues devised a model for predicting mortality and ventricular arrhythmia in 2017 [47]. This model utilized the noninvasive variables of the Khairy score and supplemented them with CMR-derived metrics related to significant systolic dysfunction (LVEF < 45% and RVEF < 30%), translating to an augmented C-index of 0.75.
4.2. Contemporary Risk Scores
More recently, four large multicenter analyses have formulated risk scores with greater sensitivity and specificity than the Khairy score for prediction of SCD in TOF patients. The Spanish ACHD network [7] evaluated over 3500 patients with a wide range of congenital heart diseases, including 360 patients with repaired TOF, to create a risk score with high sensitivity (C-index of 0.91) without marked reduction in specificity. Similarly, the PREVENTION-ACHD risk score [8] was devised to recognize high-risk features for SCD in congenital heart disease patients, and their cohort included 138 patients with repaired TOF. Both of these contemporary risk scores highlighted ischemic heart disease as a novel and significant risk factor for SCD, with a four- to eight-fold increase in odds [7][8]. Unfortunately, both of these risk scores were derived from all CHD patients. Although they provide high sensitivity and specificity for SCD across various CHD lesions, their predictive value, specifically within TOF patients, remains unknown.
A recently published prospective study by Ghonim et al. [9] included a large cohort of 550 patients with repaired TOF and incorporated detailed CMR and LGE burden to construct a score that exhibited a strong predictive capability for SCD risk. The score, consisting of eight risk factors, heavily relies on CMR measurements (68/100 points) and more specifically on the extent of RV LGE (40/100 points). Patients in the highest-risk group (≥51 points) had a 4.4% annual mortality rate and 36% mortality at ten years, while those in the low-risk group (0–20 points) had <0.2 yearly mortality and 1% 10-year mortality [9]. It is worth noting that after accounting for the degree of LGE, characteristics of surgical repair, QRS duration, and NSVT were not independently predictive of outcomes. This further validates the complex interplay between such parameters as surrogate indicators of myocardial fibrosis [48]. An important caveat is that accurate quantification of RV LGE requires a high level of expertise to guarantee reproducibility. Given how significant this value is to determining the overall score, each institution must consider its ability to accurately provide CMR metrics to avoid inappropriate implementation to their specific patient population [9].
Another study published earlier this year [49] used a machine learning algorithm that incorporated 57 variables from electronic records of patients with repaired TOF to develop a scoring system that could predict a composite outcome of mortality, resuscitated sudden death, heart failure admissions, and sustained VT. Once developed, a refined model that included the ten strongest risk factors (labeled “AiTOR”) performed well in an independent validation cohort with a C-index of 0.82. However, LGE data were only available for a small subset of patients (4%), which may reflect routine clinical practice and thus was not sufficiently powered to be included in the final model. Importantly, while this model could predict a composite of outcomes, very few sustained VTs or sudden deaths were noted, limiting the score’s utility in predicting VT and ICD.
As with any high-risk cohort, it is important to highlight that these risk scores do not encompass every patient who develops SCD. Studies have shown that even with a sensitivity of ~95%, low-risk patients continue to have an approximately 0.2% annual risk of ventricular arrhythmias and SCD [9]. It is yet uncertain whether complimentary strategies, such as continuous monitoring through wearable biosensors [49], contemporary multidisciplinary clinical surveillance techniques, and early intervention for structural and electrophysiological abnormalities, effectively reduce the remaining risk.
References
- Dobson, R.J.; Ramparsad, N.; Walker, N.L.; McConnachie, A.; Danton, M.H.D. Outcomes of adults with repaired tetralogy of Fallot from the national Scottish Cohort. Cardiol. Young 2021, 31, 1306–1314.
- James, F.W.; Kaplan, S.; Chou, T.C. Unexpected cardiac arrest in patients after surgical correction of tetralogy of Fallot. Circulation 1975, 52, 691–695.
- Quattlebaum, T.G.; Varghese, J.; A Neill, C.; Donahoo, J.S. Sudden death among postoperative patients with tetralogy of Fallot: A follow-up study of 243 patients for an average of twelve years. Circulation 1976, 54, 289–293.
- Dennis, M.; Moore, B.; Kotchetkova, I.; Pressley, L.; Cordina, R.; Celermajer, D.S. Adults with repaired tetralogy: Low mortality but high morbidity up to middle age. Open Heart 2017, 4, e000564.
- Gatzoulis, M.A.; Balaji, S.; Webber, S.A.; Siu, S.C.; Hokanson, J.S.; Poile, C.; Rosenthal, M.; Nakazawa, M.; Moller, J.H.; Gillette, P.C.; et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet 2000, 356, 975–981.
- Khairy, P.; Silka, M.J.; Moore, J.P.; DiNardo, J.A.; Vehmeijer, J.T.; Sheppard, M.N.; van de Bruaene, A.; Chaix, M.-A.; Brida, M.; Moore, B.M.; et al. Sudden cardiac death in congenital heart disease. Eur. Heart J. 2022, 43, 2103–2115.
- Oliver, J.M.; Gallego, P.; Gonzalez, A.E.; Avila, P.; Alonso, A.; Garcia-Hamilton, D.; Peinado, R.; Dos-Subirà, L.; Pijuan-Domenech, A.; Rueda, J.; et al. Predicting sudden cardiac death in adults with congenital heart disease. Heart 2021, 107, 67–75.
- Vehmeijer, J.T.; Koyak, Z.; Leerink, J.M.; Zwinderman, A.H.; Harris, L.; Peinado, R.; Oechslin, E.N.; Robbers-Visser, D.; Groenink, M.; Boekholdt, S.M.; et al. Identification of patients at risk of sudden cardiac death in congenital heart disease: The PRospEctiVE study on implaNTable cardIOverter defibrillator therapy and suddeN cardiac death in Adults with Congenital Heart Disease (PREVENTION-ACHD). Heart Rhythm. 2021, 18, 785–792.
- Ghonim, S.; Gatzoulis, M.A.; Ernst, S.; Li, W.; Moon, J.C.; Smith, G.C.; Heng, E.L.; Keegan, J.; Ho, S.Y.; McCarthy, K.P.; et al. Predicting Survival in Repaired Tetralogy of Fallot: A Lesion-Specific and Personalized Approach. JACC Cardiovasc. Imaging 2022, 15, 257–268.
- Krieger, E.V.; Zeppenfeld, K.; DeWitt, E.S.; Duarte, V.E.; Egbe, A.C.; Haeffele, C.; Lin, K.Y.; Robinson, M.R.; Sillman, C.; Upadhyay, S.; et al. Arrhythmias in Repaired Tetralogy of Fallot: A Scientific Statement From the American Heart Association. Circ. Arrhythmia Electrophysiol. 2022, 15, 776–791.
- Khairy, P.; Harris, L.; Landzberg, M.J.; Viswanathan, S.; Barlow, A.; Gatzoulis, M.A.; Fernandes, S.M.; Beauchesne, L.; Therrien, J.; Chetaille, P.; et al. Implantable cardioverter-defibrillators in tetralogy of fallot. Circulation 2008, 117, 363–370.
- Kapel, G.F.; Reichlin, T.; Wijnmaalen, A.P.; Piers, S.R.; Holman, E.R.; Tedrow, U.B.; Schalij, M.J.; Stevenson, W.G.; Zeppenfeld, K. Re-Entry using anatomically determined isthmuses: A curable ventricular tachycardia in repaired congenital heart disease. Circ. Arrhythmia Electrophysiol. 2015, 8, 102–109.
- Kapel, G.F.; Sacher, F.; Dekkers, O.M.; Watanabe, M.; Blom, N.A.; Thambo, J.-B.; Derval, N.; Schalij, M.J.; Jalal, Z.; Wijnmaalen, A.P.; et al. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired tetralogy of Fallot. Eur. Heart J. 2017, 38, 268–276.
- Nakazawa, M.; Shinohara, T.; Sasaki, A.; Echigo, S.; Kado, H.; Niwa, K.; Oyama, K.; Yokota, M.; Iwamoto, M. Arrhythmias late after repair of tetralogy of fallot—A Japanese Multicenter Study. Circ. J. 2004, 68, 126–130.
- Deanfield, J.E.; McKenna, W.J.; Hallidie-Smith, K.A. Detection of late arrhythmia and conduction disturbance after correction of tetralogy of Fallot. Heart 1980, 44, 248–253.
- Wu, M.-H.; Lu, C.-W.; Chen, H.-C.; Chiu, S.-N.; Kao, F.-Y.; Huang, S.-K. Arrhythmic burdens in patients with tetralogy of Fallot: A national database study. Heart Rhythm. 2015, 12, 604–609.
- Fujita, T.; Yoshida, A.; Ichikawa, M. A case report of paroxysmal complete atrioventricular block in a patient with dextrocardia and repaired tetralogy of Fallot. Eur. Heart J. Case Rep. 2022, 6, ytac428.
- Hokanson, J.S.; Moller, J.H. Significance of early transient complete heart block as a predictor of sudden death late after operative correction of tetralogy of fallot. Am. J. Cardiol. 2001, 87, 1271–1277.
- Friedli, B.; Bolens, M.; Taktak, M. Conduction disturbances after correction of tetralogy of Fallot: Are electrophysiologic studies of prognostic value? J. Am. Coll. Cardiol. 1988, 11, 162–165.
- Diller, G.-P.; Kempny, A.; Alonso-Gonzalez, R.; Swan, L.; Uebing, A.; Li, W.; Babu-Narayan, S.; Wort, S.J.; Dimopoulos, K.; Gatzoulis, M.A.; et al. Survival Prospects and Circumstances of Death in Contemporary Adult Congenital Heart Disease Patients Under Follow-Up at a Large Tertiary Centre. Circulation 2015, 132, 2118–2125.
- Possner, M.; Tseng, S.Y.; Alahdab, F.; Bokma, J.P.; Lubert, A.M.; Khairy, P.; Murad, M.H.; Ben Ali, W.; Prokop, L.J.; Czosek, R.J.; et al. Risk Factors for Mortality and Ventricular Tachycardia in Patients With Repaired Tetralogy of Fallot: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2020, 36, 1815–1825.
- Geva, T.; Mulder, B.; Gauvreau, K.; Babu-Narayan, S.V.; Wald, R.M.; Hickey, K.; Powell, A.J.; Gatzoulis, M.A.; Valente, A.M. Preoperative Predictors of Death and Sustained Ventricular Tachycardia after Pulmonary Valve Replacement in Patients With Repaired Tetralogy of Fallot Enrolled in the INDICATOR Cohort. Circulation 2018, 138, 2106–2115.
- Atallah, J.; Corcia, M.C.G.; Walsh, E.P. Ventricular Arrhythmia and Life-Threatening Events in Patients With Repaired Tetralogy of Fallot. Am. J. Cardiol. 2020, 132, 126–132.
- Ghai, A.; Silversides, C.; Harris, L.; Webb, G.D.; Siu, S.C.; Therrien, J. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of fallot. J. Am. Coll. Cardiol. 2002, 40, 1675–1680.
- Khairy, P.; Landzberg, M.J.; Gatzoulis, M.A.; Lucron, H.; Lambert, J.; Marçon, F.; Alexander, M.E.; Walsh, E.P. Value of programmed ventricular stimulation after tetralogy of fallot repair: A multicenter study. Circulation 2004, 109, 1994–2000.
- Vehmeijer, J.T.; Koyak, Z.; Bokma, J.P.; Budts, W.; Harris, L.; Mulder, B.J.M.; de Groot, J.R. Sudden cardiac death in adults with congenital heart disease: Does QRS-complex fragmentation discriminate in structurally abnormal hearts? EP Eur. 2018, 20, f122–f128.
- Müller, J.; Hager, A.; Diller, G.-P.; Derrick, G.; Buys, R.; Dubowy, K.-O.; Takken, T.; Orwat, S.; Inuzuka, R.; Vanhees, L.; et al. Peak oxygen uptake, ventilatory efficiency and QRS-duration predict event free survival in patients late after surgical repair of tetralogy of Fallot. Int. J. Cardiol. 2015, 196, 158–164.
- Dietl, C.A.; Cazzaniga, M.E.; Dubner, S.J.; Pérez-Baliño, N.A.; Torres, A.R.; Favaloro, R.G. Life-threatening arrhythmias and RV dysfunction after surgical repair of tetralogy of Fallot. Comparison between transventricular and transatrial approaches. Circulation 1994, 90, II7-12.
- Valente, A.M.; Gauvreau, K.; Assenza, G.E.; Babu-Narayan, S.V.; Schreier, J.; Gatzoulis, M.A.; Groenink, M.; Inuzuka, R.; Kilner, P.J.; Koyak, Z.; et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart 2014, 100, 247–253.
- Kapel, G.F.; Brouwer, C.; Jalal, Z.; Sacher, F.; Venlet, J.; Schalij, M.J.; Thambo, J.-B.; Jongbloed, M.R.; Blom, N.A.; de Riva, M.; et al. Slow Conducting Electroanatomic Isthmuses: An Important Link Between QRS Duration and Ventricular Tachycardia in Tetralogy of Fallot. JACC Clin. Electrophysiol. 2018, 4, 781–793.
- Khairy, P.; Aboulhosn, J.; Gurvitz, M.Z.; Opotowsky, A.R.; Mongeon, F.-P.; Kay, J.D.; Valente, A.M.; Earing, M.G.; Lui, G.K.; Gersony, D.R.; et al. Arrhythmia burden in adults with surgically repaired tetralogy of fallot: A multi-institutional study. Circulation 2010, 122, 868–875.
- Das, M.K.; El Masry, H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr. Opin. Cardiol. 2010, 25, 59–64.
- Bazoukis, G.; Garcia-Zamora, S.; Çinier, G.; Lee, S.; Gul, E.E.; Álvarez-García, J.; Miana, G.; Hayıroğlu, M.; Tse, G.; Liu, T.; et al. Association of electrocardiographic markers with myocardial fibrosis as assessed by cardiac magnetic resonance in different clinical settings. World J. Cardiol. 2022, 14, 483–495.
- Bokma, J.P.; Winter, M.M.; Vehmeijer, J.T.; Vliegen, H.W.; van Dijk, A.P.; van Melle, J.P.; Meijboom, F.J.; Post, M.C.; Zwinderman, A.H.; Mulder, B.J.M.; et al. QRS fragmentation is superior to QRS duration in predicting mortality in adults with tetralogy of Fallot. Heart 2017, 103, 666–671.
- Egbe, A.C.; Miranda, W.R.; Mehra, N.; Ammash, N.M.; Missula, V.R.; Madhavan, M.; Deshmukh, A.J.; Abdelsamid, M.F.; Kothapalli, S.; Connolly, H.M. Role of QRS Fragmentation for Risk Stratification in Adults With Tetralogy of Fallot. J. Am. Heart Assoc. 2018, 7, e010274.
- On, Y.K.; Kim, J.S.; Park, S.W.; Yang, J.-H.; Jun, T.-G.; Kang, I.-S.; Lee, H.J.; Choe, Y.H.; Huh, J. Relation of fragmented QRS complex to right ventricular fibrosis detected by late gadolinium enhancement cardiac magnetic resonance in adults with repaired tetralogy of fallot. Am. J. Cardiol. 2012, 109, 110–115.
- Waldmann, V.; Bouzeman, A.; Duthoit, G.; Koutbi, L.; Bessiere, F.; Labombarda, F.; Marquié, C.; Gourraud, J.B.; Mondoly, P.; Sellal, J.M.; et al. Long-Term Follow-Up of Patients With Tetralogy of Fallot and Implantable Cardioverter Defibrillator: The DAI-T4F Nationwide Registry. Circulation 2020, 142, 1612–1622.
- Diller, G.-P.; Kempny, A.; Liodakis, E.; Alonso-Gonzalez, R.; Inuzuka, R.; Uebing, A.; Orwat, S.; Dimopoulos, K.; Swan, L.; Li, W.; et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation 2012, 125, 2440–2446.
- Hanneman, K.; Crean, A.M.; Wintersperger, B.J.; Thavendiranathan, P.; Nguyen, E.T.; Kayedpour, C.; Wald, R.M. The relationship between cardiovascular magnetic resonance imaging measurement of extracellular volume fraction and clinical outcomes in adults with repaired tetralogy of Fallot. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 777–784.
- Moon, T.J.; Choueiter, N.; Geva, T.; Valente, A.M.; Gauvreau, K.; Harrild, D.M. Relation of biventricular strain and dyssynchrony in repaired tetralogy of fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am. J. Cardiol. 2015, 115, 676–680.
- Ortega, M.; Triedman, J.K.; Geva, T.; Harrild, D.M. Relation of left ventricular dyssynchrony measured by cardiac magnetic resonance tissue tracking in repaired tetralogy of fallot to ventricular tachycardia and death. Am. J. Cardiol. 2011, 107, 1535–1540.
- Orwat, S.; Diller, G.-P.; Kempny, A.; Radke, R.; Peters, B.; Kühne, T.; Boethig, D.; Gutberlet, M.; Dubowy, K.-O.; Beerbaum, P.; et al. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart 2016, 102, 209–215.
- Ghonim, S.; Ernst, S.; Keegan, J.; Giannakidis, A.; Spadotto, V.; Voges, I.; Smith, G.C.; Boutsikou, M.; Montanaro, C.; Wong, T.; et al. Three-Dimensional Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Predicts Inducibility of Ventricular Tachycardia in Adults With Repaired Tetralogy of Fallot. Circ. Arrhythmia Electrophysiol. 2020, 13, e008321.
- van Mil, S.; Heung, T.; Malecki, S.; Van, L.; Chang, J.; Breetvelt, E.; Wald, R.; Oechslin, E.; Silversides, C.; Bassett, A.S. Impact of a 22q11.2 Microdeletion on Adult All-Cause Mortality in Tetralogy of Fallot Patients. Can. J. Cardiol. 2020, 36, 1091–1097.
- Calcagni, G.; Calvieri, C.; Baban, A.; Bianco, F.; Barracano, R.; Caputo, M.; Madrigali, A.; Kikina, S.S.; Perrone, M.A.; Digilio, M.C.; et al. Syndromic and Non-Syndromic Patients with Repaired Tetralogy of Fallot: Does It Affect the Long-Term Outcome? J. Clin. Med. 2022, 11, 850.
- Vehmeijer, J.T.; Koyak, Z.; Budts, W.; Harris, L.; Silversides, C.K.; Oechslin, E.N.; Bouma, B.J.; Zwinderman, A.H.; Mulder, B.J.; de Groot, J.R.; et al. Prevention of Sudden Cardiac Death in Adults with Congenital Heart Disease: Do the Guidelines Fall Short? Circ. Arrhythmia Electrophysiol. 2017, 10, e005093.
- Bokma, J.P.; de Wilde, K.C.; Vliegen, H.W.; van Dijk, A.P.; van Melle, J.P.; Meijboom, F.J.; Zwinderman, A.H.; Groenink, M.; Mulder, B.J.M.; Bouma, B.J. Value of Cardiovascular Magnetic Resonance Imaging in Noninvasive Risk Stratification in Tetralogy of Fallot. JAMA Cardiol. 2017, 2, 678–683.
- Kakarla, J.; Denham, N.C.; Ishikita, A.; Oechslin, E.; Alonso-Gonzalez, R.; Nair, K. Risk Stratification for Sudden Cardiac Death in Repaired Tetralogy of Fallot. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 414–425.
- Ishikita, A.; McIntosh, C.; Hanneman, K.; Lee, M.M.; Liang, T.; Karur, G.R.; Roche, S.L.; Hickey, E.; Geva, T.; Barron, D.J.; et al. Machine Learning for Prediction of Adverse Cardiovascular Events in Adults With Repaired Tetralogy of Fallot Using Clinical and Cardiovascular Magnetic Resonance Imaging Variables. Circ. Cardiovasc. Imaging 2023, 16, e015205.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
985
Revisions:
2 times
(View History)
Update Date:
05 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




